Abstract
To test the error catastrophe theory of aging we determined the error frequency of protein synthesis in several strains of cultured human fibroblasts at early and late passage. Error rates were calculated from analysis of native and substituted actins on two-dimensional gels of cellular proteins after induction of mistranslation by histidine starvation in the presence of histidinol. Early-passage cells from fetal, young, and old donors and cells from subjects with the Hutchinson-Gilford and Werner syndromes of accelerated aging had similar error frequencies. Late-passage cells from fetal, young, and old normal donors had similar or lower error frequencies than corresponding early-passage cells. No correlation was observed between error frequency, donor age, or maximal life span in vitro. We also examined an immortal cell line, simian virus 40-transformed W138 fibroblasts. These cells had a significantly elevated rate of mistranslation (2.8 +/- 0.2 x 10(-4))(+/- SEM) compared to their untransformed counterpart WI38 (0.6 +/- 0.1 X 10(-4)) or all diploid cells combined (1.1 +/- 0.1 x 10(-4)). Taken together, the data fail to support the error catastrophe theory of aging.
Full text
PDF
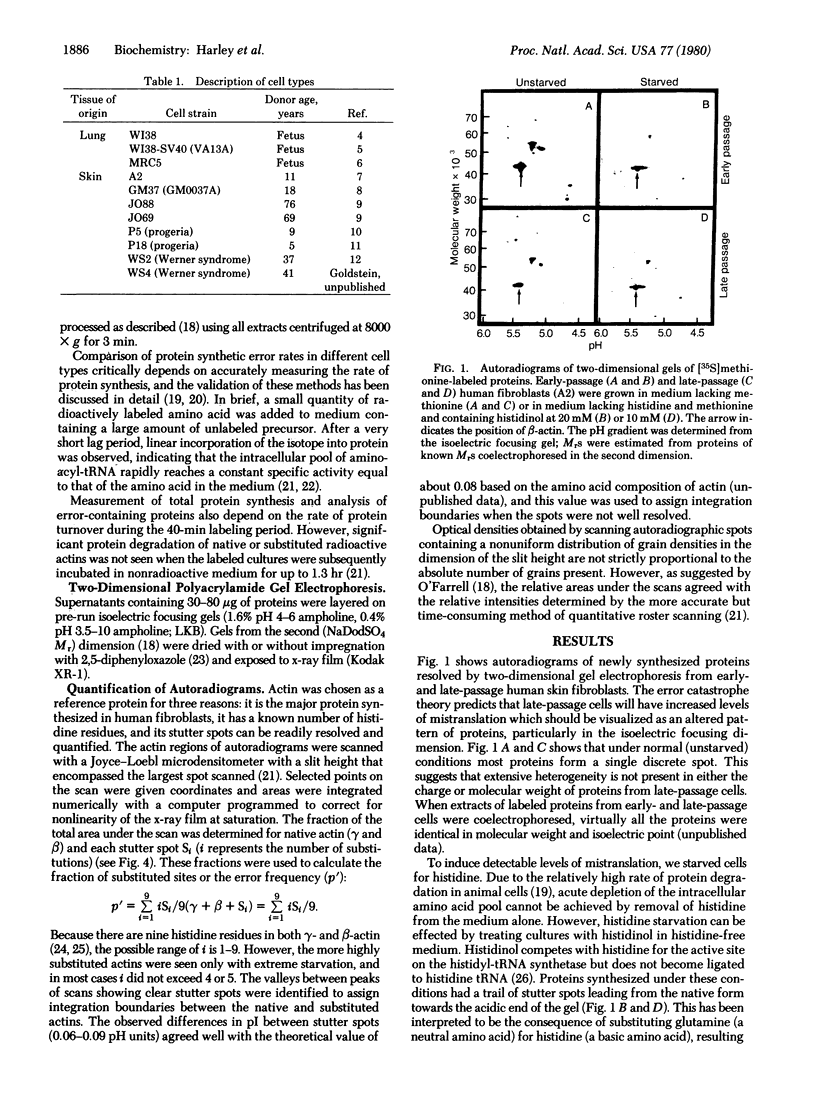
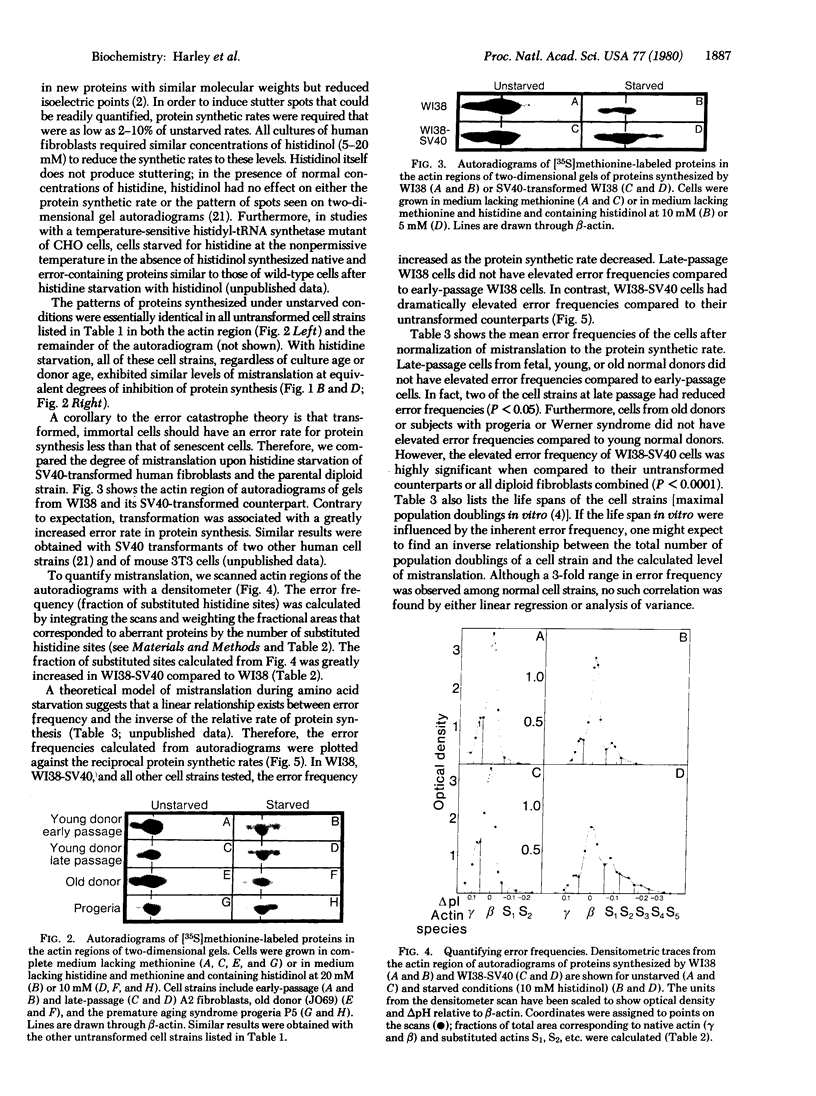


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird M. G., Samis H. V., Massie H. R., Zimmerman J. A. A brief argument in opposition to the Orgel hypothesis. Gerontologia. 1975;21(1):57–63. doi: 10.1159/000212031. [DOI] [PubMed] [Google Scholar]
- Baxter G. C., Stanners C. P. The effect of protein degradation on cellular growth characteristics. J Cell Physiol. 1978 Aug;96(2):139–145. doi: 10.1002/jcp.1040960202. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Elzinga M. The primary structure of actin from rabbit skeletal muscle. Completion and analysis of the amino acid sequence. J Biol Chem. 1975 Aug 10;250(15):5915–5920. [PubMed] [Google Scholar]
- Cristofalo V. J. Thymidine labelling index as a criterion of aging in vitro. Gerontology. 1976;22(1-2):9–27. doi: 10.1159/000212122. [DOI] [PubMed] [Google Scholar]
- Edelmann P., Gallant J. Mistranslation in E. coli. Cell. 1977 Jan;10(1):131–137. doi: 10.1016/0092-8674(77)90147-7. [DOI] [PubMed] [Google Scholar]
- Engelhardt D. L., Lee G. T., Moley J. F. Patterns of peptide synthesis in senescent and presenescent human fibroblasts. J Cell Physiol. 1979 Jan;98(1):193–198. doi: 10.1002/jcp.1040980121. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., JENSEN F. C., KOPROWSKI H. SV40-INDUCED TRANFORMATION OF HUMAN DIPLOID CELLS: CRISIS AND RECOVERY. J Cell Physiol. 1965 Feb;65:69–83. doi: 10.1002/jcp.1030650110. [DOI] [PubMed] [Google Scholar]
- Gallant J., Palmer L. Error propagation in viable cells. Mech Ageing Dev. 1979 Apr;10(1-2):27–38. doi: 10.1016/0047-6374(79)90068-x. [DOI] [PubMed] [Google Scholar]
- Gershon D. Current status of age altered enzymes: alternative mechanisms. Mech Ageing Dev. 1979 Feb;9(3-4):189–196. doi: 10.1016/0047-6374(79)90098-8. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Harley C. B. In vitro studies of age-associated diseases. Fed Proc. 1979 Apr;38(5):1862–1867. [PubMed] [Google Scholar]
- Goldstein S., Littlefield J. W. Effect of insulin on the conversion of glucose-C-14 to C-14-O2 by normal and diabetic fibroblasts in culture. Diabetes. 1969 Aug;18(8):545–549. doi: 10.2337/diab.18.8.545. [DOI] [PubMed] [Google Scholar]
- Goldstein S., Moerman E. J., Soeldner J. S., Gleason R. E., Barnett D. M. Diabetes mellitus and genetic prediabetes. Decreased replicative capacity of cultured skin fibroblasts. J Clin Invest. 1979 Mar;63(3):358–370. doi: 10.1172/JCI109311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S., Moerman E. Heat-labile enzymes in skin fibroblasts from subjects with progeria. N Engl J Med. 1975 Jun 19;292(25):1305–1309. doi: 10.1056/NEJM197506192922501. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hansen B. S., Vaughan M. H., Wang L. Reversible inhibition by histidinol of protein synthesis in human cells at the activation of histidine. J Biol Chem. 1972 Jun 25;247(12):3854–3857. [PubMed] [Google Scholar]
- Harley C. B., Goldstein S. Cultured human fibroblasts: distribution of cell generations and a critical limit. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):509–516. doi: 10.1002/jcp.1040970326. [DOI] [PubMed] [Google Scholar]
- Hirsch G. P., Holland J. M., Popp R. A. Amino acid analog incorporation into protein in dimethyl sulfoxide and mutagen-treated aging mice. Birth Defects Orig Artic Ser. 1978;14(1):431–448. [PubMed] [Google Scholar]
- Holliday R. Growth and death of diploid and transformed human fibroblasts. Fed Proc. 1975 Jan;34(1):51–55. [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- LOFTFIELD R. B. THE FREQUENCY OF ERRORS IN PROTEIN BIOSYNTHESIS. Biochem J. 1963 Oct;89:82–92. doi: 10.1042/bj0890082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Loeb L. A., Springgate C. F., Battula N. Errors in DNA replication as a basis of malignant changes. Cancer Res. 1974 Sep;34(9):2311–2321. [PubMed] [Google Scholar]
- Loftfield R. B., Vanderjagt D. The frequency of errors in protein biosynthesis. Biochem J. 1972 Aug;128(5):1353–1356. doi: 10.1042/bj1281353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee E. E., Cheung J. Y., Rannels D. E., Morgan H. E. Measurement of the rate of protein synthesis and compartmentation of heart phenylalanine. J Biol Chem. 1978 Feb 25;253(4):1030–1040. [PubMed] [Google Scholar]
- Morrow J., Garner C. An evaluation of some theories of the mechanism of aging. Gerontology. 1979;25(3):136–144. doi: 10.1159/000212332. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. The suppression of defective translation by ppGpp and its role in the stringent response. Cell. 1978 Jul;14(3):545–557. doi: 10.1016/0092-8674(78)90241-6. [DOI] [PubMed] [Google Scholar]
- ORGEL L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963 Apr;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Pollard J. W., Friesen J. D., Stanners C. P. Stuttering: high-level mistranslation in animal and bacterial cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1091–1095. doi: 10.1073/pnas.75.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein M. The formation of altered enzymes in aging animals. Mech Ageing Dev. 1979 Feb;9(3-4):197–202. doi: 10.1016/0047-6374(79)90099-x. [DOI] [PubMed] [Google Scholar]
- Russell W. C., Newman C., Williamson D. H. A simple cytochemical technique for demonstration of DNA in cells infected with mycoplasmas and viruses. Nature. 1975 Feb 6;253(5491):461–462. doi: 10.1038/253461a0. [DOI] [PubMed] [Google Scholar]
- Ryan J. M., Duda G., Cristofalo V. J. Error accumulation and aging in human diploid cells. J Gerontol. 1974 Nov;29(6):616–621. doi: 10.1093/geronj/29.6.616. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Adams M. E., Harkins J. L., Pollard J. W. Transformed cells have lost control of ribosome number through their growth cycle. J Cell Physiol. 1979 Jul;100(1):127–138. doi: 10.1002/jcp.1041000113. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Actin amino-acid sequences. Comparison of actins from calf thymus, bovine brain, and SV40-transformed mouse 3T3 cells with rabbit skeletal muscle actin. Eur J Biochem. 1978 Oct 16;90(3):451–462. doi: 10.1111/j.1432-1033.1978.tb12624.x. [DOI] [PubMed] [Google Scholar]
- Wilson D. L., Hall M. E., Stone G. C. Test of some aging hypotheses using two-dimensional protein mapping. Gerontology. 1978;24(6):426–433. doi: 10.1159/000212282. [DOI] [PubMed] [Google Scholar]
- Yatscoff R. W., Goldstein S., Freeman K. B. Conservation of genes coding for proteins synthesized in human mitochondria. Somatic Cell Genet. 1978 Nov;4(6):633–645. doi: 10.1007/BF01543155. [DOI] [PubMed] [Google Scholar]







