Abstract
The interaction of the immune system with Neisseria commensals remains poorly understood. We have previously shown that phosphoethanolamine on the lipid A portion of lipooligosaccharide (LOS) plays an important role in Toll-like receptor 4 (TLR4) signaling. For pathogenic Neisseria, phosphoethanolamine is added to lipid A by the phosphoethanolamine transferase specific for lipid A, which is encoded by lptA. Here, we report that Southern hybridizations and bioinformatics analyses of genomic sequences from all eight commensal Neisseria species confirmed that lptA was absent in 15 of 17 strains examined but was present in N. lactamica. Mass spectrometry of lipid A and intact LOS revealed the lack of both pyrophosphorylation and phosphoethanolaminylation in lipid A of commensal species lacking lptA. Inflammatory signaling in human THP-1 monocytic cells was much greater with pathogenic than with commensal Neisseria strains that lacked lptA, and greater sensitivity to polymyxin B was consistent with the absence of phosphoethanolamine. Unlike the other commensals, whole bacteria of two N. lactamica commensal strains had low inflammatory potential, whereas their lipid A had high-level pyrophosphorylation and phosphoethanolaminylation and induced high-level inflammatory signaling, supporting previous studies indicating that this species uses mechanisms other than altering lipid A to support commensalism. A meningococcal lptA deletion mutant had reduced inflammatory potential, further illustrating the importance of lipid A pyrophosphorylation and phosphoethanolaminylation in the bioactivity of LOS. Overall, our results indicate that lack of pyrophosphorylation and phosphoethanolaminylation of lipid A contributes to the immune privilege of most commensal Neisseria strains by reducing the inflammatory potential of LOS.
INTRODUCTION
The members of the genus Neisseria that colonize humans are classified into two pathogenic and eight commensal species. Infections due to the two pathogenic species, N. meningitidis and N. gonorrhoeae, represent a major public health problem around the world. N. meningitidis is the leading cause of epidemic meningitis, causing approximately 50,000 deaths worldwide each year (53). N. gonorrhoeae is a major cause of infections worldwide, estimated to be 60 million annually. In women, gonococcal infection can lead to pelvic inflammatory disease in 10 to 20% of those infected, causing chronic pain, infertility, and risk for ectopic pregnancy (22, 42, 50).
Although it can be a deadly human pathogen, N. meningitidis is carried in the nasopharynx by approximately 10% of the population and only results in disease characterized by septicemia and meningitis at low frequencies (65). Asymptomatic carriage of N. meningitidis, which is infrequent in infancy and peaks in early adulthood, is the mechanism by which the reservoir of meningococci is maintained within the population. Similarly, asymptomatic gonococcal infection of women plays an important role in maintaining transmission of the organism (16).
The eight commensal Neisseria species are N. cinerea, N. elongata, N. flavescens, N. lactamica, N. mucosa, N. polysaccharea, N. sicca, and N. subflava, with N. perflava and N. flava considered biovars of N. subflava (34). These species colonize the upper respiratory tract and are classified as part of the nonpathogenic normal flora, although scattered reports of infections by these organisms exist (17, 32). Historically, estimates of carriage rates ranged from approximately 3 to 40% depending on the species, and more recent data would suggest that the rates are even higher than previously estimated (35). In addition, high levels of colonization in individuals have been reported. Using recent advances in sequencing technology, Zaura et al. found that five oral cavity sites sampled in three healthy individuals yielded sequences of Neisseria which together represented more than 10% of the 266 species-level phylotypes harbored at each site (66), with only streptococci being more predominant. This suggests that Neisseria species represent a major proportion of the core oral cavity microbiome in healthy individuals, and as such, they must have a considerable impact on the symbiotic colonization state of the respiratory tract mucosa maintained by the innate immune system.
Differences in the reactive and immunogenic potentials of the pathogenic and commensal Neisseria species have been examined in a few studies. For example, it was shown that N. meningitidis damaged ciliated cells in human nasopharyngeal organ culture, whereas N. subflava did not (54). In a study of cultured meningioma cells, Fowler et al. reported that although both N. lactamica and N. meningitidis were found to bind to the cells, only N. meningitidis induced meningioma cell death (18). Vaughan et al. demonstrated that at the peak period of colonization with N. lactamica in early childhood there was an absence of mucosal cell-mediated immunity to N. lactamica which generated only a mitogenic response associated with production of T cell-independent polyclonal IgM, unlike the response to N. meningitidis which primed for the development T or B cell memory (63). In a subsequent study, they reported that outer membrane vesicles of N. lactamica were B cell mitogens for cultured mucosal mononuclear cells; however, porin protein PorB and lipooligosaccharide (LOS) were determined to be unlikely mitogenic candidates (62). In contrast, PorB from N. lactamica has been shown to serve as a Toll-like receptor 2 (TLR2) agonist, similar to N. meningitidis PorB, although it was less bioactive for human airway epithelial cells than the meningococcal PorB variant (39).
Phenotypic relationships among Neisseria species have been analyzed using a taxonomic approach in the past (5). More recently, the availability of the complete genome sequence for N. meningitidis strains MC58 and Z2491 and N. gonorrhoeae strain FA1090 enabled a bioinformatic microarray analysis of N. meningitidis serogroup strains and commensal Neisseria to identify genetic variations conserved within the pathogens that were lacking in all the commensal strains (51). Using this definition, the analyses revealed 55 potential virulence genes in N. meningitidis, some of which were correlated with previously acquired data from other genetic typing methods. The genes implicated in pathogenicity included those involved in pilin biosynthesis, iron response, adherence to epithelial cells, and serogroup-specific capsule biosynthesis. Pilin proteins in the pathogenic Neisseria strains undergo antigenic variation, whereas the commensal Neisseria strains lack the required silent pilin gene copies (26, 43, 51). In addition, evidence has been obtained indicating that, whereas the commensal Neisseria strains have single chromosome copies, the pathogenic Neisseria are homozygous diploids, which could make the bacteria more resistant to DNA damage (60). The diversity of Neisseria species at mucosal surfaces provides a potential opportunity for genetic exchange among the bacteria, and comprehensive genome sequencing confirmed that this could occur (40).
We previously observed significant differences in the induction of proinflammatory cytokines mediated by TLR4 signaling in response to different strains of N. gonorrhoeae and N. meningitidis and to their LOS, a major inflammatory component of the bacterial outer membrane (30, 38, 45). In particular, we found that variations in acylation of the lipid A (LA) portion of the LOS and differences in the numbers of phosphate (P) and phosphoethanolamine (PEA) groups on the LA significantly influenced inflammatory signaling through TLR4. In this study, we postulated that the engagement of the innate immune system of the respiratory tract mucosa by the LOS of commensal Neisseria may elicit a weak response, thereby promoting persistent colonization. This concept is supported by the recent report that meningococcal LOS with hexaacylated LA was significantly more inflammatory in the lungs of an in vivo mouse model than LOS with pentaacylated LA (25). In addition, the study also suggested that, although bacterial products and cytokines can upregulate MD-2, low expression levels of MD-2 in the airway epithelia play a role in the respiratory response to neisserial LOS (29). Thus, we wanted to explore the relative inflammatory potentials of the commensal and pathogenic Neisseria strains and their LOS.
We report here that inflammatory signaling in the human THP-1 monocytic cell line, as well as polymyxin B resistance, is significantly greater when treated with the pathogenic Neisseria species than with the commensal Neisseria species. We found that the difference in induction of inflammatory signaling was characterized in most commensal Neisseria species by the absence of a functional lptA and the lack of phosphoethanolaminylation and pyrophosphorylation of the LA component of the LOS.
MATERIALS AND METHODS
Bacterial strains and LOS extraction.
The sources of all of the bacterial strains, including those that were analyzed using a Blast search of the National Center for Biotechnology Information (NCBI) database, are listed in Table 1. Strains are presented as four genomic groupings based on the microarray analyses of Neisseria species by Stabler et al. (51). Briefly, N. cinerea strain 14685 and N. perflava strain 10555 were obtained in lyophilized form from the American Type Culture Collection (ATCC, Manassas, VA). N. flavescens strain 4322, N. lactamica strains 328, JJV8, 4425, and NS19, N. sicca strains 4319 and 4320, and N. subflava strain 52 were available in the Center for Immunochemistry from the collection of the late Herman Schneider, formerly of the Walter Reed Army Institute of Research. N. meningitidis serogroup A strain 7889, serogroup B strains MC58 and 7946, and serogroup C strain 89I and N. gonorrhoeae strains 1291, F62, GC56, and MS11 are clinical isolates that have been described previously (15, 30, 45). LOS was extracted and purified by a modification of the hot phenol-water method (3, 64).
Table 1.
List of strains
| Genomic groupa | Species | Strain | Sourceb or reference |
|---|---|---|---|
| 1 | N. meningitidis | 7889 | WRAIR |
| N. meningitidis | 7946 | WRAIR | |
| N. meningitidis | 7946ΔlptA | This study | |
| N. meningitidis | MC58 | 28 | |
| N. meningitidis | 89I | WRAIR | |
| N. meningitidis | NCBI | ||
| 2 | N. gonorrhoeae | 1291 | 13 |
| N. gonorrhoeae | F62 | WRAIR | |
| N. gonorrhoeae | GC56 | WRAIR | |
| N. gonorrhoeae | MS11 | WRAIR | |
| N. gonorrhoeae | NCBI | ||
| 3 | N. lactamica | 328 | WRAIR |
| N. lactamica | JJV8 | WRAIR | |
| N. lactamica | 4425 | WRAIR | |
| N. lactamica | NS19 | WRAIR | |
| 4 | N. cinerea | 14685 | ATCC |
| N. cinerea | NCBI | ||
| N. elongata | NCBI | ||
| N. flavescens | 4322 | WRAIR | |
| N. flavescens | NRL 30031 | NCBI | |
| N. flavescens | SK114 | NCBI | |
| N. mucosa | 25996 | NCBI | |
| N. perflava | 10555 | ATCC | |
| N. polysaccharea | ATCC 43768 | NCBI | |
| N. polysaccharea | 342 | NCBI | |
| N. sicca | 4319 | WRAIR | |
| N. sicca | 4320 | WRAIR | |
| N. sicca | ATCC 29256 | NCBI | |
| N. sicca | DS1 | NCBI | |
| Neisseria species (oral) | NCBI | ||
| N. subflava | 52 | WRAIR | |
| N. subflava | NJ9703 | NCBI |
Genomic groupings are based on the microarray analyses of Stabler et al. (51).
Abbreviations: WRAIR, Walter Reed Army Institute of Research; NCBI, National Center for Biotechnology Information; ATCC, American Type Culture Collection.
Bioinformatics analysis of lptA in Neisseria species.
The amino acid sequences encoded by N. meningitidis genes NMB1637 (57) (encoding a hypothetical protein), NMB1638 (lptA, encoding LPS phosphoethanolamine transferase for LA) (12), and NMB1640 (serC, encoding a putative phosphoserine aminotransferase) were used to perform a genomic Blast search (http://www.ncbi.nlm.nih.gov/sutils/genom_table.cgi) of all available Neisseria genomic DNA sequences present in the database (accession date, 31 March 2011). Genomic organizations of DNA sequences identified in individual searches were assembled by importing the DNA sequences into the computer program Geneious (Biomatters Ltd., Auckland, New Zealand).
DNA extraction and Southern blot analysis.
Chromosomal DNA was isolated using Promega's Wizard genomic DNA purification kit (Promega Corp., Madison, WI). Chromosomal DNA was digested with SspI (New England BioLabs, Ipswich, MA) and electrophoresed on a 1.2% Tris-borate-EDTA (TBE) agarose gel (47), transferred onto a charged nylon membrane (DuPont, Wilmington, DE) using the alkaline transfer method of Reed and Mann (46), and fixed onto the membrane by UV exposure (1.5 J/cm2). Probe DNA was prepared by PCR amplification of the lptA gene using primers lptAF (ATGATAAAACCGAACCTGAGGCCG) and lptAR (TCAGCGCGGACGGCGGCAGGCTGC). PCR was performed using the Expand long template PCR kit (Roche Diagnostics Corporation, Indianapolis, IN) according to the manufacturer's specifications. Primers were purchased from Integrated DNA Technologies (Coralville, IA). Visualization of hybridization was accomplished with the use of Lumi-Phos and the Genius kit (Boehringer Mannheim, Indianapolis, IN). All of the steps followed the manufacturer's protocol except as follows: membranes were washed with 1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% SDS, and 0.1× SSC, 0.1% SDS at 65°C; 1% nonfat dry milk was used as the blocking agent and in the hybridization solution; and the antidigoxigenin antibody was diluted 1:10,000 instead of 1:5,000.
Isolation of LA.
To hydrolyze the glycosidic bond between 3-deoxy-d-manno-octulosonic acid (Kdo) and LA with minimal dephosphorylation, the LOS (∼1 mg/ml) was dissolved in 12.5 mM ammonium acetate (pH 4.5) and then stirred at 100°C for 60 min (8). After cooling the sample to room temperature, 5 parts (vol/vol) of chloroform-methanol solution (2:1, vol/vol) were added to two parts of the ammonium acetate solution. The mixture was stirred for 1 min and then centrifuged at ∼5,000 × g for 20 min. The lower organic phase and interface were transferred into a glass vial and dried under a stream of nitrogen gas. The dry LA was stored at −20°C.
Electrospray ionization (ESI)-MS and tandem MS (MS/MS).
The LA was dissolved in chloroform-methanol solution (1:2, vol/vol) at concentrations of ∼0.2 to 1.0 mg/ml and analyzed by negative-ion nanoelectrospray using an LCQ Deca quadrupole ion trap mass spectrometer (MS) (Thermo Scientific, San Jose, CA). The needle voltage was typically set to 1.8 to 2.8 kV, and the temperature of the heated capillary was 150 to 180°C. For selection of parent ions for collision-induced dissociation, the isolation width parameter was set to 2 to 3.
Negative-ion MALDI-TOF MS.
Negative-ion matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) MS was performed on a Synapt G2 high-definition (HD) MS system (Waters, Manchester, United Kingdom) with an orthogonal TOF mass analyzer in sensitivity mode. The Nd:Yag laser was operated with a wavelength of 355 nm at 100 to 200 Hz. MassLynx software was used to digitally smooth and correct the baseline of the spectra. The instrument was calibrated by using the exact mass for the monoisotopic (M-H)− ions for bovine insulin at m/z 5,728.5931, insulin β-chain at m/z 3,492.6357, renin substrate at m/z 1,756.9175, and angiotensin II at m/z 1,044.5267.
Preparation of intact LOS for MALDI-TOF MS.
Intact LOS samples were prepared for MS analysis using a method described previously (55). Briefly, purified LOS (4 to 10 mg/ml) was suspended in a methanol-water (1:3) solution with 5 mM EDTA, and an aliquot was desalted with a few cation exchange beads (Dowex 50WX8-200) that had been converted to the ammonium form. A spot containing a layer of matrix was formed by deposition of 1 or 2 drops (∼1.0 μl each) of a solution composed of 2,4,6-trihydroxyacetophenone (200 mg/ml; Sigma-Aldrich, St. Louis, MO) in methanol, with nitrocellulose transblot membrane (15 mg/ml; Bio-Rad, Hercules, CA) in acetone-isopropanol (1:1, vol/vol) mixed in a 4:1 (vol/vol) ratio, within inscribed circles on the stainless steel sample plate. After the desalted sample solution was mixed with 100 mM dibasic ammonium citrate (9:1, vol/vol), 1.0 to 2.0 μl was deposited on top of the circular spot of dried matrix.
TNF-α response of human monocytes.
The human monocytic leukemia cell line THP-1 was obtained from ATCC. The cells were cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere and differentiated with 10 ng/ml phorbol myristate acetate (Sigma-Aldrich) for 18 h. Differentiated cells were seeded in 96-well plates at a concentration of 104 cells per well and treated for 18 h with LOS (100 ng/ml) or with live bacteria (multiplicity of infection of 1) grown overnight on plates with Difco GC medium base containing 1% IsoVitaleX (Becton, Dickinson, Franklin Lakes, NJ) in the presence of 5% CO2. The doses of LOS and bacteria were based on our previous studies of their relative bioactivities (30, 31, 38). Cell culture supernatants were assayed for tumor necrosis factor alpha (TNF-α) levels by enzyme-linked immunosorbent assay (ELISA) as recommended by the manufacturer (human TNF-α ELISA Ready-SET-Go; eBioscience, San Diego, CA).
Polymyxin B MIC assay.
The MICs of polymyxin B (Sigma-Aldrich) for Neisseria strains were determined by adding 2-fold dilutions of the antibiotic in a concentration range of 0.8 to 200 μg/ml to plates with Difco GC medium base containing Kellogg's supplements as we described previously (52). A single colony was struck onto each plate, and the plates were incubated for 24 h at 37°C and scored for growth. The MIC was defined as the lowest concentration of the antibiotic that prevented colony formation on the plate.
Construction of N. meningitidis 7946 lptA mutant.
The lptA gene was amplified by PCR from strain MC58 using primers described by Cox et al. (12) and cloned into the EcoRI restriction sites of pUC19. The kanamycin resistance gene was amplified by PCR and inserted into the MluI restriction site. The plasmid carrying the inactivated lptA was then transformed into meningococcal strain 7946. Transformants were selected on GC agar plates that contained 50 μg/ml kanamycin, and mutants were confirmed by PCR and by MALDI-TOF MS analysis of purified intact LOS.
Statistical analysis.
Statistical analyses were performed using Sigma-Stat for Windows version 3.10 (Systat Software, San Jose, CA). Groups of data were analyzed by the Student-Newman-Keuls analysis of variance test for multiple pairwise comparisons. P values of <0.05 were considered significant for all comparisons.
RESULTS
Bioinformatics analysis of genomic sequences of commensal Neisseria strains for the presence of lptA.
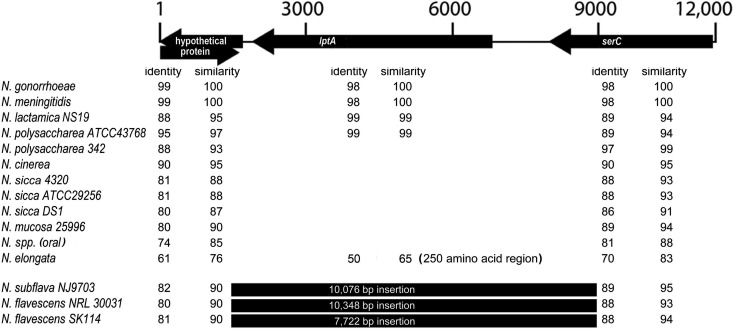
We have previously shown that PEA moieties on the LA from N. meningitidis and N. gonorrhoeae LOS play an important role in activation of the TLR4 signaling pathway (31, 38). For pathogenic Neisseria strains, PEA residues are added to the LA by LptA, a PEA transferase specific for LA (12, 37). To investigate whether the genomes of commensal Neisseria strains contain lptA, the DNA and translated protein sequences of lptA were used to search the genomic sequences of numerous commensal Neisseria strains available at NCBI for the presence of lptA. Sequences corresponding to lptA were found in all gonococcal and meningococcal strains (Fig. 1). In addition, this gene was found in N. lactamica, but it was absent from all other commensal strains except one of two strains of N. polysaccharea. We used the flanking sequences of lptA to reconstruct the chromosomal organization of lptA and found that it was conserved in all strains. We also found that all commensal Neisseria strains possessed the two flanking open reading frames and that most of these were present as linked genes. In a small subset of strains, these genes were separated by an insertion of between ∼8,000 and 10,000 bp. Taken together, the bioinformatics data indicate that lptA is absent in the majority of commensal strains.
Fig 1.
Genomic organization of the lptA region of Neisseria species. The amino acid sequences encoded by N. meningitidis genes NMB1637 (hypothetical protein), NMB1638 (LptA), and NMB1640 (SerC) were used to perform a genomic Blast search, and the overall organization of these genes as found in N. meningitidis is shown at the top of the figure. The numbers across the top of the figure indicate the size of the region in nucleotides. If a number is seen in a row, it indicates that the gene was present in that strain (for gonococcal strains, the information was derived from 14 different strains; for meningococcal strains, the information was derived from 3 different strains). The column labeled “Identity” indicates the percent identity seen between the gene found in that strain and the probe sequence. Similarity indicates the degree of conserved substitution identity, also as a percentage.
Southern hybridization to confirm the absence of lptA in various commensal Neisseria strains.
We performed Southern hybridization using the lptA sequence as a probe to confirm the absence of lptA in four commensal strains, N. cinerea strain 14685, N. flavescens strain 4322, N. perflava strain 10555, and N. subflava strain 52, as shown in Figure 2. NMB1638, the meningococcal lptA, is encoded by a 1,635-bp fragment of DNA (57). Analysis of the DNA sequence of MC58 indicated that lptA contains two internal SspI sites. The expected sizes of the bands generated after digestion are 355 and 892 bp. The data in Figure 2 demonstrate that N. gonorrhoeae strain MS11 and N. meningitidis strains 89I and 7946 possessed the expected hybridization bands that correspond to the presence of an intact lptA. However, none of the four commensal strains analyzed possessed DNA sequences that hybridized with the probe. These data, in combination with the bioinformatics analyses described above, strongly support the conclusion that commensal Neisseria strains are characterized by the lack of lptA.
Fig 2.
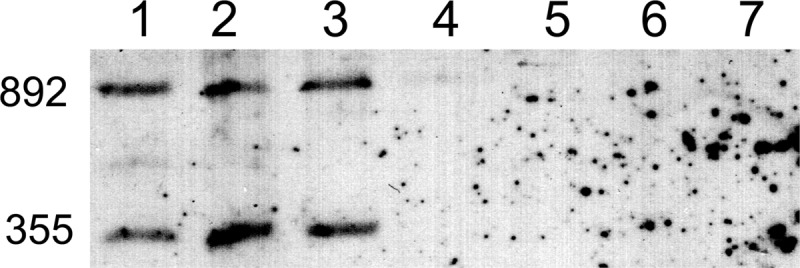
Southern hybridization to detect lptA. After digestion of chromosomal DNA with SspI, the DNA was transferred to a charged nylon membrane and probed with a digoxigenin-labeled probe specific for lptA. The lanes represent DNA isolated from the following strains: lane 1, N. gonorrhoeae strain MS11; lane 2, N. meningitidis strain 7946; lane 3, N. meningitidis strain 89I; lane 4, N. cinerea strain 14685; lane 5, N. flavescens strain 4322; lane 6, N. perflava strain 10555; and lane 7, N. subflava strain 52. The numbers on the left side of the figure indicate the fragment sizes in base pairs.
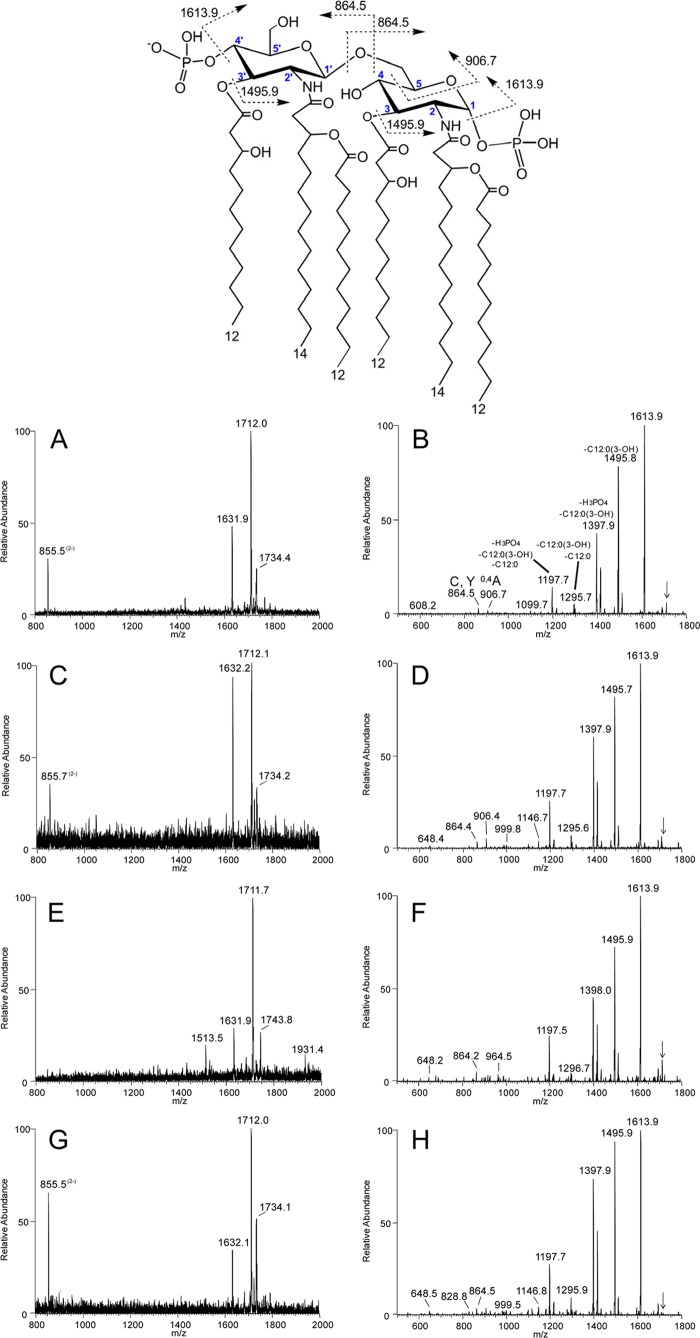
Negative-ion ESI-MS and MS/MS analyses of LA of Neisseria commensals reveal DPLA.
We performed negative-ion ESI-MS and MS/MS analyses of LA from N. cinerea strain 14685 (Fig. 3A and B), N. flavescens strain 4322 (Fig. 3C and D), N. perflava strain 10555 (Fig. 3E and F), and N. subflava strain 52 (Fig. 3G and H). An illustration of the conserved structure of the bisphosphorylated LA from Neisseria, with phosphates on the 1 and 4′ positions of the diglucosamine disaccharide, is shown (Fig. 3, top). The base peak at m/z 1,712 in all of the ESI-MS spectra (Fig. 3A, C, E, and G) is consistent with LA containing two phosphate moieties (diphosphoryl LA [DPLA]); a peak for LA containing one phosphate moiety (monophosphoryl LA [MPLA]) is also observed at m/z 1,632. The peaks at m/z 855 are consistent with the doubly charged DPLA (calculated exact mass at m/z 855.6).
Fig 3.
Negative-ion ESI-MS and MS/MS analyses of LA of Neisseria commensals. ESI-MS and MS/MS spectra of LA from N. cinerea strain 14685 (A and B, respectively), N. flavescens strain 4322 (C and D), N. perflava strain 10555 (E and F), and N. subflava strain 52 (G and H) have peaks for DPLA and MPLA [calculated exact masses of the monoisotopic peaks for (M-H)− at m/z 1,712.1 and 1,632.2, respectively], as well as peaks for the doubly charged DPLA (calculated exact mass at m/z 855.6). The MS/MS spectra (B, D, F, and H) of (M-H)− ions at m/z 1,712 (indicated by arrows) of the species are very similar and confirm that the LA moieties are DPLA. Fatty acid (hydroxylaurate, −216 Da, and laurate, −200 Da) and phosphate (H3PO4, −98 Da) losses and cross-ring fragment ions are noted on the MS/MS spectrum of N. cinerea (B). These data support diphosphoryl substitution and lack of PEA on the LA of commensal Neisseria. Top, illustration of the conserved structure of the diphosphorylated LA from Neisseria (m/z 1,712.1), shown with phosphates on the 1 and 4′ positions of diglucosamine. The dotted lines in the structure indicate the potential cleavage products of LA. The numbers shown in blue for the carbon atoms on the N-acetylglucosamine moieties are used to identify proposed sites of cross-ring cleavage, such as that producing the 0,4A-type fragment ions observed at m/z 906 in the spectra shown in panels B and D.
In addition, there are less-abundant peaks for sodium adducts of the DPLA (M-2H+Na)− at m/z 1,734 in the spectra of N. cinerea (Fig. 3A), N. flavescens, (Fig. 3C), and N. subflava (Fig. 3G). The peak at m/z 1,513.5 seen in the spectrum of the N. perflava LA (Fig. 3E) is in accord with prompt fragment ions from the molecular ions for the DPLA at m/z 1,712 due to loss of hydroxylaurate (3-OH C12:0, −198 Da). The peak at m/z 1,931.4 in the N. perflava spectrum is consistent with the presence of a single Kdo on the DPLA that could occur from incomplete hydrolysis.
The MS/MS spectra of (M-H)− ions at m/z 1,712 (Fig. 3B, D, F, and H) are similar and help to confirm the identity of the structures as hexaacylated DPLA. Assignments for some ions are provided in the spectrum shown in Figure 3B. The base peak at m/z 1,613.9 in each spectrum is due to loss of H3PO4 (98 Da) from the parent ions. Prominent peaks are observed at m/z 1,496 and m/z 1,398 for elimination of hydroxylauric acid as a free acid (−216 Da) and loss of hydroxylaurate with H3PO4, respectively. Less abundant are peaks at m/z 1,296 due to loss of hydroxylaurate and laurate (C12:0, −200 Da) and at m/z 1,198 for loss of hydroxylaurate, laurate, and H3PO4. Peaks for fragments formed by glycosidic bond cleavage that could be reducing terminal Y-type fragment ions or nonreducing terminal C-type fragment ions, as illustrated in the structure in Figure 3, can be observed in each spectrum at m/z 864. Losses of hydroxylaurate from the fragment ions at m/z 864 are in accord with the ions observed at m/z 648 in the spectra in Figure 3D, F, and H. Cross-ring 0,4A-type fragment ions are observed at m/z 906 in the spectra shown in Figure 3B and D. These glycosidic and cross-ring fragments are consistent with the bisphosphorylated LA structure. Taken together, the ESI-MS and MS/MS analyses of the purified LA show that all four commensal Neisseria strains primarily express 2P LA and do not pyrophosphorylate or phosphoethanolaminylate their LOS.
MALDI-TOF MS of intact LOS from commensal and meningococcal Neisseria strains.
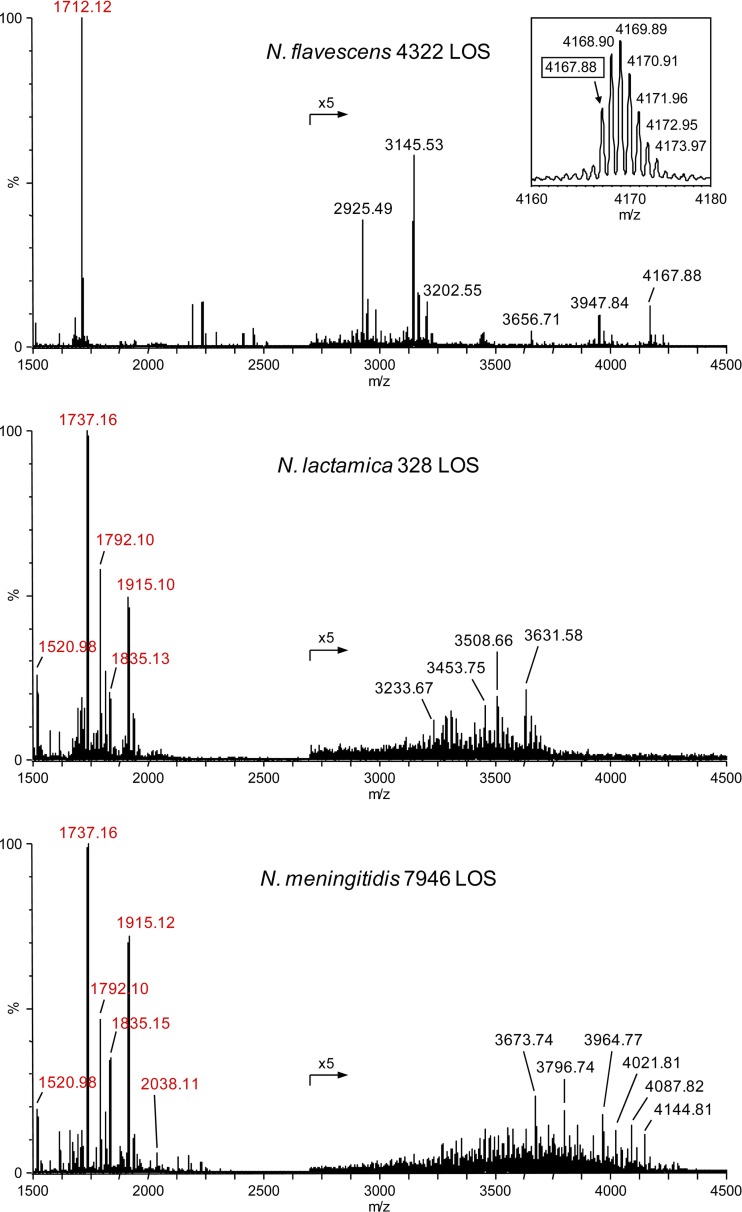
MALDI-TOF MS of intact LOS was performed to confirm lptA functionality or lack thereof and to evaluate phosphorylation of the unmodified LOS structures, as pyrophosphoryl bonds could be cleaved during acid hydrolysis, although the conditions used were chosen to minimize this possibility (8) and, in previous work, we readily detected PEA substituents on LA (30, 31). Spectra were acquired of the LOS from N. cinerea strain 14685, N. flavescens strain 4322, N. perflava strain 10555, N. subflava strain 52, and N. meningitidis strain 7946 using a methodology we described previously (30, 31). In addition, we analyzed the LOS from N. lactamica strain 328, since our bioinformatics analyses indicated that, although it is also a commensal Neisseria strain, it may express lptA.
High-resolution negative-ion mass spectra of intact LOS were acquired on a Synapt G2 HDMS with a MALDI source with an orthogonal TOF mass analyzer. Figure 4 illustrates spectra of intact LOS from N. flavescens strain 4322 (top), N. lactamica strain 328 (middle), and wild-type N. meningitidis strain 7946 (bottom). The observed masses of peaks for Y-type fragment ions containing LA are labeled in red. Table 2 presents calculated exact monoisotopic masses for peaks of LA fragment ions in the spectra that are consistent with the ions observed and our previously published data (10). The high mass resolution of the data obtained on the Synapt G2 HDMS is apparent from the inset with data for the intact N. flavescens LOS in the top spectrum in Figure 4, showing the molecular ion region for peaks with a monoisotopic mass at m/z 4,167.88.
Fig 4.
High-resolution negative-ion MALDI-TOF mass spectra of intact LOS from N. flavescens strain 4322 (top), N. lactamica strain 328 (middle), and N. meningitidis strain 7946 (bottom). The labeled masses represent the monoisotopic ions, as illustrated by the peak at 4,167.88 in the inset showing the envelope of (M-H)− ions for the intact LOS from N. flavescens 4322. The numbers presented in red in each of the spectra label peaks for prompt fragment ions for LA. The phosphoryl substitution of LA from N. lactamica was very similar to that of LA from N. meningitidis. The base peak in the spectrum of N. flavescens at m/z 1,712.12 is consistent with prompt fragment ions composed of DPLA. In the spectrum for N. lactamica 328 LOS, peaks for prompt fragment ions for LA are observed at m/z 1,915.10 for TPLA with one PEA, 1,835.13 for DPLA with one PEA, and 1,792.10 for TPLA. The base peak at m/z 1,737.16 is due to additional loss of H4P2O7 from 1,915.10 or of H3PO4 from 1,835.13.
Table 2.
Masses of (M-H)− lipid A fragment ions of intact LOS
| Strain | Experimental (M-H)− | Proposed composition | Calculated (M-H)− | Difference in ppma |
|---|---|---|---|---|
| N. flavescens 4322 | 1,712.12 | DPLA | 1,712.12 | 0.0 |
| N. lactamica 328 | 1,915.10 | TPLA PEA | 1,915.09 | 4.0 |
| 1,835.13 | DPLA PEA | 1,835.13 | 2.2 | |
| 1,792.10 | TPLA | 1,792.08 | 9.0 | |
| 1,737.16 | TPLA PEA−(2P+H2O)b | 1,737.15 | 6.2 | |
| N. meningitidis 7946 | 2,038.11 | TPLA 2PEA | 2,038.10 | 4.5 |
| 1,915.12 | TPLA PEA | 1,915.09 | 4.0 | |
| 1,835.15 | DPLA PEA | 1,835.13 | 13.1 | |
| 1,792.10 | TPLA | 1,792.08 | 9.0 | |
| 1,737.16 | TPLA PEA−(2P+H2O)b | 1,737.15 | 6.2 |
ppm, parts per million.
Prompt fragmentation with loss of H4P2O7.
In the bottom panel of Figure 4, the peak for the molecular ions at m/z 4,144.81 for the intact N. meningitidis 7946 LOS that we have analyzed previously is in accord with the peak observed at m/z 1,915.10 for triphosphoryl LA (TPLA) with a single PEA and a lacto-N-neotetraose α-chain with a single PEA, sialic acid, O-acetyl group, and glycine (Gly; calculated m/z 4,144.79). These data are consistent with those we reported previously regarding the structure of the meningococcal 7946 LOS (10).
An advantage of this methodology of MALDI-TOF analysis of intact LOS is that it produces molecular ions for intact LOS and in-source prompt fragment ions that correspond to both the oligosaccharide and LA domains. Thus, in addition to the peaks for Y-type fragment ions for LA in each of the spectra, there are peaks for apparent molecular ions for the intact LOS (Fig. 4, labeled in black). Peaks for some of the B-type oligosaccharide fragment ions predicted from the difference between the m/z values of the peaks for the molecular ions and the LA fragment ions were detected in each spectrum but have not been labeled.
In the spectra of the LOS from N. lactamica strain 328 and N. meningitidis strain 7946, the base peaks at m/z 1,737.16 (calculated m/z 1,737.15) are consistent with LA fragment ions that contain a single P and a PEA but that have lost H2O. This is probably due to facile loss of phosphate (H3PO4, −98 Da) from the DPLA with a single PEA or loss of diphosphate (H4P2O7, −178 Da) from TPLA with a single PEA (33). Prominent peaks for Y-type LA fragment ions can be observed in the spectra from both N. lactamica and N. meningitidis at m/z 1,792.1, 1,835.1, and 1,915.1 for TPLA, DPLA with a PEA, and TPLA with a PEA, respectively. Interestingly, in the spectrum of N. meningitidis strain 7946, there also is a peak at m/z 2,038.11 that is consistent with a TPLA with two PEA moieties (calculated m/z 2,038.10).
The base peak observed in the spectrum of the intact N. flavescens LOS at m/z 1,712.12 is consistent with Y-type LA fragment ions of LOS with a DPLA moiety. This was also observed as the base peak in the spectra of the LOS from N. cinerea strain 14685, N. perflava strain 10555, and N. subflava strain 52 (data not shown). Absent from the spectra of all four of these commensal strains were peaks at m/z 1,792.1 for fragment ions of TPLA, at m/z 1,737.1, 1,756.1, or m/z 1,835.1 for fragment ions of MPLA or DPLA with a PEA substituent, and at m/z 1,915.1 for TPLA with a PEA. Thus, the MALDI-TOF analyses of intact LOS from N. cinerea strain 14685, N. perflava strain 10555, N. flavescens strain 4322, and N. subflava strain 52 further confirm that these four commensal Neisseria strains primarily express DPLA and do not pyrophosphorylate or phosphoethanolaminylate their LOS.
Overall, the LA peaks observed are consistent with our previous MS analyses of pathogenic Neisseria (30, 31). As shown in Figure 4, there were prominent peaks for LA with 3 or more phosphoryl moieties in the spectrum of the pathogenic N. meningitidis strain 7946. In contrast, as illustrated by the spectrum of the commensal N. flavescens strain 4322 LOS, there was a dearth of peaks for other than DPLA in the spectra of the LOS from the commensal strains. This difference is indicative of the higher degree of phosphorylation and phosphoethanolaminylation that appears to be characteristic of pathogenic Neisseria strains as compared to most commensal Neisseria strains.
The similarity between the abundance of the peaks for LA ions in the spectra of N. lactamica strain 328 and N. meningitidis strain 7946 is striking. The phosphoryl substitution on the LOS from N. lactamica strain JJV8 also appeared very similar based on negative-ion MALDI analysis of the intact LOS (data not shown). These data are consistent with the expression of lptA in N. lactamica as shown by the bioinformatics data and, furthermore, indicate that the gene is functional, as there are PEA moieties on the LA.
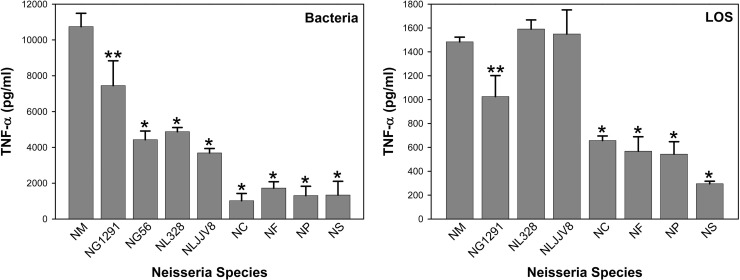
Differential induction of TNF-α by whole bacteria and LOS from commensal and pathogenic Neisseria strains.
The ability of various Neisseria strains to induce TNF-α in THP-1 cells was examined. As shown in Figure 5, N. meningitidis induced significantly higher levels of TNF-α than the two strains of N. gonorrhoeae (P < 0.01 for 1291 and P < 0.001 for GC56) or the six strains of commensals N. lactamica, N. cinerea, N. flavescens, N. perflava, and N. subflava (P < 0.001 for all six comparisons). Both gonococcal strains also induced higher levels of expression of TNF-α than the commensals, except for N. lactamica (P < 0.05 for all comparisons). Of particular note is that gonococcal strain GC56, which had the lowest induction potential for TNF-α of all the pathogenic Neisseria strains that we have studied to date (30, 38, 45), induced a level of TNF-α that was significantly higher than those induced by the commensal N. cinerea, N. flavescens, N. perflava, and N. subflava strains (P < 0.05) but similar to those induced by both strains of N. lactamica. For the commensal bacteria N. cinerea, N. flavescens, N. perflava, and N. subflava, the TNF-α levels induced were, on average, approximately 12% of the level induced by meningococci. In contrast, the TNF-α levels for N. lactamica were significantly higher than those of the other commensals (P < 0.05 for all comparisons), which is consistent with its relatively high levels of expression of LA PEA, TPLA, and TPLA with PEA as shown by the MALDI MS data. Interestingly, despite sharing LA structural similarities with meningococci and gonococci, the TNF-α levels for N. lactamica were, on average, approximately 40% of the meningococcal level and 57% of the level of gonococcal strain 1291.
Fig 5.
Induction of TNF-α in THP-1 cells by commensal and pathogenic Neisseria bacteria and LOS. Whole bacteria and LOS from N. meningitidis strain 89I (NM), N. gonorrhoeae disease isolate strains 1291 (NG1291) and GC56 (NG56), and commensals N. lactamica 328 (NL328), N. lactamica JJV8 (NLJJV8), N. cinerea 14685 (NC), N. flavescens 4322 (NF), N. perflava 10555 (NP), and N. subflava 52 (NS) were analyzed. The results presented are the means ± standard deviations from at least three independent experiments (*, P < 0.001, and **, P < 0.01, compared with the results for 89I). For bacteria, comparison of results between both gonococcal strains and the four commensal strains N. cinerea, N. flavescens, N. perflava, and N. subflava has a P value of <0.05, and comparison of results between both strains of N. lactamica and the four commensal strains N. cinerea, N. flavescens, N. perflava, and N. subflava has a P value of <0.05.
We tested the potential of purified LOS to induce TNF-α expression in THP-1 cells, as shown in Figure 5. LOS from N. meningitidis induced significantly higher levels of TNF-α than LOS from N. gonorrhoeae (P < 0.01) and the commensal N. cinerea, N. flavescens, N. perflava, and N. subflava strains (P < 0.001). The TNF-α levels induced by the LOS from these four commensals were, on average, approximately 35% of the levels induced by the LOS of the meningococci. Similarly, the gonococcal LOS induced higher levels of TNF-α than the LOS from these four commensal strains (P < 0.001). These data are consistent with our previous data, which indicated that purified LOS induces similar relative amounts of TNF-α but lower absolute amounts of TNF-α than the whole bacteria (38). In contrast, the relative inflammatory potential of the N. lactamica LOS was similar to that of the LOS from N. meningitidis strain 89I and greater than that of the LOS from N. gonorrhoeae strain 1291 (P < 0.01 for 1291). The similarity between the relative inflammatory potentials of the LOS from N. lactamica and from the pathogenic N. meningitidis is consistent with the similarity of the phosphoryl substitution on LA as shown in the MALDI-TOF analyses. Taken together, these data indicate that, compared to other Neisseria commensals that we tested, N. lactamica uniquely uses a mechanism other than expressing LA with an altered structure to reduce its inflammatory potential and maintain its commensalism.
Sensitivity of commensal and pathogenic Neisseria strains to polymyxin B.
Previously, insertional inactivation of lptA in N. gonorrhoeae was shown to significantly increase the sensitivity of gonococci to polymyxin B, which indicated that PEA on the gonococcal LA influences the efficacy of polymyxin B (4, 37). Thus, we compared the polymyxin B sensitivities of the commensal species to those of N. gonorrhoeae, because our bioinformatics and MS data showed the presence of PEA on N. lactamica LA and its absence on the LA of all but one of the other commensal strains. As shown in Table 3, the MIC of polymyxin B for N. gonorrhoeae ranged from 32- to 64-fold greater than the MICs for the commensals N. cinerea, N. flavescens, N. perflava, N. sicca, and N. subflava. Similarly, the MICs for the two strains of N. lactamica were 16- to 32-fold greater than those for the other five commensal species, which is consistent with phosphoethanolaminylation of the N. lactamica LA. This suggests a role for PEA on the LA of N. lactamica in resistance to cationic antimicrobial peptides (CAMPs) such as polymyxin B, as shown previously for pathogenic Neisseria (4, 12, 37).
Table 3.
MICs of polymyxin B for various Neisseria species
| Neisseria species and strain | Polymyxin B MIC (μg/ml) |
|---|---|
| N. gonorrhoeae MS11 | 50 |
| N. lactamica NS19 | 25 |
| N. lactamica 4425 | 25 |
| N. cinerea 14685 | 1.6 |
| N. flavescens 4322 | 1.6 |
| N. perflava 10555 | 1.6 |
| N. sicca 4319 | <0.8 |
| N. sicca 4320 | <0.8 |
| N. subflava 52 | <0.8 |
lptA null mutants of N. meningitidis 7946 bacteria and LOS have reduced inflammatory potential.
Our TNF-α and structural data showed that LOS and bacteria of the four commensal N. cinerea, N. flavescens, N. perflava, and N. subflava strains had reduced inflammatory potentials compared to those of LOS and bacteria of pathogenic N. meningitidis and N. gonorrhoeae, lacked PEA on LA, and did not express lptA. To confirm the biological significance of phosphoryl substitution and, specifically, phosphoethanolaminylation of LA in TNF-α induction, an lptA null mutant of N. meningitidis strain 7946 was generated and the effect of the mutation on the inflammatory potentials of the bacteria and LOS was investigated.
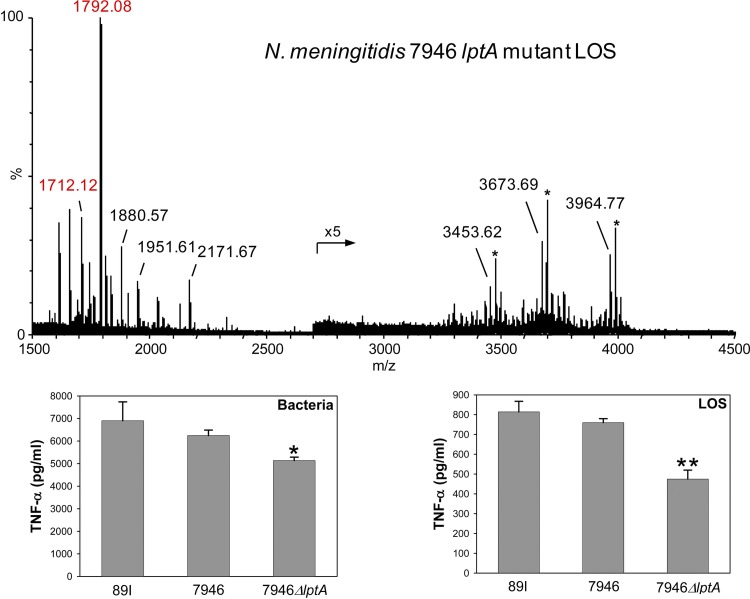
To confirm the absence of PEA in the LA of the 7946 lptA mutant, a high-resolution negative-ion MALDI-TOF mass spectrum of intact LOS from the mutant was obtained and is illustrated in Figure 6. As in the other MALDI spectra shown, observed masses of peaks for Y-type fragment ions containing LA are labeled in red. As expected due to the lack of expression of lptA, there are clear differences in the peaks observed for LA in the spectrum of the 7946 lptA mutant LOS and in the spectrum of the wild-type 7946 LOS shown at the bottom of Figure 4. In the spectrum of the mutant LOS, the base peak at m/z 1,792.08 is consistent with Y-type LA fragment ions of LOS with a TPLA moiety. A less-abundant peak at m/z 1,712.12 is in accord with a DPLA. Noticeably absent are peaks at m/z 1,737, 1,835, or 1,915 due to DPLA or TPLA with a single PEA moiety. Also lacking are ions at m/z 2,038 for a TPLA with two PEA groups.
Fig 6.
ΔlptA mutants of N. meningitidis strain 7946 bacteria and LOS induce less TNF-α in THP-1 cells than the wild-type 7946 strain or N. meningitidis strain 89I bacteria or LOS. In the high-resolution negative-ion MALDI-TOF mass spectrum of intact LOS from the mutant, the labeled masses represent the monoisotopic ions, and the numbers presented in red label peaks for prompt fragment ions for LA. Peaks at m/z 1,792.08 and m/z 1,712.12 are consistent with the expression of TPLA and DPLA. As anticipated, there was no evidence of PEA substitution on the LA. The graphs of TNF-α induction by 89I, wild-type 7946, and 7946 ΔlptA mutant bacteria and LOS show that the absence of LA PEA significantly reduces the induction of TNF-α (*, P < 0.01, and **, P < 0.001, compared with the results for 7946 and 89I). The results presented are the means ± standard deviations from at least three independent experiments.
The peak at m/z 2,171.67 corresponds to oligosaccharide fragment ions and differs from those at 1,951.61 and 1,880.57 by the loss of Kdo (−220 Da) and sialic acid (−291 Da), respectively. Similarly, observed at m/z 3,964.77 are molecular ions that differ from the peaks observed at m/z 3,673.69 by the loss of sialic acid and at m/z 3,453.62 by the loss of both sialic acid and Kdo. Also, sodium adducts of the molecular ions (marked with asterisks) can be observed in this spectrum. The ions at m/z 3,964.77 differ by 180.04 Da from the molecular ions observed for the wild-type 7946 LOS at m/z 4,144.81, which corresponds to the expected loss of expression of PEA (123 Da) and the loss of 57 Da that is indicative of the loss of expression of the Gly moiety on the oligosaccharide. Thus, the spectrum of the intact 7946 lptA mutant LOS confirms the lack of expression of PEA on the LA. In addition, it indicates that the predominant form of the LA lacking PEA is triphosphorylated. The predominant substitution on the LA from wild-type 7946 also appeared to be triphosphoryl, which suggests that the expression of PEA and phosphorylation are independently regulated.
The 7946 lptA mutant bacteria induced significantly less expression of TNF-α than the wild-type 7946 or the 89I bacteria in THP-1 cells (Fig. 6; P < 0.01 for both comparisons). In addition, the LOS isolated from the 7946 lptA mutant also induced less TNF-α than the LOS from wild-type 7946 and 89I (P < 0.001 for both comparisons). These data provide strong support for our hypothesis that, in addition to its acylation state, the inflammatory potential of LA is positively correlated with the extent of phosphoryl substitution (30, 31).
DISCUSSION
The relationship between the immune system and Neisseria commensals remains poorly understood. Previous studies from our laboratory have reported that specific structural features of the LOS of N. gonorrhoeae and N. meningitidis are associated with increased inflammatory signaling. In particular, we found that the degree of phosphorylation, phosphoethanolaminylation, and hexaacylation of LA from the LOS of pathogenic Neisseria directly influences signal transduction through TLR4 and the expression of inflammatory cytokines (30, 31, 38) and may be of critical importance to the progression of the infection, or conversely, to the persistence of Neisseria commensalism. For commensal Neisseria, we postulated that the maintenance of symbiosis with the human respiratory tract mucosa is dependent on reduced agonistic activity of the LA portion of LOS for TLR4, thereby dampening innate immune sensing.
The results of this study show that whole bacteria of commensal Neisseria strains are substantially less inflammatory than those of pathogenic N. meningitidis and N. gonorrhoeae strains, as revealed by the induction of TNF-α in human THP-1 cells. MS analyses demonstrated that four commensal strains, one each of N. cinerea, N. flavescens, N. perflava, and N. subflava, expressed diphosphoryl LA without pyrophosphorylation or phosphoethanolaminylation, unlike the LA of pathogenic Neisseria species, which in addition to diphosphoryl substituents, has both pyrophosphorylation and phosphoethanolaminylation (30, 31, 36, 37). Similarly, the absence of LA phosphoethanolaminylation in a meningococcal lptA mutant resulted in significantly diminished induction of TNF-α both by bacteria and LOS. The mutant LOS was primarily triphosphorylated, in contrast to the diphosphorylated LA of the four commensal strains, and its induction of TNF-α was intermediate to the levels of induction of these commensal strains and the wild-type meningococcal LOS, supporting our hypothesis that increased phosphorylation of Neisseria LA, either pyrophosphorylation or phosphoethanolaminylation, is correlated with greater inflammatory potential. In addition, we found that the gonococcal LOS was significantly less inflammatory than the meningococcal LOS, consistent with our previous results (30, 38). This difference may account for some of the substantially greater severity of morbidity and higher levels of mortality in cases of meningococcemia than in cases of disseminated gonococcal infection (6, 53).
There is very little known about the regulation of pyrophosphorylation of LA in Neisseria, but a gene encoding the PEA transferase that is specific for LA has been identified. Using the lptA NMB1638 DNA sequence identified by Cox and coworkers as a probe for Southern hybridizations (12), we were able to confirm that lptA was absent in the four commensal strains that we had previously studied by MS. Furthermore, we sought to determine the genetic basis for phosphoethanolaminylation of LA in various Neisseria species by using bioinformatics to analyze lptA. We identified lptA in all gonococcal and meningococcal strains that have DNA sequences in the NCBI database (more than 20 sequences in total), and the nucleotide sequence of the gene was virtually identical in all strains, with only 4 nucleotide differences seen among all of the pathogens. The gene was similarly conserved in N. lactamica. Furthermore, the genomic organization among the gonococcus, the meningococcus, and N. lactamica was conserved. The conservation of lptA expression among the pathogenic Neisseria contrasted with the results from analysis of nearly all of the commensal strains other than N. lactamica, for which no genes with significant homology to lptA were found; one of two strains of N. polysaccharea was found to possess the gene, and one strain of N. elongata possessed a 750-bp region with 50% identity to a portion of lptA that would be unlikely to encode a functional transferase protein.
It is interesting to note that in a phenotypic taxonomic study of Neisseria, Barrett and Sneath found that N. lactamica, N. polysaccharea, and N. elongata grouped with N. meningitidis and N. gonorrhoeae and apart from the other commensal Neisseria species (5), suggesting a broader association in addition to lptA. A microarray genomic analysis of phylogenetic relationships between Neisseria species showed that N. meningitidis strains were clustered closely, with commensal Neisseria species at the greatest distance from them and N. gonorrhoeae between these two groups (51). The closer phylogenetic proximity of N. gonorrhoeae to the commensal strains than to N. meningitidis is significant in light of the results presented here, which show the inflammatory potential of gonococci to be intermediate to those of the meningococcal and commensal strains. Our report of the presence of lptA in N. lactamica is also consistent with the finding that N. lactamica is phylogenetically closer to N. gonorrhoeae than to any of the other commensal strains analyzed (51).
Our MS data showed that the LA from N. lactamica LOS was similar to LA from N. meningitidis in its phosphorylation and acylation. The potential of the LOS from N. lactamica to induce TNF-α was also similar to that of the LOS from N. meningitidis. However, live N. lactamica bacteria had significantly less inflammatory potential than N. meningitidis bacteria in THP-1 cells. The difference between the inflammatory potential of live N. lactamica bacteria and that of its LOS was striking. Our results are supported by recent data showing that live N. lactamica bacteria had significantly less inflammatory potential than killed bacteria in human nasopharyngeal epithelial cells, whereas live N. meningitidis bacteria were significantly more inflammatory than killed bacteria (58). The data suggest that live N. lactamica bacteria can specifically interact with host cells to dampen inflammatory responses in a manner that is distinct from the interactions of pathogenic N. meningitidis with host cells. This hypothesis is supported by the report that differential genetic regulation by N. meningitidis and N. lactamica was found after bacterial interaction with host epithelial cells (24) but that only 167 genes were common to the 347 and 285 genes differentially induced by N. meningitidis and N. lactamica, respectively.
Thus, overall, our results indicate that the lack of expression of PEA and pyrophosphoryl groups on LA reduces the inflammatory potential of most commensal Neisseria and, therefore, may facilitate their symbiotic relationship with human hosts, in which the bacteria are able to evade recognition by the innate immune system. In addition, our previous observation that two commensal strains (one N. cinerea and one N. flavescens) did not activate the inflammasome signaling pathway as robustly as N. gonorrhoeae can now be accounted for by LA structural data (14). Furthermore, our results suggest that the expression of LA with reduced inflammatory potential may similarly facilitate the ability of some carrier strains of meningococci or asymptomatic gonococcal strains to evade the immune system and survive within their human host for long periods of time. This implies that Neisseria may possess regulatory mechanisms that downregulate phosphorylation.
The presence of pentaacylated LA due to lpxL1 mutations in a surprisingly large ∼9% of meningococcal disease isolates from 464 patients was shown previously to be correlated with infections that were characterized by less rash and tissue factor-mediated coagulopathy (21). Studies of lpxL1 mutant meningococcal strains in mice confirmed the significance of acylation in relation to inflammatory potential (20). Thus, our results showing that most strains of commensal Neisseria analyzed did not contain lptA are another example of naturally occurring variation in the LA of Neisseria species that may affect whether bacterial colonization of the nasopharynx progresses into a symptomatic clinical presentation.
Our analyses demonstrated that the five commensal species N. cinerea, N. flavescens, N. perflava, N. sicca, and N. subflava, none of which were found to contain lptA, were significantly more sensitive to polymyxin B than N. gonorrhoeae strain MS11. In contrast, N. lactamica was similar to N. gonorrhoeae in polymyxin B resistance, which is consistent with its expression of lptA. These findings expand on those in the study of Anand et al., which reported that N. meningitidis, N. gonorrhoeae, and N. lactamica were equally resistant to colistin (polymyxin E), whereas N. subflava was significantly more sensitive (2). Previously, lptA mutations in N. meningitidis were shown to increase sensitivity to polymyxin B (12, 37, 61). Sensitivity to CAMPs that are part of the innate immune system is thought to be reflected by sensitivity to polymyxin B (23, 37). In addition, an lptA null mutation in N. gonorrhoeae strain FA19 was shown to confer sensitivity to complement-mediated killing by normal human serum, and it was possible to restore serum resistance by complementation with wild-type lptA (37). Thus, our data regarding the sensitivity of commensal Neisseria strains to polymyxin B further confirm the absence of phosphoethanolaminylation of the LA of most strains and suggests that they are more likely to be more sensitive to CAMPs, as well as to complement-mediated lysis.
A previous report showed wide variations in sensitivity to colistin among clinical isolates of N. polysaccharea that could be divided into two groups, one of which displayed a 60-fold greater resistance to the antibiotic than the other (2). These data support our bioinformatic analysis of two strains of N. polysaccharea, which showed that only one of the two strains expressed lptA. Phosphoethanolaminylation of the LA in some strains of N. polysaccharea would be expected to greatly increase their resistance to polymyxin B compared to that of strains lacking PEA. Taken in toto, these data provide a genetic explanation for colistin resistance and suggest a mechanism by which N. meningitidis, N. gonorrhoeae, and N. lactamica can grow on Thayer-Martin medium (1, 59), which contains colistin, while the majority of commensal Neisseria strains cannot.
Using intestinal commensalism as a model, immune tolerance exists toward commensal bacteria, as they survive indefinitely within the mucosal environment of the gut without eliciting immune pathology. Several mechanisms have been proposed which suggest that this is an active process in which TLR signaling pathway molecules, such as TLR4 and TRAF6, are directly targeted by the gut flora, resulting in attenuated inflammatory responses (48). For example, the LA of the Bacteroidetes species that dominate the gut microbiota is pentaacylated, making it a weak agonist of TLR4/MD-2 on gut immune cells (27).
We have shown that due to the lack of a functional lptA, the LA of most strains of commensal Neisseria is not phosphoethanolaminylated and also lacks apparent pyrophosphorylation. Coupled with our previous results, these data indicate that the absence of these groups on the LA is critical to the reduced inflammatory potential of the commensal bacteria (30–32, 38). Our data support our hypothesis that the bisphosphorylated LA of the asymptomatic Neisseria commensal strains elicits a weak innate immune response that promotes persistent colonization. The potential significance of LA phosphorylation in physiological reactions is underscored by the finding that NF-κB activation by mono- but not diphosphorylated hexaacylated LA from Salmonella enterica serovar Typhimurium causes predominantly Toll-interleukin 1 receptor domain-containing adapter-inducing beta interferon (TRIF)-mediated rather than myeloid differentiation factor 88 (MyD88)-mediated signal transduction in mice (41). Monophosphoryl LA has been approved for use as a vaccine adjuvant in several products and is undergoing clinical trials as a component of cancer vaccines due to its stimulation of adaptive immunity and lack of proinflammatory activity (9, 11, 19). Recent data indicate that the balance between NF-κB signal transduction though the MyD88 or TRIF pathways may be affected by negative regulators of TLR signaling, including microRNA (44, 49).
Natural variations in pili, Opa proteins, porins, and capsules are thought to play important roles in the virulence and transmissibility of N. meningitidis (56). Our results indicate that lack of phosphoethanolaminylation and pyrophosphorylation of LA may be a structural component which contributes to the immune privilege of the Neisseria carriage state by reducing the inflammatory potential of the LOS and increasing the sensitivity of the bacteria to the activity of the host CAMPs. Given the importance of LOS in Neisseria infections (7), our data also pinpoint these structural differences in phosphoryl substitution as potentially one of the determinants of pathology in infections caused by Neisseria. A more complete understanding of the phenotypic and molecular basis of the diminished pathogenic potential of Neisseria commensals could inform efforts to define the relationships between asymptomatic carriage and the virulence of disease caused by N. meningitidis and N. gonorrhoeae, to develop new prognostic indicators or targets for drug development, and to develop new adjuvants or vaccines.
ACKNOWLEDGMENTS
This work was supported by a Merit Review Award from the Research Service of the U.S. Department of Veterans Affairs (G.A.J.) and by National Institutes of Health grants AI063927 (G.A.J.), AI053728 and AI065605 (J.M.G.), and AI073667 (D.C.S.) from the National Institute of Allergy and Infectious Diseases. Additional support for this project was provided by National Institutes of Health grant 5U19AI031496 to the Southeastern Sexually Transmitted Infections Cooperative Research Center, and administrative support was provided by the Northern California Institute for Research and Education.
We also gratefully acknowledge the UCSF Mass Spectrometry Core Facility, which is supported by the Sandler Family Foundation, the Gordon and Betty Moore Foundation, and NIH/NCI Cancer Center Support Grant P30 CA082103 and the NIH/National Center for Research Resources Shared Instrumentation grant S10RR029446-01 (H. E. Witkowska), and the UCSF Mass Spectrometry Facility (A. L. Burlingame, Director) which is supported by NIH/NCRR grant P41RR001614.
Footnotes
Published ahead of print 4 September 2012
This article is paper number 111 from the Center for Immunochemistry, Veterans Affairs Medical Center, San Francisco, California, USA.
REFERENCES
- 1. Ahmad F, Young H, McLeod DT, Croughan MJ, Calder MA. 1987. Characterisation of Branhamella catarrhalis and differentiation from Neisseria species in a diagnostic laboratory. J. Clin. Pathol. 40:1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anand CM, Ashton F, Shaw H, Gordon R. 1991. Variability in growth of Neisseria polysaccharea on colistin-containing selective media for Neisseria spp. J. Clin. Microbiol. 29:2434–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Apicella MA, Griffiss JM, Schneider H. 1994. Isolation and characterization of lipopolysaccharides, lipooligosaccharides, and lipid A. Methods Enzymol. 235:242–252 [DOI] [PubMed] [Google Scholar]
- 4. Balthazar JT, et al. 2011. Lipooligosaccharide structure is an important determinant in the resistance of Neisseria gonorrhoeae to antimicrobial agents of innate host defense. Front.Microbiol. 2:30 doi:10.3389/fmicb.2011.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barrett SJ, Sneath PH. 1994. A numerical phenotypic taxonomic study of the genus Neisseria. Microbiology 140:2867–2891 [DOI] [PubMed] [Google Scholar]
- 6. Bleich AT, Sheffield JS, Wendel GD, Jr, Sigman A, Cunningham FG. 2012. Disseminated gonococcal infection in women. Obstet. Gynecol. 119:597–602 [DOI] [PubMed] [Google Scholar]
- 7. Brandtzaeg P, et al. 2001. Neisseria meningitidis lipopolysaccharides in human pathology. J. Endotoxin Res. 7:401–420 [PubMed] [Google Scholar]
- 8. Caroff M, Tacken A, Szabo L. 1988. Detergent-accelerated hydrolysis of bacterial endotoxins and determination of the anomeric configuration of the glycosyl phosphate present in the “isolated lipid A” fragment of the Bordetella pertussis endotoxin. Carbohydr. Res. 175:273–282 [DOI] [PubMed] [Google Scholar]
- 9. Casella CR, Mitchell TC. 2008. Putting endotoxin to work for us: monophosphoryl lipid A as a safe and effective vaccine adjuvant. Cell. Mol. Life Sci. 65:3231–3240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng H, et al. 2011. Human lipooligosaccharide IgG that prevents endemic meningococcal disease recognizes an internal lacto-N-neotetraose structure. J. Biol. Chem. 286:43622–43633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cluff CW. 2010. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: clinical results. Adv. Exp. Med. Biol. 667:111–123 [DOI] [PubMed] [Google Scholar]
- 12. Cox AD, et al. 2003. Phosphorylation of the lipid A region of meningococcal lipopolysaccharide: identification of a family of transferases that add phosphoethanolamine to lipopolysaccharide. J. Bacteriol. 185:3270–3277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dudas KC, Apicella MA. 1988. Selection and immunochemical analysis of lipooligosaccharide mutants of Neisseria gonorrhoeae. Infect. Immun. 56:499–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duncan JA, et al. 2009. Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome. J. Immunol. 182:6460–6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Estabrook MM, Jarvis GA, Griffiss JM. 2007. Affinity-purified human immunoglobulin G that binds a lacto-N-neotetraose-dependent lipooligosaccharide structure is bactericidal for serogroup B Neisseria meningitidis. Infect. Immun. 75:1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farley TA, Cohen DA, Elkins W. 2003. Asymptomatic sexually transmitted diseases: the case for screening. Prev. Med. 36:502–509 [DOI] [PubMed] [Google Scholar]
- 17. Feder HM, Jr, Garibaldi RA. 1984. The significance of nongonococcal, nonmeningococcal Neisseria isolates from blood cultures. Rev. Infect. Dis. 6:181–188 [DOI] [PubMed] [Google Scholar]
- 18. Fowler MI, Yin KY, Humphries HE, Heckels JE, Christodoulides M. 2006. Comparison of the inflammatory responses of human meningeal cells following challenge with Neisseria lactamica and with Neisseria meningitidis. Infect. Immun. 74:6467–6478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fox CB, Friede M, Reed SG, Ireton GC. 2010. Synthetic and natural TLR4 agonists as safe and effective vaccine adjuvants. Subcell. Biochem. 53:303–321 [DOI] [PubMed] [Google Scholar]
- 20. Fransen F, et al. 2010. The structure of Neisseria meningitidis lipid A determines outcome in experimental meningococcal disease. Infect. Immun. 78:3177–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fransen F, et al. 2009. Naturally occurring lipid A mutants in Neisseria meningitidis from patients with invasive meningococcal disease are associated with reduced coagulopathy. PLoS Pathog. 5:e1000396 doi:10.1371/journal.ppat.1000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gerbase AC, Rowley JT, Heymann DH, Berkley SF, Piot P. 1998. Global prevalence and incidence estimates of selected curable STDs. Sex. Transm. Infect. 74(Suppl 1):S12–S16 [PubMed] [Google Scholar]
- 23. Goytia M, Shafer WM. 2010. Polyamines can increase resistance of Neisseria gonorrhoeae to mediators of the innate human host defense. Infect. Immun. 78:3187–3195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grifantini R, et al. 2002. Gene expression profile in Neisseria meningitidis and Neisseria lactamica upon host-cell contact: from basic research to vaccine development. Ann. N. Y. Acad. Sci. 975:202–216 [DOI] [PubMed] [Google Scholar]
- 25. Hadina S, Weiss JP, McCray PB, Jr, Kulhankova K, Thorne PS. 2008. MD-2-dependent pulmonary immune responses to inhaled lipooligosaccharides: effect of acylation state. Am. J. Respir. Cell Mol. Biol. 38:647–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hagblom P, Segal E, Billyard E, So M. 1985. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature 315:156–158 [DOI] [PubMed] [Google Scholar]
- 27. Honda K, Takeda K. 2009. Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2:187–196 [DOI] [PubMed] [Google Scholar]
- 28. Jennings MP, et al. 1993. Cloning and molecular analysis of the galE gene of Neisseria meningitidis and its role in lipopolysaccharide biosynthesis. Mol. Microbiol. 10:361–369 [PubMed] [Google Scholar]
- 29. Jia HP, et al. 2004. Endotoxin responsiveness of human airway epithelia is limited by low expression of MD-2. Am. J. Physiol. Lung Cell. Mol. Physiol. 287:L428–L437 [DOI] [PubMed] [Google Scholar]
- 30. John CM, Liu M, Jarvis GA. 2009. Profiles of structural heterogeneity in native lipooligosaccharides of Neisseria and cytokine induction. J. Lipid Res. 50:424–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. John CM, Liu M, Jarvis GA. 2009. Natural phosphoryl and acyl variants of lipid A from Neisseria meningitidis strain 89I differentially induce tumor necrosis factor-α in human monocytes. J. Biol. Chem. 284:21515–21525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Johnson AP. 1983. The pathogenic potential of commensal species of Neisseria. J. Clin. Pathol. 36:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jones JW, Shaffer SA, Ernst RK, Goodlett DR, Turecek F. 2008. Determination of pyrophosphorylated forms of lipid A in Gram-negative bacteria using a multivaried mass spectrometric approach. Proc. Natl. Acad. Sci. U. S. A. 105:12742–12747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knapp JS. 1988. Historical perspectives and identification of Neisseria and related species. Clin. Microbiol. Rev. 1:415–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Knapp JS, Hook EW., III 1988. Prevalence and persistence of Neisseria cinerea and other Neisseria spp. in adults. J. Clin. Microbiol. 26:896–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kulshin VA, et al. 1992. Structural characterization of the lipid A component of pathogenic Neisseria meningitidis. J. Bacteriol. 174:1793–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lewis LA, et al. 2009. Phosphoethanolamine substitution of lipid A and resistance of Neisseria gonorrhoeae to cationic antimicrobial peptides and complement-mediated killing by normal human serum. Infect. Immun. 77:1112–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu M, John CM, Jarvis GA. 2010. Phosphoryl moieties of lipid A from Neisseria meningitidis and N. gonorrhoeae lipooligosaccharides play an important role in activation of both MyD88- and TRIF-dependent TLR4-MD-2 signaling pathways. J. Immunol. 185:6974–6984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu X, Wetzler LM, Nascimento LO, Massari P. 2010. Human airway epithelial cell responses to Neisseria lactamica and purified porin via Toll-like receptor 2-dependent signaling. Infect. Immun. 78:5314–5323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marri PR, et al. 2010. Genome sequencing reveals widespread virulence gene exchange among human Neisseria species. PLoS One 5:e11835 doi:10.1371/journal.pone.0011835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mata-Haro V, et al. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316:1628–1632 [DOI] [PubMed] [Google Scholar]
- 42. McNabb SJ, et al. 2008. Summary of notifiable diseases—United States, 2006. MMWR Morb. Mortal. Wkly. Rep. 55:1–92 [PubMed] [Google Scholar]
- 43. Nassif X, et al. 1993. Antigenic variation of pilin regulates adhesion of Neisseria meningitidis to human epithelial cells. Mol. Microbiol. 8:719–725 [DOI] [PubMed] [Google Scholar]
- 44. Piao W, et al. 2009. Endotoxin tolerance dysregulates MyD88- and Toll/IL-1R domain-containing adapter inducing IFN-β-dependent pathways and increases expression of negative regulators of TLR signaling. J. Leukoc. Biol. 86:863–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pridmore AC, et al. 2003. Activation of toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains. Infect. Immun. 71:3901–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reed KC, Mann DA. 1985. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 13:7207–7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 48. Sansonetti PJ, Medzhitov R. 2009. Learning tolerance while fighting ignorance. Cell 138:416–420 [DOI] [PubMed] [Google Scholar]
- 49. Schmidt WM, Spiel AO, Jilma B, Wolzt M, Müller M. 2009. In vivo profile of the human leukocyte microRNA response to endotoxemia. Biochem. Biophys. Res. Commun. 380:437–441 [DOI] [PubMed] [Google Scholar]
- 50. Soper DE. 2010. Pelvic inflammatory disease. Obstet. Gynecol. 116:419–428 [DOI] [PubMed] [Google Scholar]
- 51. Stabler RA, et al. 2005. Identification of pathogen-specific genes through microarray analysis of pathogenic and commensal Neisseria species. Microbiology 151:2907–2922 [DOI] [PubMed] [Google Scholar]
- 52. Stein DC, Danaher RJ, Cook TM. 1991. Characterization of a gyrB mutation responsible for low-level nalidixic acid resistance in Neisseria gonorrhoeae. Antimicrob. Agents Chemother. 35:622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stephens DS, Greenwood B, Brandtzaeg P. 2007. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet 369:2196–2210 [DOI] [PubMed] [Google Scholar]
- 54. Stephens DS, et al. 1986. Analysis of damage to human ciliated nasopharyngeal epithelium by Neisseria meningitidis. Infect. Immun. 51:579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sturiale L, et al. 2005. New conditions for matrix-assisted laser desorption/ionization mass spectrometry of native bacterial R-type lipopolysaccharides. Rapid Commun. Mass Spectrom. 19:1829–1834 [DOI] [PubMed] [Google Scholar]
- 56. Taha MK, et al. 2002. The duality of virulence and transmissibility in Neisseria meningitidis. Trends Microbiol. 10:376–382 [DOI] [PubMed] [Google Scholar]
- 57. Tettelin H, et al. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809–1815 [DOI] [PubMed] [Google Scholar]
- 58. Tezera LB, Hampton J, Jackson SK, Davenport V. 2011. Neisseria lactamica attenuates TLR-1/2-induced cytokine responses in nasopharyngeal epithelial cells using PPAR-γ. Cell Microbiol. 13:554–568 [DOI] [PubMed] [Google Scholar]
- 59. Thayer JD, Martin JE., Jr 1966. Improved medium selective for cultivation of N. gonorrhoeae and N. meningitidis. Public Health Rep. 81:559–562 [PMC free article] [PubMed] [Google Scholar]
- 60. Tobiason DM, Seifert HS. 2010. Genomic content of Neisseria species. J. Bacteriol. 192:2160–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tzeng YL, et al. 2005. Cationic antimicrobial peptide resistance in Neisseria meningitidis. J. Bacteriol. 187:5387–5396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Vaughan AT, et al. 2010. Neisseria lactamica selectively induces mitogenic proliferation of the naive B cell pool via cell surface Ig. J. Immunol. 185:3652–3660 [DOI] [PubMed] [Google Scholar]
- 63. Vaughan AT, Gorringe A, Davenport V, Williams NA, Heyderman RS. 2009. Absence of mucosal immunity in the human upper respiratory tract to the commensal bacteria Neisseria lactamica but not pathogenic Neisseria meningitidis during the peak age of nasopharyngeal carriage. J. Immunol. 182:2231–2240 [DOI] [PubMed] [Google Scholar]
- 64. Westphal O, Jann K. 1965. Bacterial lipopolysaccharides. Extraction with phenol-water and further applications of the procedure, p 83–91 In Whistler RL. (ed), Methods in carbohydrate chemistry, vol 5 Academic Press, New York, NY [Google Scholar]
- 65. Yazdankhah SP, et al. 2004. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J. Clin. Microbiol. 42:5146–5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zaura E, Keijser BJ, Huse SM, Crielaard W. 2009. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 9:259. [DOI] [PMC free article] [PubMed] [Google Scholar]