Abstract
In Escherichia coli, several systems are known to transport glucose into the cytoplasm. The main glucose uptake system under batch conditions is the glucose phosphoenolpyruvate:carbohydrate phosphotransferase system (glucose PTS), but the mannose PTS and the galactose and maltose transporters also can translocate glucose. Mutant strains which lack the enzyme IIBC (EIIBC) protein of the glucose PTS have been investigated previously because their lower rate of acetate formation offers advantages in industrial applications. Nevertheless, a systematic study to analyze the impact of the different glucose uptake systems has not been undertaken. Specifically, how the bacteria cope with the deletion of the major glucose uptake system and which alternative transporters react to compensate for this deficit have not been studied in detail. Therefore, a series of mutant strains were analyzed in aerobic and anaerobic batch cultures, as well as glucose-limited continuous cultivations. Deletion of EIIBC disturbs glucose transport severely in batch cultures; cyclic AMP (cAMP)-cAMP receptor protein (CRP) levels rise, and induction of the mgl operon occurs. Nevertheless, Mgl activity is not essential for growth of these mutants, since deletion of this transporter did not affect the growth rate; the activities of the remaining transporters seem to be sufficient. Under conditions of glucose limitation, mgl is upregulated 23-fold compared to levels for growth under glucose excess. Despite the strong induction of mgl upon glucose limitation, deletion of this transport system did not lead to further changes. Although the galactose transporters are often regarded as important for glucose uptake at micromolar concentrations, the glucose as well as mannose PTS might be sufficient for growth at this relatively low dilution rate.
INTRODUCTION
The transport of carbon sources, especially glucose, is an important field of research that has been previously investigated in Escherichia coli. The main uptake system under conditions of glucose excess is the phosphoenolpyruvate:carbohydrate phosphotransferase system (PTS). It plays an important role not only in the transport and phosphorylation of carbon sources but also in the regulation of carbon metabolism and chemotaxis (for an overview, see reviews in references 11a, 47a, 50, and 50a). Glucose transport into the cytoplasm by EIICBGlc (encoded by ptsG) is coupled to its phosphorylation. The phosphate group is derived from phosphoenolpyruvate (PEP) and is transferred via a cascade of proteins, enzyme I (EI), HPr, EIIA, and EIIB. EI and HPr are general PTS proteins, while the EII proteins are carbohydrate specific. Like the glucose PTS, the mannose PTS (encoded by manXYZ) has been shown to play a role in transportation and phosphorylation of glucose under aerobic conditions with glucose as the sole carbon source (26), although PtsM has a lower affinity for glucose than PtsG (Km PtsM, 1.3 mM [9]; Km PtsG, 10 to 20 μM [26]). Besides the phosphotransferase systems, the galactose transport systems, the galactose ABC transporter (encoded by mglBAC; Kd Mgl [dissociation constant for Mgl], 0.2 μM) and galactose permease (encoded by galP; Km GalP, 10.2 μM [37]), are known to be able to transport glucose into the cytoplasm; in particular, the Mgl system has been reported to be active under conditions of glucose limitation (9, 13, 25). The maltose ABC transporter (encoded by malEFG) can also translocate glucose, and its expression is elevated under glucose-limited conditions in a chemostat (2, 39). The galactose transport systems and the maltose transporter release unphosphorylated glucose into the cytoplasm, and hence phosphorylation of glucose occurs independently via a glucokinase that transfers phosphate from ATP to form glucose-6-phosphate.
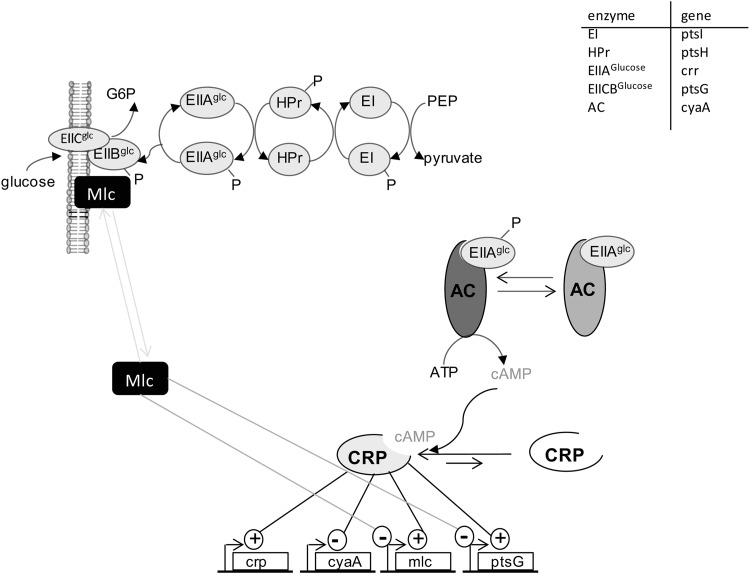
There are several global transcriptional regulators involved in controlling carbohydrate uptake and metabolism in E. coli. The complex of CRP (cAMP receptor protein) or CAP (catabolite gene activator protein) and cAMP regulates mainly catabolic genes and plays an important role in catabolite repression. During carbon starvation conditions, the PTS enzyme EIIAGlc is phosphorylated. EIIAGlc-P activates adenylate cyclase (Cya) (11, 43, 51), which produces cAMP. The intracellular concentration of cAMP-CRP increases and leads to the activation of catabolic genes and also of crp itself, as well as repression of cya (Fig. 1). The activity of the repressor Mlc (“makes large colonies,” also called DgsA) is linked to the phosphorylation state of the PTS as well. It binds to unphosphorylated EIIBCGlc and is thus inactivated (32, 38, 62). In the presence of EIIBCGlc-P, Mlc is released and represses transcription of ptsG, ptsHI-crr, the man operon (manXYZ), malT, and its own promoter. Beside the transcriptional control of ptsG by Mlc and cAMP-CRP, the amount of EIIBC protein is also regulated at the posttranscriptional level via mRNA stability in response to glycolytic flux (31, 47).
Fig 1.
Main regulation of uptake of glucose via the phosphoenolpyruvate phosphotransferase system.
The maltose and the galactose transport systems are subject to carbon catabolite repression and therefore need the cAMP-CRP complex for activation of gene expression. The maltose regulon is controlled by the transcriptional activator MalT (21). In the absence of the inducer maltotriose, MalT is inactivated (29, 42, 48). The galactose transport systems Mgl and GalP are mainly regulated by the repressor GalR and the isorepressor GalS and by CRP-cAMP (34, 57–59). GalR is the main regulator for galP expression, and GalS for mgl expression (20). Under glucose-limited conditions, production of the endogenous inducers galactose and maltotriose, as well as elevated cAMP concentrations, leads to induction of the mgl and mal operons (8, 9, 13). Galactose inactivates the Gal repressors (20), while maltotriose activates MalT, the activator of the maltose transport system. Adaptive evolution analysis has been performed with chemostat cultures with the K-12 wild-type (18, 25, 35, 41, 60) and PTS deletion strains (16, 17). Genes of the galactose and maltose transporter were especially upregulated. Mutational analysis under aerobic and oxygen-limited conditions revealed an enrichment of ptsG mutations in anaerobic cultures, in contrast to mutations for enhanced mgl expression aerobically (35), indicating the different importances of these systems depending on oxygen availability.
Mutant strains defective in the glucose PTS (deletion of ptsG, ptsH, ptsI, or crr) have been previously investigated (4, 5, 17, 26, 39, 45). These mutants have a reduced growth rate in glucose minimal medium and exhibit a strongly reduced rate of acetate formation (45) even in complex media, where the growth rate is unaffected (4). Lower acetate production is an advantage for industrial applications, and hence PTS mutants, as well as strains with additional manipulations, such as the upregulation of the mgl operon, galP (3, 7, 24, 33), and/or the glucokinase (24), have already been analyzed. Nevertheless, a systematic approach to studying the impact of all systems capable of translocating glucose has not been attempted. In particular, the relative contributions of the various transport systems have not been systematically investigated. Experiments from different laboratories are often difficult to compare because of different culturing conditions or strain backgrounds; in particular, cAMP levels might vary (40). Common laboratory E. coli K-12 strains differ from each other, as reported for MG1655 and MC4100 (15), and even MG1655 strains from different stocks are known to show genetic variations (19, 55). Therefore, it is necessary for a systematic comparison of mutant strains to have the same experimental procedure and strain background. Different knockout strains, as well as double and triple deletion strains, were analyzed for their growth capabilities, glucose consumption, and by-product formation rates under aerobic and anaerobic conditions. The transcription of remaining transporter systems was examined via real-time reverse transcription-PCR (RT-PCR). The impact of glucose excess versus glucose limitation was investigated in batch and glucose-limited chemostat cultures.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
All strains are derivatives of E. coli K-12 MG1655. Genes (Table 1) were deleted according to the method of Datsenko and Wanner (6) using plasmid pKD4 or pKD3, leaving the start codon and seven codons at the 3′ end of the target gene. The resistance cassettes were eliminated as described previously (6). For double or triple knockout strains, P1 transduction was used to eliminate the second and third genes. Strains were characterized in batch (four independent cultivations per strain) and chemostat cultures with differing oxygen availabilities (two cultivations per strain; two samples after a minimum of 20 and 40 h at steady state). LB0 (LB medium without glucose and Ca2Cl; 10 g/liter tryptone, 5 g/liter yeast extract, and 5 g/liter NaCl) precultures were used for inoculation of minimal medium precultures. The minimal medium used was based on that of Evans et al. (12). For batch experiments, 20 mM glucose, 0.05 M NaxHxPO4 buffer (sodium phosphate buffer; mixture of NaH2PO4 and Na2HPO4), 0.01 M KCl, 0.00125 M MgCl2, 0.1 M NH4Cl, 0.002 M Na2SO4, 0.02 mM CaCl2, 0.001 mM selenite, 0.38 g/liter nitrilotriacetic acid, and 5 ml/liter trace element solution (0.412 g/liter FeCl3, 5.4 g/liter MnCl3, 0.172 g/liter CuCl2, 0.476 g/liter CoCl2, 0.064 g/liter H3BO3, 0.004 g/liter Na2MoO4, and 10 ml/liter 37% HCl), pH 7, were used. Aerobic batch cultivations were performed in 500-ml conical flasks with baffles on a rotary shaker at 250 rpm with 50 ml Evans medium. Anaerobic batch cultivations were performed in sealed flasks filled with 100 ml Evans medium and stirred slowly on a magnetic stirrer to avoid sedimentation. After inoculation, the medium was sparged with argon for 15 min to remove residual oxygen. Samples were drawn via a three-way tap, and a balloon with argon was attached to prevent influx of air during sampling. The optical density was determined at 420 nm (OD420). Samples for analyzing residual glucose and accumulated by-products in the supernatant were taken every 45 to 60 min; cells for determining mRNA expression rates at an optical density of approximately 1.0 and 1.5, as well as cell culture for cAMP measurements, were used.
Table 1.
Strains used in this work
| Deletion/designation | Strain description |
|---|---|
| None (MG1655) | λ− F− rph-1 Fnr+ |
| Mgl | MG1655 ΔmglBAC |
| GalP | MG1655 ΔgalP |
| Mgl, GalP | MG1655 ΔmglBAC ΔgalP |
| Mal | MG1655 ΔmalEFG |
| Man | MG1655 ΔmanXYZ |
| PtsG | MG1655 ΔptsG |
| PtsG, Mgl | MG1655 ΔptsG ΔmglBAC |
| PtsG, Mgl, GalP | MG1655 ΔptsG ΔmglBAC ΔgalP |
| PtsG, Mal | MG1655 ΔptsG ΔmalEFG |
| PtsG, Man | MG1655 ΔptsG ΔmanXYZ |
For continuous cultivations in a stirred-tank reactor, Evans medium was used with 20 mM glucose as the carbon source; the pH was maintained at 6.9. The medium for bioreactor experiments was not buffered; 0.01 M NaH2PO4 was added instead of 0.05 M NaxHxPO4 buffer. Chemostat experiments were carried out in Infors bioreactors (Infors GmbH, Einsbach, Germany) with a 400-ml working volume at a temperature of 37°C. The agitation rate was set to 550 rpm, and the aeration rate was adjusted to 0.5 vvm (gas volume flow per unit of liquid volume per minute) (0.2 liter/min) with air. Cultivations were started at an optical density of 0.2 (OD420, representing ca. 108 cells/ml) in batch mode and switched to continuous fermentation at an OD420 of 1 to 2; the dilution rate was set to 0.2 h−1. Samples were taken after steady state was reached for a minimum of four residence times. The establishment of steady state was validated by observing several parameters, including the optical density, redox value, and carbon dioxide and oxygen concentrations in the exhaust gasses.
Measurement of substrate and by-product concentrations.
A 1-ml sample of cell culture was centrifuged for 5 min at 13,000 rpm (4°C), and the supernatant was stored at −20°C. Enzymatic test kits from Boehringer (Mannheim)/R-Biopharm were used for analyzing glucose, acetate, formate, succinate, lactate, and ethanol. Tests (except ethanol) were adapted for use in microtiter plates.
Measurement of cAMP.
Samples of 0.2 ml culture were immediately frozen at −20°C and stored until measurement. The analysis was performed as recommended for the Amersham cAMP Biotrak enzyme immunoassay system (GE Healthcare).
Gene expression analysis by RT-PCR.
A culture volume containing ∼109 cells was quenched in twice the volume of RNAprotect solution (Qiagen, Hilden), vortexed for 5 s, and incubated at room temperature for 5 min. After centrifugation for 5 min at 13,000 rpm (4°C), the pellet was stored at −80°C. For RNA isolation and purification, the Master Pure RNA purification kit (Epicentre, Madison, WI) was used. For determining the purity and concentration of mRNA, the optical density at 260 and 280 nm was measured using a NanoDrop spectrophotometer. Transcription of isolated mRNA to cDNA was performed with the RevertAid H Minus First Strand cDNA synthesis kit (Fermentas). Two samples were taken from each experiment and purified. Two mRNA aliquots per sample were transcribed to cDNA and pooled; from these pools, three RT-PCR runs were carried out. Relative quantitative RT-PCR of different cDNA samples was carried out in a Rotor-Gene 6000 instrument from Corbett Life Science using Mesa green qPCR MasterMix Plus (Eurogentec) with SYBR green as a detection agent. Investigated genes with corresponding enzymes and relevant primer pairs are listed in Table 2. Primers were designed for the first gene of the appropriate operon.
Table 2.
Genes and primer pairs used for real-time RT-PCR
| Gene | Primer sequences |
|---|---|
| atpA | 5′-ATTCCCGGGCGACGTTTTCTAC-3′ |
| 3′-ACTTCACTTTCCCTTTTGGCCA-5′ | |
| crp | 5′-CGAAAACCGCCTGTGAAGT-3′ |
| 3′-GTGTCTACCGCGCAGCAG −5′ | |
| cyaA | 5′-AGCGAGTGGGGTTGGGACGAA-3′ |
| 3′-GCAGAGGCGGTCAGGCGTC-5′ | |
| galP | 5′-GATGCCGAACGCGTGCTGCTAC-3′ |
| 3′-TTGTCGTTGAAGGCGGCGCG-5′ | |
| galR | 5′-GCGGTCGAACAGGTGGCTTAT-3′ |
| 3′-ACTCGTTGACTAGGCGGTA-5′ | |
| galS | 5′-TCGTTGCGTTTGCCTGGATAAT-3′ |
| 3′-CCAATAGAAAGAAGGTCGGTGC-5′ | |
| glk | 5′-CGCGCTCGGCAAGAAAGAT-3′ |
| 3′-ACTGTTAGGGCCCGGAAGAGC-5′ | |
| ihfB | 5′-GCCAAGACGGTTGAAGATGC-3′ |
| 3′-CGCCAAAGCCGTCAAAGAG-5′ | |
| malE | 5′-CGGTCTCGCTGAAGTCGGTAAG-3′ |
| 3′-CGATGCGAGTTAGACCGGACAA-5′ | |
| malT | 5′-GCAGGCCGGACGTAAAAGTGAC-3′ |
| 3′-ATTATGCGACGGCCTTGACCTT-5′ | |
| manX | 5′-ACCAATGGGGCCAAACGACTAC-3′ |
| 3′-AATCACTACTTCACCGACGCCT-5′ | |
| mglB | 5′-ACGTGCCGGTGGTTTTCTTCAA-3′ |
| 3′-CGATTTGTGACCCGCCGCTTA-5′ | |
| mlc | 5′-ACACAGGTCGACCCGTATGG-3′ |
| 3′-GAGTAACACAGTCCGCCGTAAC-5′ | |
| ptsG | 5′-GGTTTTCCACGGCGACATTCC-3′ |
| 3′-ACGATTTGGTCTTTTGGCGCG-5′ | |
| ptsH | 5′-CCAGCAAGAAGTTACCATTACC-3′ |
| 3′-CTGAGACCCGGACTGAGTTC-5′ | |
| recA | 5′-CGCTTGGGGCAGGTGGTCT-3′ |
| 3′-TTTTGGTGCGACTGCGACGT-5′ | |
| rpoD | 5′-TCTGCGTATGCGTTTCGGTATC-3′ |
| 3′-TTGACGCAGTGGGCTCGGCA-5′ |
Amplification conditions were as follows: 95°C for 10 min and 40 cycles at 95°C for 15 s and 60°C for 1 min. A negative control without template was carried out for each gene in each PCR run, and a control for DNA contamination was implemented by using the purified mRNA samples as the template. Relative quantification and error propagation were calculated with the software program qBasePLUS (Biogazelle), with efficiency correlation, normalization to a stably expressed reference gene, and interrun calibrators to correct for run-to-run differences (22). The genes recA, rpoD, and atpA were used as reference genes (30), and results for samples were normalized to expression of the wild-type strain. Changes in gene expression levels were considered significant when differences were at least 2-fold. Values were calculated and plotted as log2.
RESULTS
Gene expression under anaerobic and aerobic conditions in glucose batch cultures.
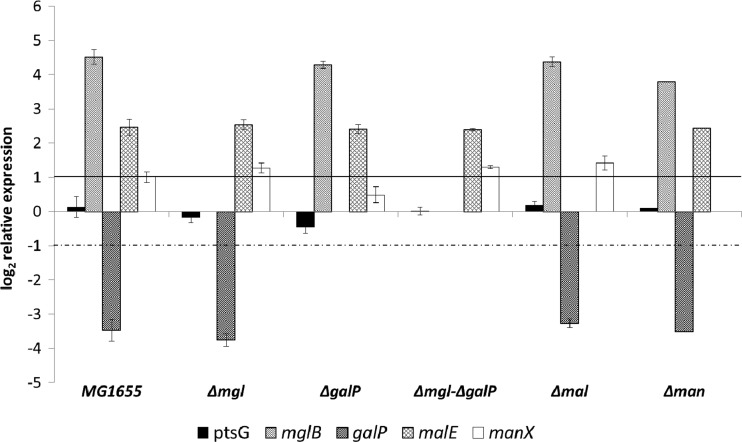
The transcription of genes coding for several transporters capable of transporting glucose from the periplasm into the cytoplasm was compared under aerobic and anaerobic growth conditions with excess glucose; the expression showed a dependency on oxygen availability (Fig. 2). Transcription of galP, mglB, and malE was greater under aerobic conditions, whereas ptsG expression was higher anaerobically (Fig. 2). Expression of manX was similar in the presence or absence of oxygen (Fig. 2).
Fig 2.
Expression of genes coding for carbohydrate uptake systems in E. coli MG1655 grown under anaerobic versus aerobic conditions with glucose excess, normalized to anaerobic conditions. ptsG encodes EIIBCGlc; mglB, encodes a subunit of the galactose ABC transporter MglBAC; galP encodes galactose permease; malE encodes a subunit of the maltose ABC transporter MalEFG; manX encodes EIIABMan. Average data from 4 independent cultivations per strain and condition are given. Gene expression was normalized to the expression level under anaerobic conditions. The horizontal lines mark the significance levels; only changes above the solid line or below the dotted one were regarded as significant.
Growth characteristics of mutant strains in anaerobic and aerobic batch cultures.
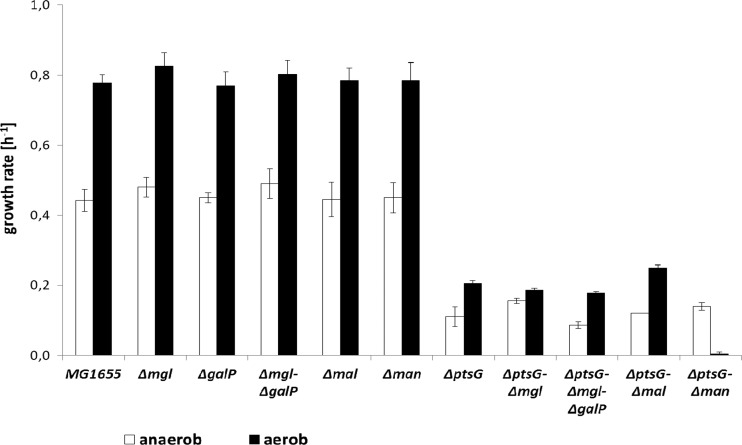
Growth of E. coli MG1655 and the isogenic mutant strains, lacking one or more potential glucose transport systems, was analyzed in minimal medium under conditions of glucose excess. Deletion of the galactose transporters (MglBAC and GalP), the maltose transporter (MalEFG) or the mannose PTS (ManXYZ) did not lead to significant changes in the growth rate under aerobic or anaerobic conditions (Fig. 3). However, the ptsG mutant exhibited a drastically reduced aerobic growth rate (0.21 h−1, compared to 0.78 h−1 for the parent, representing a doubling time of 3.3 h instead of 53 min); under anaerobic conditions, the growth rate was 0.14 h−1, compared to 0.47 h−1 for the parent strain (5-h doubling time, versus 1.5 h). To analyze further the effects in ptsG deletion strains, double and triple mutants lacking additional glucose transporters were generated. An additional disruption of the galactose ABC transporter (mglBAC) did not further impair the growth rate of the ptsG mutant. However, deletion of both galactose transporters (mglBAC and galP) reduced the growth rate of anaerobic cultures even further (0.09 h−1). Deletion of the mannose PTS (manXYZ) in the ptsG mutant led to a significant decrease in the aerobic growth rate (μ < 0.01 h−1), while it did not show an obvious effect under anaerobic conditions (Fig. 3).
Fig 3.
Growth rate of E. coli MG1655 and isogenic mutant strains in glucose minimal medium under anaerobic and aerobic conditions. The mutant strains bear one or more deletions of the galactose transport system (MglBAC, GalP), maltose transporter (MalEFG), mannose PTS (ManXYZ), or glucose PTS (PtsG).
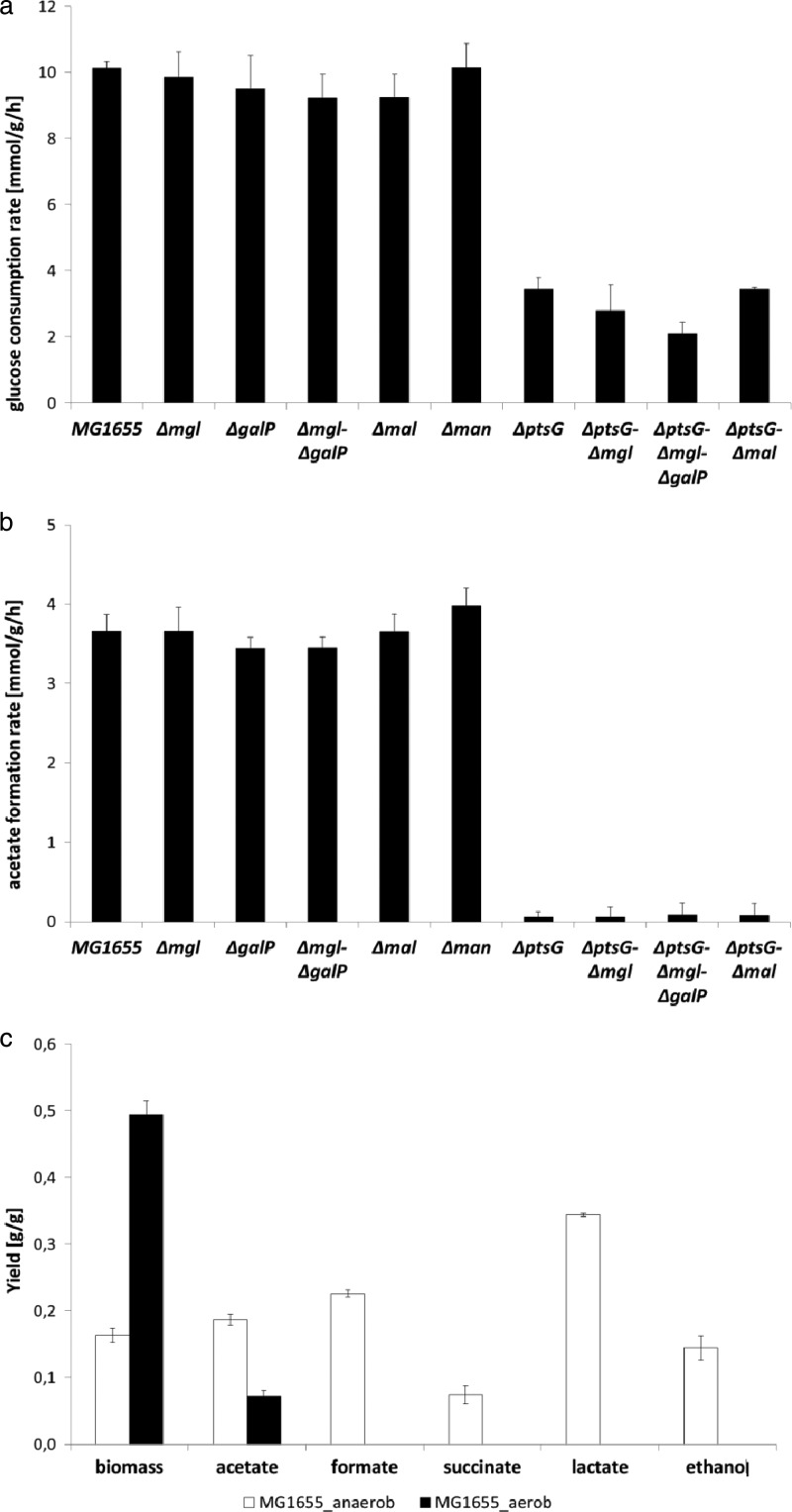
When provided with excess glucose under aerobic conditions, E. coli MG1655 excretes acetate as an overflow metabolite because of an imbalance between glucose uptake and central metabolism. Glucose consumption rates for cultures grown under aerobic conditions and by-product yields of aerobic and anaerobic cultures are presented in Fig. 4. The aerobic glucose consumption rate was lowered only for ptsG mutant strains, presumably reflecting their lower growth rates under these conditions (Fig. 4a). The parent strain, as well as the strains with deletions of mgl and/or galP, mal, or man, excreted acetate as an overflow metabolite, whereas the ptsG single, double and triple mutant strains excreted only traces of acetate (Fig. 4b). The biomass yield (g biomass per g glucose) was unaffected by the mutations at about 0.5 g/g (not shown). In addition to acetate, the only by-product observed in aerobic cultures, formate, succinate, lactate, and ethanol were also excreted into the medium under anaerobic conditions (Fig. 4c). The by-product yields of the mutant strains were similar to those of the wild-type strain (not shown).
Fig 4.
(a and b) Aerobic glucose consumption rate (a) or aerobic by-product yield (b) of E. coli MG1655 and isogenic mutant strains. (c) Anaerobic and aerobic biomass and metabolite yield of MG1655 in glucose minimal medium. The mutant strains bear one or more deletions in the galactose transport system (Mgl and GalP), a component of the maltose transporter (MalE), the mannose PTS (ManX), or the glucose PTS (PtsG).
Gene expression analysis of mutant strains in batch cultures.
RT-PCR of selected genes was used to identify any compensatory reprogramming of gene expression caused by mutation of the potential glucose transporters. Neither the galactose transport systems Mgl and GalP nor the maltose or mannose transporters seem to contribute to glucose transport under conditions of glucose excess. The growth rates were similar to that of the parent strain, and the expression of the remaining glucose transport systems was also unchanged under aerobic as well as anaerobic conditions.
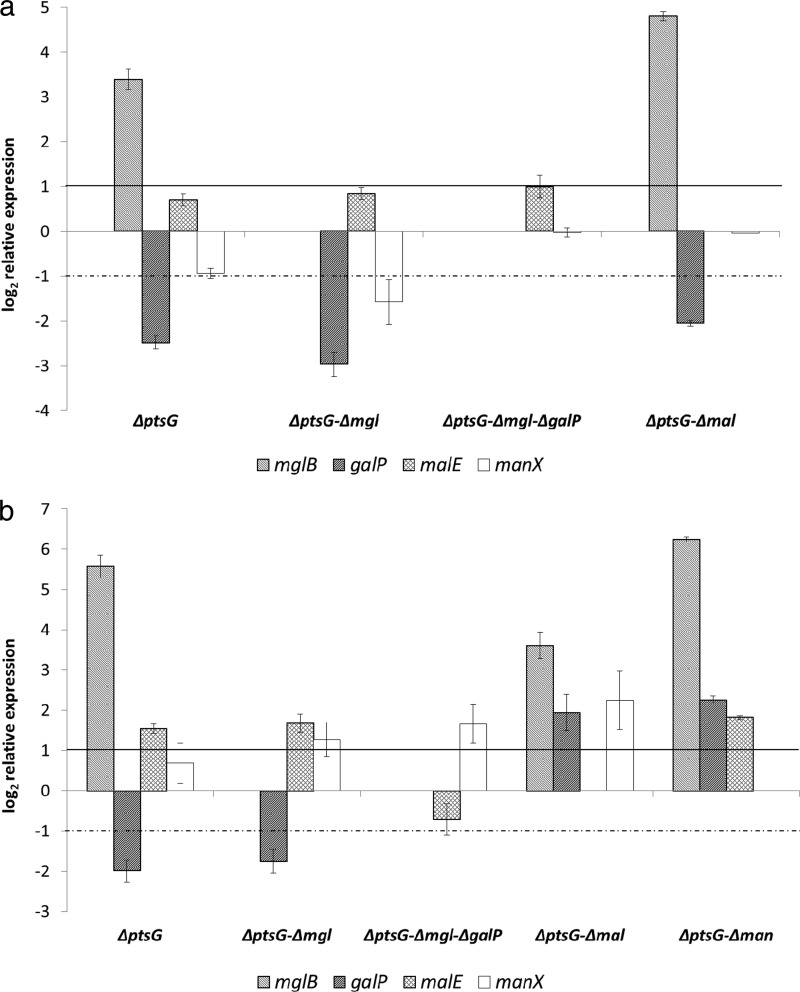
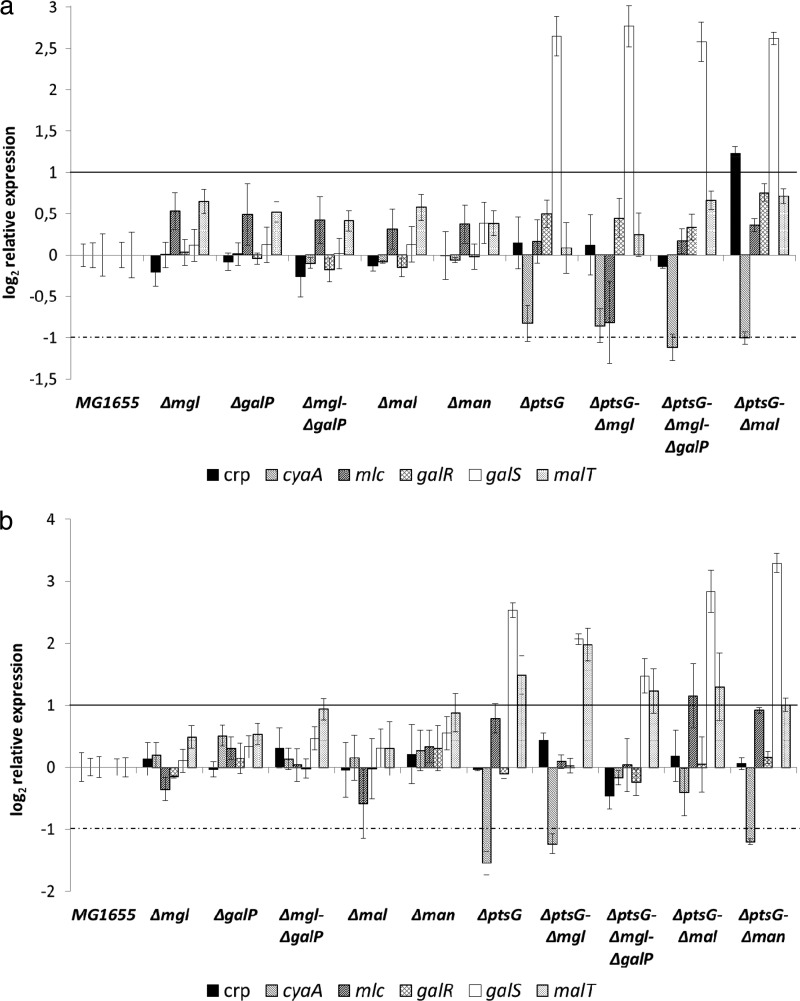
Only the ptsG mutant strains showed significant changes in the expression pattern of the other transporters (Fig. 5): the mgl operon was upregulated 10-fold under aerobic conditions (Fig. 5a). Interestingly, a deletion of ptsG caused downregulation of galP and manX (0.2- and 0.5-fold). Deletion of the malEFG operon in the ptsG mutant further enhanced the upregulation of mglB. Because of the strong effect of the ptsG and mal mutations on the expression of the mglBAC operon, mutant strains additionally lacking the galactose transporters were constructed; interestingly, only minor changes were observed, considering growth and gene expression; malE expression increased about 2-fold in the ptsG mgl galP strain compared to that for the wild type. Because of the very low growth rate of the ptsG manXYZ strain under aerobic conditions, the gene expression pattern could not be determined. Since the galactose and maltose transporters release unphosphorylated glucose into the cytoplasm, which requires phosphorylation via a glucokinase, glk expression was also analyzed. No significant changes in the transcription level were observed.
Fig 5.
Expression of transport systems in E. coli MG1655 and isogenic mutant strains in glucose minimal medium under aerobic conditions (a) or anaerobic conditions (b). The mutant strains bear deletions of the glucose PTS (PtsG), the galactose transport system (MglBAC and GalP), a component of the maltose transporter (MalEFG), or the mannose PTS (ManXYZ). Average data from 4 independent cultivations per strain and condition are shown. Gene expression was normalized to the expression level of the wild type. The horizontal lines mark the significance levels; only changes above the solid line or below the dotted one were regarded as significant.
Under anaerobic conditions, deletion of ptsG led to an even stronger upregulation of mglB (48-fold) (Fig. 5b). In addition, malE showed enhanced expression (2.9-fold). The inactivation of the galactose transporter Mgl in the ptsG mutant increased malE and manX expression (3.2- and 2.4-fold); deletion of both galactose transporters increased only manX expression. The ptsG malEFG double mutant exhibited an interesting expression pattern anaerobically: mglB expression was lower than that in the single-knockout strain (12-fold compared to 48-fold), but galP and manX were upregulated (3.8- and 4.7-fold). An enhanced transcription level of galP was also found in the ptsG manXYZ double deletion strain, where in addition to the strong upregulation of mgl, all remaining transport systems were upregulated. Anaerobic expression of glucokinase was increased in the ptsG mutant strains, about 2-fold in the single mutant and ptsG mgl and ptsG mgl galP strains, 4.4-fold in the ptsG malEFG mutant, and 2.7-fold in the ptsG manXYZ mutant strain, where both PTS were disrupted.
To analyze how changes in transporter gene expression might be mediated, the transcriptional profiles of several regulators that are known to control catabolic genes in response to carbon source and concentration, as well as cya, encoding adenylate cyclase, were examined in the transporter mutants and compared to that of the wild type (Fig. 6). As expected, significant changes were observed only in the ptsG mutant strains; galS expression increased under aerobic (Fig. 6a) and anaerobic (Fig. 6b) conditions. GalS is the isorepressor of the Mgl and GalP galactose transport systems. Accordingly, transcription of galP was decreased in these strains (Fig. 5a and b), whereas mgl expression was enhanced. Anaerobically, transcription of malT, encoding the maltose activator, was enhanced, especially in the ptsG mglBAC double mutant strain (3.9-fold), and accordingly, expression of the maltose transporter gene malE was increased (Fig. 5b). The cya gene encodes adenylate cyclase, the enzyme catalyzing cAMP synthesis in response to glucose starvation. Expression of cyaA was downregulated in the ptsG mutant strains under aerobic and anaerobic conditions. It is known that cyaA is negatively regulated by Crp, whose expression was similar to the wild-type level. To test whether decreased cyaA expression really affected cAMP levels, cAMP concentrations were measured (Table 3). Because more than 99.9% of the intracellular cAMP is excreted into the medium, the measurement of extracellular cAMP can be expected to be representative (36). Generally, cAMP concentrations were lower under anaerobic conditions for all strains, and for all the ptsG mutant strains, cAMP concentrations were higher than that in the parent (Table 3). None of the other mutations had a significant effect on cAMP production. The additional deletion of the maltose transporter in the ptsG mutant led to a further enhancement of cAMP concentrations under aerobic conditions. The activity of adenylate cyclase is therefore, despite its reduced expression rate, higher than that in the wild type; Cya is mainly regulated posttranscriptionally (27), and thus mRNA levels might not correlate with activity. The phosphorylation state of EIIAGlc, the main effector of adenylate cyclase, is significantly higher in the ptsG mutants (about 75% [see the supplementary data for reference 1; also unpublished results for strain LJ110), explaining the observed higher cAMP concentrations.
Fig 6.
Expression of several regulators involved in carbon catabolism in E. coli MG1655 and isogenic mutant strains. (a) Expression under aerobic conditions. (b) Expression under anaerobic conditions. The mutant strains bear one or more deletions in the galactose transport system (Mgl and GalP), a component of the maltose transporter (MalE), or the mannose PTS (ManX). Average data from 4 independent cultivations per strain and condition are shown. Gene expression was normalized to the expression level of the wild type. The horizontal lines mark the significance levels; only changes above the solid line or below the dotted one were regarded as significant.
Table 3.
cAMP concentrations, calculated per OD420
| Strain or genotype | cAMP concn/OD (nmol/ml) |
|
|---|---|---|
| Aerobic | Anaerobic | |
| MG1655 | 0.040 ± 0.003 | 0.033 ± 0.008 |
| Δmgl | 0.045 ± 0.001 | 0.038 ± 0.010 |
| ΔgalP | 0.041 ± 0.003 | 0.027 ± 0.001 |
| Δmgl ΔgalP | 0.047 ± 0.002 | 0.039 ± 0.010 |
| Δmal | 0.040 ± 0.003 | 0.028 ± 0.002 |
| Δman | 0.038 ± 0.004 | 0.034 ± 0.000 |
| ΔptsG | 0.221 ± 0.031 | 0.129 ± 0.011 |
| ΔptsG Δmgl | 0.203 ± 0.040 | 0.114 ± 0.025 |
| ΔptsG Δmgl ΔgalP | 0.142 ± 0.017 | 0.095 ± 0.002 |
| ΔptsG Δmal | 0.319 ± 0.021 | 0.095 ± 0.018 |
| ΔptsG Δman | Not determined | 0.095 ± 0.049 |
Changes in gene expression with glucose excess versus glucose limitation.
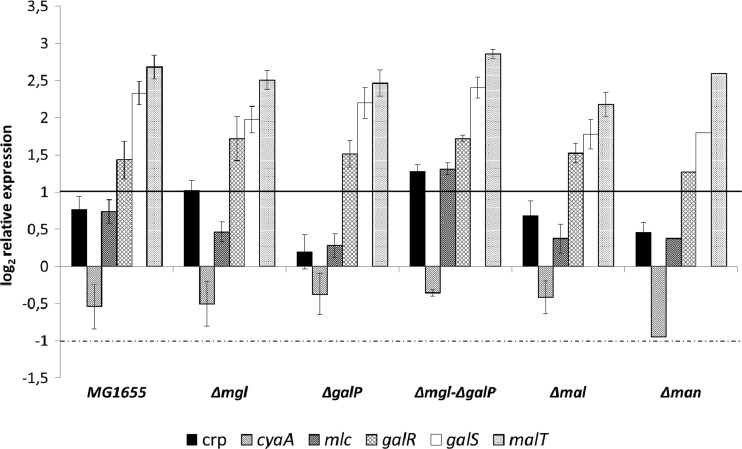
Besides analyzing growth properties and gene expression patterns under glucose-excess conditions, limiting glucose concentrations were investigated. The ptsG mutants were not grown in chemostat cultures, because the maximal growth rates observed under batch conditions were especially low and were near the chosen dilution rate of 0.2 h−1. Chemostat cultures with the remaining mutant strains were performed only under aerobic conditions. Figure 7 represents changes in the expression of the main transporter systems, comparing conditions with glucose excess to those of glucose limitation (steady-state conditions in chemostats).
Fig 7.
Changes in gene expression rates for E. coli MG1655 and isogenic mutant strains grown under glucose limitation versus growth with glucose excess. The mutant strains bear one or more deletions in the galactose transport system (Mgl and GalP), a component of the maltose transporter (MalE), or the mannose PTS (ManX). Average data from 2 independent cultivations per strain and condition are shown. Gene expression was normalized to the expression level under glucose-excess conditions for each strain. The horizontal lines mark the significance levels; only changes above the solid line or below the dotted one were regarded as significant.
Significant changes in the expression of the glucose uptake systems occurred; in the parent, transcription of the mgl operon increased 23-fold under glucose limitation; that of malE increased 5.5-fold, and that of manX increased 2-fold (Fig. 7). While ptsG expression remained nearly unchanged, galP expression decreased to a very low level (0.09-fold). Expression of glk, coding for the glucokinase necessary to phosphorylate glucose which is not phosphorylated via the PTS, was upregulated 2.1-fold (not shown). This might indicate its increased use under glucose-limiting conditions, where glucose transport occurs not only via the PTS but also via the high-affinity ABC transporter Mgl.
Because of the upregulation of mgl under glucose-limiting conditions, deletion of the operon was expected to cause a severe effect leading to an increased expression of an alternative transport system. Interestingly, this was not the case, raising the question of whether upregulation of mgl is not specific and hence not coupled to increased transport by the Mgl system. Deletion of galP, the second galactose transporter, did not alter gene expression compared to that of the parent strain, and even the double-knockout mgl galP strain showed no significant changes. Deletion of the maltose transporter showed a slightly enhanced expression of manX (2.7-fold), whereas deletion of the mannose PTS caused no further changes; only mgl expression was lower than that in the wild type, but it was nevertheless 13.9-fold higher than that under batch conditions. Interestingly, expression of galP, coding for the low-affinity galactose permease, was lower under glucose limitation than under glucose-excess conditions in all strains.
Expression of transcription factors, affecting sugar transport, was also analyzed under conditions of glucose excess compared to conditions of limitation (Fig. 8). In the wild type, the galactose repressor GalR and the isorepressor GalS, as well as the maltose regulator MalT, were upregulated under glucose limitation (2.7- and 5-fold and 6.4-fold, respectively). These regulators were upregulated in all strains. Expression of mlc, coding for the main regulator of the PTS enzymes, increased in the mgl galP double mutant, as did expression of crp, by about 2.5-fold. The expression of crp was also slightly enhanced in the mgl single mutant (2-fold). Unexpectedly, only cyaA showed no significant change in the expression pattern when changing from glucose-excess to glucose-limiting conditions. Nevertheless, cAMP concentrations increased 5- to 7-fold in all tested strains upon shifting from glucose excess to glucose limitation, as described by Notley and Ferenci, who reported 5-fold-enhanced cAMP levels in chemostat cultures compared to conditions with glucose excess (39). This can be explained by the fact that regulation of the activity of Cya is more important than control of the expression level.
Fig 8.
Changes in gene expression rates for E. coli MG1655 and isogenic mutant strains grown under glucose limitation versus growth with glucose excess. The mutant strains bear one or more deletions in the galactose transport system (Mgl and GalP), a component of the maltose transporter (MalE), or the mannose PTS (ManX). Average data from 2 independent cultivations per strain and condition are shown. Gene expression was normalized to the expression level under glucose-excess conditions for each strain. The horizontal lines mark the significance levels; only changes above the solid line or below the dotted one were regarded as significant.
Analysis of mutant strains in glucose-limited continuous cultures.
None of the strains excreted acetate under these steady-state conditions. The residual glucose concentration remained below the detection limit. Although the high-affinity mglBAC transporter is generally regarded as the main contributor to glucose transport under glucose-limiting conditions (9, 13), its deletion caused no significant changes. Also, no change in the transcription pattern of the analyzed regulators was observed.
DISCUSSION
Differences in gene expression under anaerobic versus aerobic conditions.
Uptake of glucose by different transport systems that are capable of translocating glucose into the cytoplasm is regulated not only by the concentration of glucose but also by oxygen availability, although this has not been well studied until now. Genes coding for enzymes of the PTS, ptsG and ptsHI-crr, are controlled by the oxygen-responsive regulator ArcA, repressing ptsG transcription about 2-fold under anaerobic conditions (28). In addition to the transcriptional control, a reduced mRNA turnover controls the amount of protein, resulting in enhanced EIIBCGlc (and EIIAGlc) concentrations anaerobically (53). The increased abundance of ptsG mRNA under anaerobic conditions observed in this study could result from higher mRNA stability under anaerobic than under aerobic conditions. In the work of Seeto et al. (51), ptsG levels were similar in aerobic and anaerobic batch cultures, but those authors used ptsG-lacZ fusions and compared β-galactosidase activities. The fusion might influence mRNA degradation, which is delayed under anaerobic conditions (53).
Growth characteristics of mutant strains in anaerobic and aerobic batch cultures.
Deleting ptsG, encoding the membrane subunit of the glucose PTS, caused a severe decrease in the growth rate and in glucose consumption under all conditions tested, whereas a deletion of genes coding for the EIIBC of the mannose PTS, the maltose operon, or the galactose transporters did not lead to significant changes in the ability to grow on glucose, confirming that the glucose PTS represents the main glucose transporter aerobically and also anaerobically (5, 49). Disruption of both the glucose and mannose PTS nearly abolished aerobic growth on glucose, indicating that glucose transport in the ptsG deletion strain takes place mainly via the mannose permease. Interestingly, this was observed only under aerobic conditions; the anaerobic growth rate was higher and was similar to that of the ptsG single mutant strain. Accordingly, the PTSMan does not seem to contribute to glucose transport under anaerobic conditions, as described by Roehl and Vinopal for most E. coli K-12 strains (49). In the literature, ptsG mutant strains have been reported to exhibit 20- to 40%-reduced growth rates compared to those of the parent strains in minimal media (4, 5, 44, 45). Picon et al. (44, 45) described a ptsG manXYZ double mutant that exhibited a growth rate of 0.13 h−1 (19% of that of the parent strain), which is significantly higher than that observed in our experiments (Fig. 1). In some experiments, we observed that ptsG strains started to grow with the described growth rate of 0.21 h−1, but after some hours the growth accelerated and reached a reproducible growth rate of 0.49 h−1 (± 0.024). After inoculation into fresh medium, these cells continued to grow faster, indicating the selection of spontaneous mutants. Possibly, the strains described in the literature were already mutated strains. Isolated and analyzed cells from these fast-growing cultures revealed upregulated malE, manX, and ptsH transcripts (data not shown), leading to the hypothesis that a mutation in the mlc gene might be the reason for reduced repression of the operons. Expression of mlc itself was unchanged; that of mgl was slightly lower than that in the “normal” ptsG strain.
Aerobic acetate excretion occurs as result of an imbalance between glucose uptake and limited flux through the tricarboxylic acid (TCA) cycle or respiratory chain (61). Acetate excretion was nearly abolished in all PtsG-defective strains (Fig. 4b). This could be due to the low glucose uptake rates in these mutants avoiding overflow metabolism (45). Acetate production still occurred in some ptsG mutant strains described in the literature (4, 45), probably due to a different strain background (MC4100). In the above-mentioned fast-growing ptsG strains, acetate excretion was observed, but the acetate yield was low.
Gene expression analysis of mutant strains in batch cultures.
As already mentioned, the observed growth behavior indicated that neither the galactose transport systems nor the mannose or maltose transporters seemed to contribute to growth on glucose under conditions of glucose excess. Disrupting these systems did not lead to changes in growth or in the expression of other transporters. Only deletion of ptsG resulted in changed expression levels: the mgl operon was upregulated, while galP and manX were downregulated. A further deletion of mglBAC as well as galP did not change the expression of the other transporters significantly. Although increased expression of the mannose transporter was found in a PtsG-defective strain in the past (45), we could not confirm this at the transcriptional level; manX expression was even lower than that in the wild type, and instead the galactose transporter Mgl was upregulated. Even after the deletion of this transporter, manXYZ expression did not change, but instead expression of the maltose transporter increased. These findings fit with data in the literature; as with a ptsG strain, Mlc cannot be sequestered to the membrane and therefore is active (46, 56) and represses the man operon (46), ptsHI-crr, malT, and its own expression. All Mlc-regulated genes are additionally under carbon catabolite repression (54). In the case of the Mlc promoter, activation by cAMP-CRP is dominant over Mlc repression (54), which might explain the unchanged expression level of mlc. The mal operon is influenced indirectly by Mlc via repression of MalT, the activator of the mal regulon (10, 46). Repression of malT could not be verified, since transcription levels were similar to the wild-type level.
Although mgl was 10-fold upregulated, the growth rate of the ptsG mutant was low. Maybe a reason for the reduced growth in this case is not the reduced uptake but a less efficient subsequent phosphorylation of glucose due to limited glucokinase activity. Hernandez-Montalvo (24) described a PTS− mutant with overexpressed galactose permease with impaired growth until galactokinase levels were also enhanced.
Expression of adenylate cyclase (cyaA) was downregulated in ptsG mutant strains. Despite the low expression level of the adenylate cyclase, cAMP levels were higher in these strains, probably due to the slow growth with higher ratios of PEP to pyruvate or the prolonged growth to reach the same optical density. To be able to compare the strains, cAMP concentrations were analyzed at the same cell density; very slow growing strains therefore had more time to accumulate cAMP. The expression of galP, regulated mainly by GalR (20), exhibited lower values, while the Mgl galactose transport system, repressed by GalS (20), was upregulated in the ptsG mutant strains, although the expression of galS was enhanced. Repression of both regulators can be released by the presence of an endogenous inducer, and GalS responds to lower concentrations of galactose than GalR (20, 23, 52). The intracellular galactose concentration in these mutants seems to be high enough to allow induction of the GalS-repressed genes but too low to release repression by GalR. Elevated cAMP levels induce the expression of many transport systems in E. coli, especially the ATP-driven ABC transporters (14), to which the Mgl system belongs, as well as the maltose transporter. Endogenous inducers like galactose and maltotriose are formed from low internal concentrations of glucose. They can bind and thereby inactivate the repressor and isorepressor for galactose assimilation, GalR (34) and GalS (57), respectively, and activate MalT, the activator of the mal operons, for uptake and utilization of maltose (21). Only the glucose and mannose PTS release phosphorylated glucose into the cytoplasm. Glucose translocated by other transport systems needs subsequent phosphorylation. In the PtsG mutant strains, the concentration of internal glucose could therefore be higher if glucose is not transported only via the mannose PTS, which might lead to the observed strong induction of the Mgl system. Interestingly, the magnitude of endogenously produced inducer seems to differ in aerobic and anaerobic cells. Expression of mglB, as well as galP, is enhanced in anaerobically grown ptsG mutant strains compared to that in aerobic cultures (Fig. 5a and b), and even malE shows slightly increased expression. EIIBCGlc, the product of ptsG, is relevant not only for the uptake but also for phosphorylation of glucose. It is therefore likely that PtsG is an important factor determining the production of endogenous inducers.
Changes in gene expression with glucose excess versus glucose limitation.
Comparing glucose-excess with glucose-limiting conditions, the composition of transporters in the membrane changed: expression of the galactose transporter Mgl increased strongly, as did expression of the maltose transporter and the mannose permease; galP expression dropped to a very low level under the latter conditions. The increased expression of the galactose and maltose operon under glucose-limited conditions (also reported by others [9, 25, 60]) is due to increased cAMP levels and the presence of endogenous inducer (galactose and maltotriose) (8, 13, 39). The decreased level of galP could be a result of the repression via GalR, which is inactivated by higher concentrations of the endogenous inducer galactose than GalS (20). PTS mutants are observed to have a growth advantage in oxygen- and glucose-limited but not oxygen-supplied cultures (35), again hinting at differences in fluxes under aerobic and anaerobic cultures, as observed under conditions of glucose excess.
Analysis of mutant strains in glucose-limited continuous cultures.
No significant changes were observable in the mutant strains under glucose limitations. According to the experiments of Death and Ferenci (9), about one-third of the substrate in glucose-limited chemostats is transported via the Mgl system and the rest by the PTS. At the dilution rate of 0.2 h−1, glucose uptake solely via the glucose PTS might be sufficient. The mannose PTS, playing a minor role under conditions of glucose excess, is even less important under limiting glucose conditions (13, 27). Therefore, deleting this system does not lead to problems in glucose uptake and growth.
Conclusions.
The glucose PTS is the main glucose uptake system under conditions of glucose excess and works alongside the high-affinity transport system Mgl under conditions of glucose limitation. Deletion of ptsG, the gene encoding EIIBC, disturbs glucose transport severely and reduces the maximal growth rate. cAMP-CRP levels rise, and induction of the mgl operon occurs. Nevertheless, Mgl activity is not essential, since deletion of this transporter did not affect the growth rate. Reduced glucose concentrations altered the transporter composition of the membrane; although ptsG expression remained nearly unchanged, mgl expression increased drastically and malE and manX transcription also rose; the glucose PTS seems to be the main uptake system under these conditions, too. Again, despite the strong upregulation of mgl, deletion of this transport system did not lead to further changes. The remaining transport systems seemed to be sufficient to allow growth at the chosen growth rate.
ACKNOWLEDGMENTS
We thank Christine Richter for excellent technical support. We thank all partners of the SUMO consortium for fruitful discussions. The members of the SUMO (Systems Understanding of Microbial Oxygen response) project are K. Bettenbrock, B. Cseke, M. Ederer, J. Green, M. Holcombe, W. Jia, D. Knies, R. K. Poole, M. D. Rolfe, G. Sanguinetti, O. Sawodny, S. Stagge, S. Steinsiek, and J. Teixera de Mattos.
This work was supported by the BMBF as part of the research program SysMO (Systems Biology of Microorganisms) within the project SUMO.
Footnotes
Published ahead of print 24 August 2012
REFERENCES
- 1. Bettenbrock K, et al. 2006. A quantitative approach to catabolite repression in Escherichia coli. J. Biol. Chem. 281: 2578–2584 [DOI] [PubMed] [Google Scholar]
- 2. Boos W, Shuman H. 1998. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol. Mol. Biol. Rev. 62: 204–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen RZ, Yap WMGJ, Postma PW, Bailey JE. 1997. Comparative studies of Escherichia coli strains using different glucose uptake systems: metabolism and energetics. Biotechnol. Bioeng. 56: 583–590 [DOI] [PubMed] [Google Scholar]
- 4. Chou CH, Bennett GN, San KY. 1994. Effect of modified glucose uptake using genetic engineering techniques on high-level recombinant protein production in Escherichia coli dense cultures. Biotechnol. Bioeng. 44: 952–960 [DOI] [PubMed] [Google Scholar]
- 5. Curtis SJ, Epstein W. 1975. Phosphorylation of D-glucose in Escherichia coli mutants defective in glucosephosphotransferase, mannosephosphotransferase, and glucokinase. J. Bacteriol. 122: 1189–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97: 6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. De Anda R, et al. 2006. Replacement of the glucose phosphotransferase transport system by galactose permease reduces acetate accumulation and improves process performance of Escherichia coli for recombinant protein production without impairment of growth rate. Metab. Eng. 8: 281–290 [DOI] [PubMed] [Google Scholar]
- 8. Death A, Ferenci T. 1994. Between feast and famine—endogenous inducer synthesis in the adaptation of Escherichia coli to growth with limiting carbohydrates. J. Bacteriol. 176: 5101–5107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Death A, Ferenci T. 1993. The importance of the binding-protein-dependent Mgi system to the transport of glucose in Escherichia coli growing on low sugar concentrations. Res. Microbiol. 144: 529–537 [DOI] [PubMed] [Google Scholar]
- 10. Decker K, Plumbridge J, Boos W. 1998. Negative transcriptional regulation of a positive regulator: the expression of malT, encoding the transcriptional activator of the maltose regulon of Escherichia coli, is negatively controlled by Mlc. Mol. Microbiol. 27: 381–390 [DOI] [PubMed] [Google Scholar]
- 11. De Reuse H, Danchin A. 1991. Positive regulation of the Pts operon of Escherichia coli—genetic evidence for a signal transduction mechanism. J. Bacteriol. 173: 727–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a. Deutscher J, Francke C, Postma PA. 2006. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol. Mol. Biol. Rev. 70: 939–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Evans C, Herbert D, Tempest D. 1970. The continuous cultivation of micro-organisms. 2. Construction of a chemostat, p 277–327. Methods in microbiology, vol 2 Academic Press Inc., London, United Kingdom [Google Scholar]
- 13. Ferenci T. 1996. Adaptation to life at micromolar nutrient levels: the regulation of Escherichia coli glucose transport by endoinduction and cAMP. FEMS Microbiol. Rev. 18: 301–317 [DOI] [PubMed] [Google Scholar]
- 14. Ferenci T. 1999. Regulation by nutrient limitation. Curr. Opin. Microbiol. 2: 208–213 [DOI] [PubMed] [Google Scholar]
- 15. Ferenci T, et al. 2009. Genomic sequencing reveals regulatory mutations and recombinational events in the widely used MC4100 lineage of Escherichia coli K-12. J. Bacteriol. 191: 4025–4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flores N, et al. 2005. Adaptation for fast growth on glucose by differential expression of central carbon metabolism and gal regulon genes in an Escherichia coli strain lacking the phosphoenolpyruvate:carbohydrate phosphotransferase system. Metab. Eng. 7: 70–87 [DOI] [PubMed] [Google Scholar]
- 17. Flores N, Xiao J, Berry A, Bolivar F, Valle F. 1996. Pathway engineering for the production of aromatic compounds in Escherichia coli. Nat. Biotechnol. 14: 620–623 [DOI] [PubMed] [Google Scholar]
- 18. Franchini AG, Egli T. 2006. Global gene expression in Escherichia coli K-12 during short-term and long-term adaptation to glucose-limited continuous culture conditions. Microbiology 152: 2111–2127 [DOI] [PubMed] [Google Scholar]
- 19. Freddolino PL, Amini S, Tavazoie S. 2012. Newly identified genetic variations in common Escherichia coli MG1655 stock cultures. J. Bacteriol. 194: 303–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Geanacopoulos M, Adhya S. 1997. Functional characterization of roles of GalR and GalS as regulators of the gal regulon. J. Bacteriol. 179: 228–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hatfield D, Hofnung M, Schwartz M. 1969. Genetic analysis of maltose A region in Escherichia coli. J. Bacteriol. 98: 559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. 2007. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 8: R19 doi:10.1186/gb-2007-8-2-r19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henderson PJF. 1980. The interrelationship between proton coupled and binding protein dependent transport systems in bacteria. Biochem. Soc. Trans. 8: 678–679 [DOI] [PubMed] [Google Scholar]
- 24. Hernandez-Montalvo V, et al. 2003. Expression of galP and glk in a Escherichia coli PTS mutant restores glucose transport and increases glycolytic flux to fermentation products. Biotechnol. Bioeng. 83: 687–694 [DOI] [PubMed] [Google Scholar]
- 25. Hua Q, Yang C, Oshima T, Mori H, Shimizu K. 2004. Analysis of gene expression in Escherichia coli in response to changes of growth-limiting nutrient in chemostat cultures. Appl. Environ. Microbiol. 70: 2354–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunter IS, Kornberg HL. 1979. Glucose transport of Escherichia coli growing in glucose-limited continuous culture. Biochem. J. 178: 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Inada T, Takahashi H, Mizuno T, Aiba H. 1996. Down regulation of cAMP production by cAMP receptor protein in Escherichia coli: an assessment of the contributions of transcriptional and posttranscriptional control of adenylate cyclase. Mol. Gen. Genet. 253: 198–204 [DOI] [PubMed] [Google Scholar]
- 28. Jeong JY, et al. 2004. Expression of ptsG encoding the major glucose transporter is regulated by ArcA in Escherichia coli. J. Biol. Chem. 279: 38513–38518 [DOI] [PubMed] [Google Scholar]
- 29. Joly N, Bohm A, Boos W, Richet E. 2004. MalK, the ATP-binding cassette component of the Escherichia coli maltodextrin transporter, inhibits the transcriptional activator MalT by antagonizing inducer binding. J. Biol. Chem. 279: 33123–33130 (Erratum, 279:44229.). [DOI] [PubMed] [Google Scholar]
- 30. Karu AE, Belk ED. 1982. Induction of E. coli recA protein via recBC and alternate pathways—quantitation by enzyme-linked immunosorbent assay (ELISA). Mol. Gen. Genet. 185: 275–282 [DOI] [PubMed] [Google Scholar]
- 31. Kimata K, Tanaka Y, Inada T, Aiba H. 2001. Expression of the glucose transporter gene, ptsG, is regulated at the mRNA degradation step in response to glycolytic flux in Escherichia coli. EMBO J. 20: 3587–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee SJ, Boos W, Bouche JP, Plumbridge J. 2000. Signal transduction between a membrane-bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J. 19: 5353–5361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu J, et al. 2012. Combinatorial modulation of galP and glk gene expression for improved alternative glucose utilization. Appl. Microbiol. Biotechnol. 93: 2455–2462 [DOI] [PubMed] [Google Scholar]
- 34. Majumdar A, Rudikoff S, Adhya S. 1987. Purification and properties of Gal repressor-pL-galR fusion in pKC31 plasmid vector. J. Biol. Chem. 262: 2326–2331 [PubMed] [Google Scholar]
- 35. Manche K, Notley-McRobb L, Ferenci T. 1999. Mutational adaptation of Escherichia coli to glucose limitation involves distinct evolutionary pathways in aerobic and oxygen-limited environments. Genetics 153: 5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matin A, Matin MK. 1982. Cellular levels, excretion, and synthesis rates of cyclic-AMP in Escherichia coli grown in continuous culture. J. Bacteriol. 149: 801–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McDonald TP, Walmsley AR, Henderson PJF. 1997. Asparagine 394 in putative helix 11 of the galactose-H+ symport protein (GalP) from Escherichia coli is associated with the internal binding site for cytochalasin B and sugar. J. Biol. Chem. 272: 15189–15199 [DOI] [PubMed] [Google Scholar]
- 38. Nam TW, et al. 2001. The Escherichia coli glucose transporter enzyme IICBGlc recruits the global repressor Mlc. EMBO J. 20: 491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Notley L, Ferenci T. 1995. Differential expression of Mal genes under cAMP and endogenous inducer control in nutrient-stressed Escherichia coli. Mol. Microbiol. 16: 121–129 [DOI] [PubMed] [Google Scholar]
- 40. Notley-McRobb L, Death A, Ferenci T. 1997. The relationship between external glucose concentration and cAMP levels inside Escherichia coli: implications for models of phosphotransferase-mediated regulation of adenylate cyclase. Microbiol. 143(Pt 6): 1909–1918 [DOI] [PubMed] [Google Scholar]
- 41. Notley-McRobb L, Ferenci T. 1999. Adaptive mgl-regulatory mutations and genetic diversity evolving in glucose-limited Escherichia coli populations. Environ. Microbiol. 1: 33–43 [DOI] [PubMed] [Google Scholar]
- 42. Panagiotidis CH, Boos W, Shuman HA. 1998. The ATP-binding cassette subunit of the maltose transporter MalK antagonizes MalT, the activator of the Escherichia coli mal regulon. Mol. Microbiol. 30: 535–546 [DOI] [PubMed] [Google Scholar]
- 43. Park YH, Lee BR, Seok YJ, Peterkofsky A. 2006. In vitro reconstitution of catabolite repression in Escherichia coli. J. Biol. Chem. 281: 6448–6454 [DOI] [PubMed] [Google Scholar]
- 44. Picon A, de Mattos MJT, Postma PW. 2008. Protein production by Escherichia coli wild-type and ΔptsG mutant strains with IPTG induction at the onset. J. Ind. Microbiol. Biotechnol. 35: 213–218 [DOI] [PubMed] [Google Scholar]
- 45. Picon A, de Mattos MJT, Postma PW. 2005. Reducing the glucose uptake rate in Escherichia coli affects growth rate but not protein production. Biotechnol. Bioeng. 90: 191–200 [DOI] [PubMed] [Google Scholar]
- 46. Plumbridge J. 1999. Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol. Microbiol. 33: 260–273 [DOI] [PubMed] [Google Scholar]
- 47. Plumbridge J. 2002. Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr. Opin. Microbiol. 5: 187–193 [DOI] [PubMed] [Google Scholar]
- 47a. Postma PW, Lengeler JW, Jacobson GR. 1993. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol. Rev. 57: 543–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Reyes M, Shuman HA. 1988. Overproduction of MalK protein prevents expression of the Escherichia coli mal regulon. J. Bacteriol. 170: 4598–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roehl RA, Vinopal RT. 1980. Genetic locus, distant from ptsM, affecting enzyme Iia-Iib function in Escherichia coli K-12. J. Bacteriol. 142: 120–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saier MH. 1989. Protein-phosphorylation and allosteric control of inducer exclusion and catabolite repression by the bacterial phosphoenolpyruvate-sugar phosphotransferase system. Microbiol. Rev. 53: 109–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50a. Saier MH, Reizer J. 1994. The bacterial phosphotransferase system—new frontiers 30 years later. Mol. Microbiol. 13: 755–764 [DOI] [PubMed] [Google Scholar]
- 51. Seeto S, Notley-McRobb L, Ferenci T. 2004. The multifactorial influences of RpoS, Mlc and cAMP on ptsG expression under glucose-limited and anaerobic conditions. Res. Microbiol. 155: 211–215 [DOI] [PubMed] [Google Scholar]
- 52. Semsey S, Krishna S, Sneppen K, Adhya S. 2007. Signal integration in the galactose network of Escherichia coli. Mol. Microbiol. 65: 465–476 [DOI] [PubMed] [Google Scholar]
- 53. Shin D, Cho N, Kim YJ, Seok YJ, Ryu S. 2008. Up-regulation of the cellular level of Escherichia coli PTS components by stabilizing reduced transcripts of the genes in response to the low oxygen level. Biochem. Biophys. Res. Commun. 370: 609–612 [DOI] [PubMed] [Google Scholar]
- 54. Shin D, Lim S, Seok Y, Ryu S. 2001. Heat shock RNA polymerase (E sigma(32)) is involved in the transcription of mlc and crucial for induction of the Mlc regulon by glucose in Escherichia coli. J. Biol. Chem. 276: 25871–25875 [DOI] [PubMed] [Google Scholar]
- 55. Soupene E, et al. 2003. Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J. Bacteriol. 185: 5611–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tanaka Y, Kimata K, Aiba H. 2000. A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J. 19: 5344–5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tokeson JPE, Garges S, Adhya S. 1991. Further inducibility of a constitutive system—ultrainduction of the gal operon. J. Bacteriol. 173: 2319–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Weickert MJ, Adhya S. 1993. Control of transcription of gal repressor and isorepressor genes in Escherichia coli. J. Bacteriol. 175: 251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weickert MJ, Adhya S. 1992. Isorepressor of the gal regulon in Escherichia coli. J. Mol. Biol. 226: 69–83 [DOI] [PubMed] [Google Scholar]
- 60. Wick LM, Quadroni M, Egli T. 2001. Short- and long-term changes in proteome composition and kinetic properties in a culture of Escherichia coli during transition from glucose-excess to glucose-limited growth conditions in continuous culture and vice versa. Environ. Microbiol. 3: 588–599 [DOI] [PubMed] [Google Scholar]
- 61. Wolfe AJ. 2005. The acetate switch. Microbiol. Mol. Biol. Rev. 69: 12–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zeppenfeld T, Larisch C, Lengeler JW, Jahreis K. 2000. Glucose transporter mutants of Escherichia coli K-12 with changes in substrate recognition of IICBGlc and induction behavior of the ptsG gene. J. Bacteriol. 182: 4443–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]