Abstract
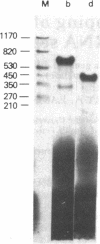
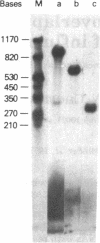
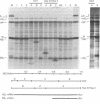
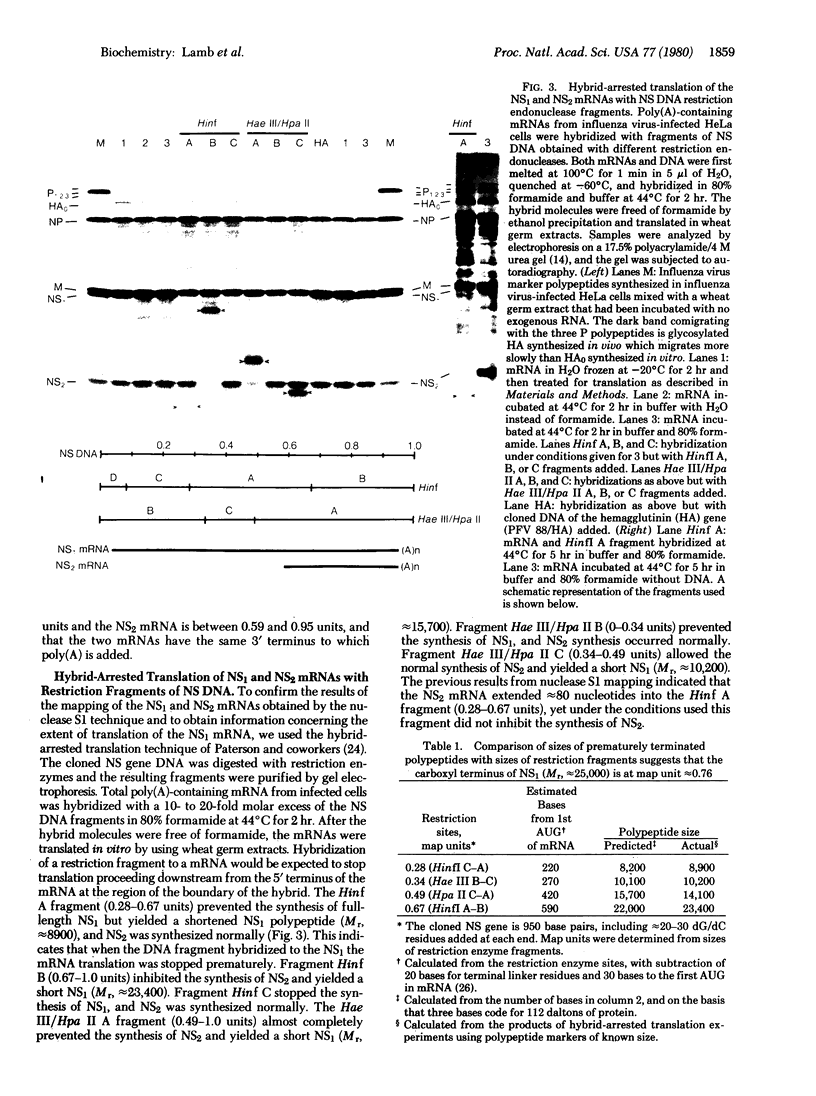
RNA segment 8 of the influenza virus genome is unique in coding for two polypeptides, NS1 (Mr, approximately 25,000) and NS2 (Mr, approximately 11,000). These polypeptides are synthesized from separate mRNA species. By using cloned DNA derived from RNA segment 8 (NS DNA) the two mRNAs have been mapped on segment 8 by hybridization of mRNAs with restriction endonuclease fragments of the DNA and nuclease S1 digestion methods. These data indicate that the body of the NS1 mRNA (approximately 850 nucleotides) maps at 0.05-0.95 units of the cloned NS DNA and the body of the NS2 mRNA (approximately 340 nucleotides) maps at 0.59-0.95 unitssuggesting that the two mRNAs are 3' coterminal and share the same poly(A) addition site. These positions of the mRNAs on the viral genome segment were confirmed in hybrid-arrested translation experiments using fragments of the cloned NS DNA to inhibit the synthesis in vitro of NS1 or NS2 polypeptides. In addition, in these translation experiments the use of certain DNA fragments resulted in premature termination of the NS1 polypeptide. From these data, it could be estimated that the termination of translation of NS1 is at approximately 0.76 map unit. Thus, the coding regions of the two mRNAs overlap by approximately 144-159 nucleotides, the equivalent of approximately 48-53 amino acids. Peptide mapping experiments indicated that polypeptides NS1 and NS2 do not share methionine- or leucine-containing tryptic peptides. The results obtained indicate the translation of the NS2 mRNA occurs in a reading frame different from that used for NS1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bouloy M., Plotch S. J., Krug R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4886–4890. doi: 10.1073/pnas.75.10.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Compans R. W. Distinct carbohydrate components of influenza virus glycoproteins in smooth and rough cytoplasmic membranes. Virology. 1973 Oct;55(2):541–545. doi: 10.1016/0042-6822(73)90199-2. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. New virus-specific antigens in cells infected with influenza virus. Virology. 1969 Oct;39(2):224–234. doi: 10.1016/0042-6822(69)90042-7. [DOI] [PubMed] [Google Scholar]
- Follett E. A., Pringle C. R., Wunner W. H., Skehel J. J. Virus replication in enucleate cells: vesicular stomatitis virus and influenza virus. J Virol. 1974 Feb;13(2):394–399. doi: 10.1128/jvi.13.2.394-399.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay A. J., Lomniczi B., Bellamy A. R., Skehel J. J. Transcription of the influenza virus genome. Virology. 1977 Dec;83(2):337–355. doi: 10.1016/0042-6822(77)90179-9. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., Barrett T., Brown C. M., Almond J. W. The smallest genome RNA segment of influenza virus contains two genes that may overlap. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3790–3794. doi: 10.1073/pnas.76.8.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C., Carroll A. R., Lamb R. A., Mahy B. W. Polypeptides specified by the influenza virus genome I. Evidence for eight distinct gene products specified by fowl plague virus. Virology. 1976 Oct 15;74(2):489–503. doi: 10.1016/0042-6822(76)90355-x. [DOI] [PubMed] [Google Scholar]
- Inglis S. C., McGeoch D. J., Mahy B. W. Polypeptides specified by the influenza virus genoma. 2. Assignement of protein coding functions to individual genome segments by in vitro translation. Virology. 1977 May 15;78(2):522–536. doi: 10.1016/0042-6822(77)90128-3. [DOI] [PubMed] [Google Scholar]
- Klessig D. F. Two adenovirus mRNAs have a common 5' terminal leader sequence encoded at least 10 kb upstream from their main coding regions. Cell. 1977 Sep;12(1):9–21. doi: 10.1016/0092-8674(77)90181-7. [DOI] [PubMed] [Google Scholar]
- Krug R. M., Broni B. A., Bouloy M. Are the 5' ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell. 1979 Oct;18(2):329–334. doi: 10.1016/0092-8674(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Dhar R., Khoury G. Mapping the spliced and unspliced late lytic SV40 RNAs. Cell. 1978 Aug;14(4):971–982. doi: 10.1016/0092-8674(78)90351-3. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Khoury G. Deletion mutants of simian virus 40 defective in biosynthesis of late viral mRNA. Proc Natl Acad Sci U S A. 1979 Jan;76(1):71–75. doi: 10.1073/pnas.76.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Markoff L. J., Zimmerman S., Cohen B., Berndt J. A., Chanock R. M. Cloning DNA sequences from influenza viral RNA segments. Proc Natl Acad Sci U S A. 1980 Jan;77(1):210–214. doi: 10.1073/pnas.77.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Segment 8 of the influenza virus genome is unique in coding for two polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4908–4912. doi: 10.1073/pnas.76.10.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. Synthesis of influenza virus proteins in infected cells: translation of viral polypeptides, including three P polypeptides, from RNA produced by primary transcription. Virology. 1976 Oct 15;74(2):504–519. doi: 10.1016/0042-6822(76)90356-1. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Etkind P. R., Choppin P. W. Evidence for a ninth influenza viral polypeptide. Virology. 1978 Nov;91(1):60–78. doi: 10.1016/0042-6822(78)90355-0. [DOI] [PubMed] [Google Scholar]
- Lazarowitz S. G., Compans R. W., Choppin P. W. Influenza virus structural and nonstructural proteins in infected cells and their plasma membranes. Virology. 1971 Dec;46(3):830–843. doi: 10.1016/0042-6822(71)90084-5. [DOI] [PubMed] [Google Scholar]
- McGeoch D., Fellner P., Newton C. Influenza virus genome consists of eight distinct RNA species. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3045–3049. doi: 10.1073/pnas.73.9.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor P. D., Dimmock N. J. Inhibition of synthesis of influenza virus proteins: evidence of two host-cell-dependent events during multiplication. Virology. 1975 Sep;67(1):114–123. doi: 10.1016/0042-6822(75)90409-2. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E. Groups of adenovirus type 2 mRNA's derived from a large primary transcript: probable nuclear origin and possible common 3' ends. J Virol. 1978 Mar;25(3):811–823. doi: 10.1128/jvi.25.3.811-823.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Schulman J. L. Differences in RNA patterns of influenza A viruses. J Virol. 1976 Mar;17(3):876–884. doi: 10.1128/jvi.17.3.876-884.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotch S. J., Krug R. M. Segments of influenza virus complementary RNA synthesized in vitro. J Virol. 1978 Feb;25(2):579–586. doi: 10.1128/jvi.25.2.579-586.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons M. W. A reexamination of influenza single-and double-stranded RNAs by gel electrophoresis. Virology. 1976 Feb;69(2):789–792. doi: 10.1016/0042-6822(76)90508-0. [DOI] [PubMed] [Google Scholar]
- Ritchey M. B., Palese P., Schulman J. L. Mapping of the influenza virus genome. III. Identification of genes coding for nucleoprotein, membrane protein, and nonstructural protein. J Virol. 1976 Oct;20(1):307–313. doi: 10.1128/jvi.20.1.307-313.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. S. 5' and 3' terminal nucleotide sequences of the RNA genome segments of influenza virus. Nucleic Acids Res. 1979 Aug 24;6(12):3745–3757. doi: 10.1093/nar/6.12.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Harms E., Rohde W., Orlich M., Rott R. Correlation between RNA fragments of fowl plague virus and their corresponding gene functions. Virology. 1976 Oct 15;74(2):332–344. doi: 10.1016/0042-6822(76)90340-8. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Hay A. J. Nucleotide sequences at the 5' termini of influenza virus RNAs and their transcripts. Nucleic Acids Res. 1978 Apr;5(4):1207–1219. doi: 10.1093/nar/5.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehel J. J. Polypeptide synthesis in influenza virus-infected cells. Virology. 1972 Jul;49(1):23–36. doi: 10.1016/s0042-6822(72)80004-7. [DOI] [PubMed] [Google Scholar]
- Ziff E., Fraser N. Adenovirus type 2 late mRNA's: structural evidence for 3'-coterminal species. J Virol. 1978 Mar;25(3):897–906. doi: 10.1128/jvi.25.3.897-906.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]