Abstract
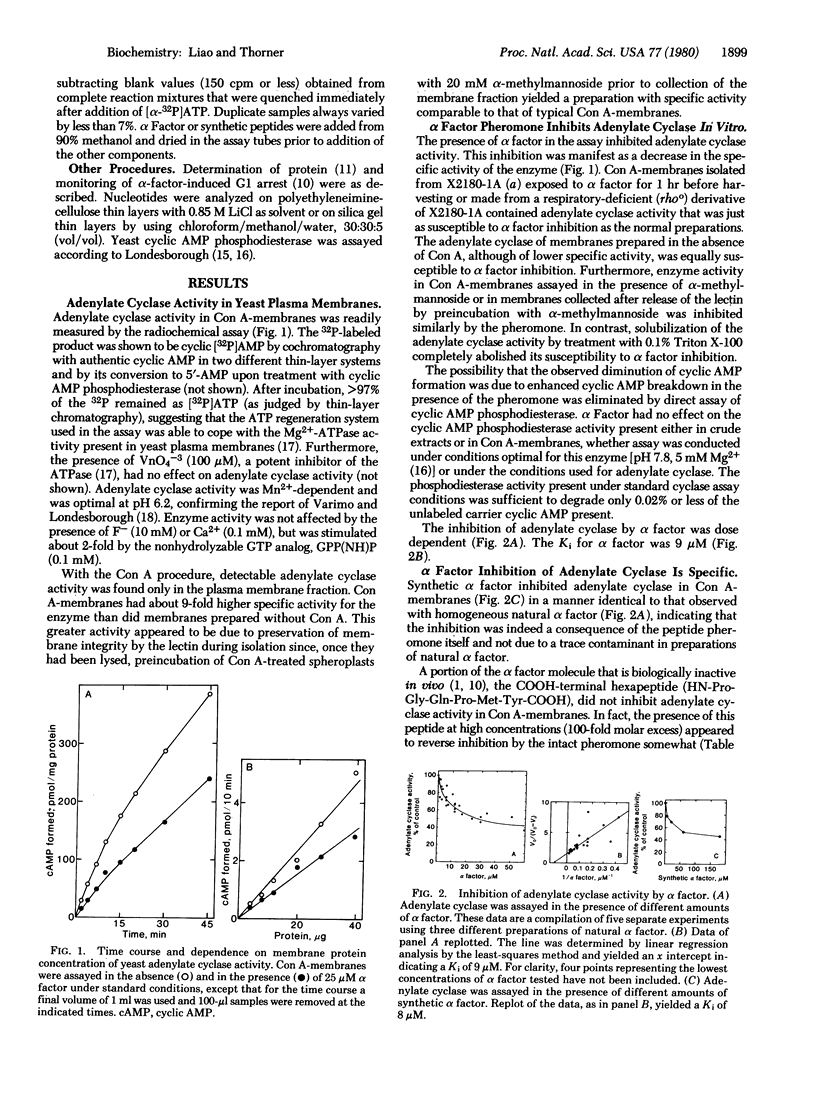
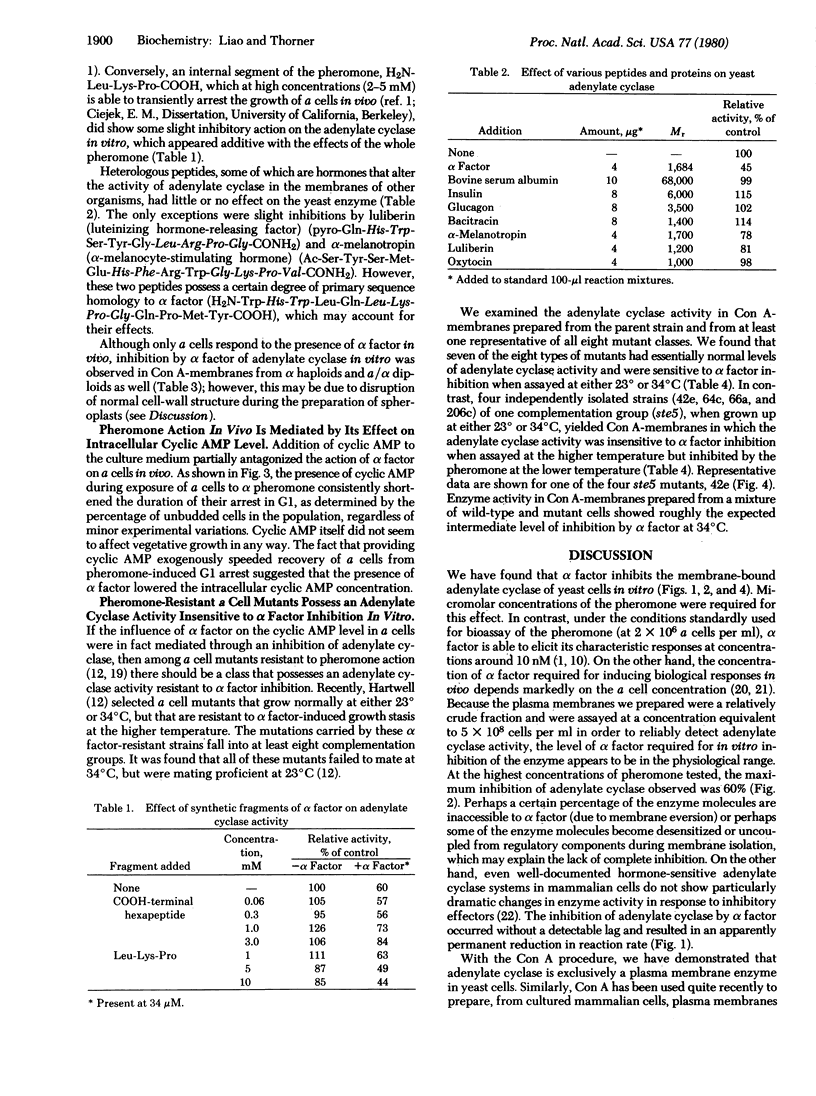
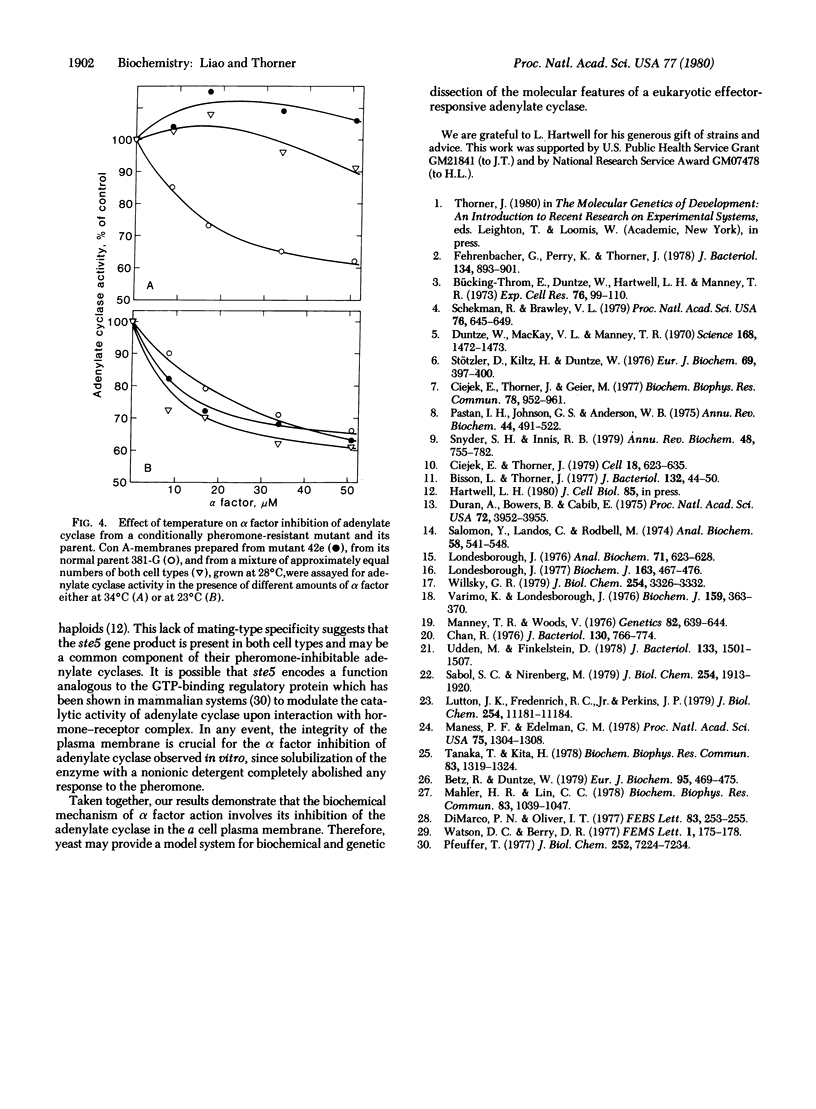
The pheromone alpha factor, secreted by Saccharomyces cerevisiae cells of the alpha mating type, serves to synchronize the opposite mating type (a cells) at G1 as a prelude to fusion of the two cell types. We found that, in vitro, alpha factor inhibited the membrane-bound adenylate cyclase of these cells in a dose-dependent manner. Moreover, one class (ste5) of a cell mutants that grow normally at either 23 degrees or 34 degrees C but that are unable to respond to alpha factor or to mate at the higher temperature possessed an adenylate cyclase activity that was not inhibited by alpha factor at 34 degrees C but was fully sensitive to inhibition at 23 degrees C. Furthermore, addition of cyclic AMP to a cell culture medium shortened the period of pheromone-induced G1 arrest. We conclude that inhibition of adenylate cyclase activity by alpha factor may constitute, at least in part, the biochemical mode of action of the pheromone in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz R., Duntze W. Purification and partial characterization of a factor, a mating hormone produced by mating-type-a cells from Saccharomyces cerevisiae. Eur J Biochem. 1979 Apr;95(3):469–475. doi: 10.1111/j.1432-1033.1979.tb12986.x. [DOI] [PubMed] [Google Scholar]
- Bisson L., Thorner J. Thymidine 5'-monophosphate-requiring mutants of Saccharomyces cerevisiae are deficient in thymidylate synthetase. J Bacteriol. 1977 Oct;132(1):44–50. doi: 10.1128/jb.132.1.44-50.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücking-Throm E., Duntze W., Hartwell L. H., Manney T. R. Reversible arrest of haploid yeast cells in the initiation of DNA synthesis by a diffusible sex factor. Exp Cell Res. 1973 Jan;76(1):99–110. doi: 10.1016/0014-4827(73)90424-2. [DOI] [PubMed] [Google Scholar]
- Chan R. K. Recovery of Saccharomyces cerevisiae mating-type a cells from G1 arrest by alpha factor. J Bacteriol. 1977 May;130(2):766–774. doi: 10.1128/jb.130.2.766-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E., Thorner J., Geier M. Solid phase peptide synthesis of alpha-factor, a yeast mating pheromone. Biochem Biophys Res Commun. 1977 Oct 10;78(3):952–961. doi: 10.1016/0006-291x(77)90514-9. [DOI] [PubMed] [Google Scholar]
- Ciejek E., Thorner J. Recovery of S. cerevisiae a cells from G1 arrest by alpha factor pheromone requires endopeptidase action. Cell. 1979 Nov;18(3):623–635. doi: 10.1016/0092-8674(79)90117-x. [DOI] [PubMed] [Google Scholar]
- Di Marco P. N., Oliver I. T. Problems associated with the determination of the intracellular distribution of adenosine 3',5'-cyclic monophosphate in rat liver. FEBS Lett. 1977 Nov 15;83(2):253–255. doi: 10.1016/0014-5793(77)81016-8. [DOI] [PubMed] [Google Scholar]
- Duntze W., MacKay V., Manney T. R. Saccharomyces cerevisiae: a diffusible sex factor. Science. 1970 Jun 19;168(3938):1472–1473. doi: 10.1126/science.168.3938.1472. [DOI] [PubMed] [Google Scholar]
- Durán A., Bowers B., Cabib E. Chitin synthetase zymogen is attached to the yeast plasma membrane. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3952–3955. doi: 10.1073/pnas.72.10.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher G., Perry K., Thorner J. Cell-cell recognition in Saccharomyces cerevisiae: regulation of mating-specific adhesion. J Bacteriol. 1978 Jun;134(3):893–901. doi: 10.1128/jb.134.3.893-901.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londesborough J. Characterization of an adenosine 3':5'-cyclic monophosphate phosphodiesterase from baker's yeast. Its binding to subcellular particles, catalytic properties and gel-filtration behaviour. Biochem J. 1977 Jun 1;163(3):467–476. doi: 10.1042/bj1630467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londesborough J. Quantitative estimation of 3'5' cyclic AMP phosphodiesterase using anion exchange resin in a batch process. Anal Biochem. 1976 Apr;71(2):623–628. doi: 10.1016/s0003-2697(76)80037-1. [DOI] [PubMed] [Google Scholar]
- Lutton J. K., Frederich R. C., Jr, Perkins J. P. Isolation of adenylate cyclase-enriched membranes from mammalian cells using concanavalin A. J Biol Chem. 1979 Nov 25;254(22):11181–11184. [PubMed] [Google Scholar]
- Mahler H. R., Lin C. C. Exogenous adenosine 3': 5'-monophosphate can release yeast from catabolite repression. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1039–1047. doi: 10.1016/0006-291x(78)91500-0. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Edelman G. M. Inactivation and chemical alteration of mating factor alpha by cells and spheroplasts of yeast. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1304–1308. doi: 10.1073/pnas.75.3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manney T. R., Woods V. Mutants of Saccharomyces cerevisiae resistant to the alpha mating-type factor. Genetics. 1976 Apr;82(4):639–644. doi: 10.1093/genetics/82.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I. H., Johnson G. S., Anderson W. B. Role of cyclic nucleotides in growth control. Annu Rev Biochem. 1975;44:491–522. doi: 10.1146/annurev.bi.44.070175.002423. [DOI] [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Sabol S. L., Nirenberg M. Regulation of adenylate cyclase of neuroblastoma x glioma hybrid cells by alpha-adrenergic receptors. I. Inhibition of adenylate cyclase mediated by alpha receptors. J Biol Chem. 1979 Mar 25;254(6):1913–1920. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schekman R., Brawley V. Localized deposition of chitin on the yeast cell surface in response to mating pheromone. Proc Natl Acad Sci U S A. 1979 Feb;76(2):645–649. doi: 10.1073/pnas.76.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Innis R. B. Peptide neurotransmitters. Annu Rev Biochem. 1979;48:755–782. doi: 10.1146/annurev.bi.48.070179.003543. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Kita H. Site of action of mating factor in a-mating type cell of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1319–1324. doi: 10.1016/0006-291x(78)91365-7. [DOI] [PubMed] [Google Scholar]
- Udden M. M., Finkelstein D. B. Reaction order of Saccharomyces cerevisiae alpha-factor-mediated cell cycle arrest and mating inhibition. J Bacteriol. 1978 Mar;133(3):1501–1507. doi: 10.1128/jb.133.3.1501-1507.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varimo K., Londesborough J. Solubilization and other studies on adenylate cyclase of baker's yeast. Biochem J. 1976 Nov;159(2):363–370. doi: 10.1042/bj1590363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R. Characterization of the plasma membrane Mg2+-ATPase from the yeast, Saccharomyces cerevisiae. J Biol Chem. 1979 May 10;254(9):3326–3332. [PubMed] [Google Scholar]



