Abstract
Cannabinoids exert powerful action on various forms of synaptic plasticity. These retrograde messengers modulate GABA and glutamate release from presynaptic terminals by acting on presynaptic CB1 receptors. In particular, they inhibit long-term potentiation (LTP) elicited by electrical stimulation of excitatory pathways in rat hippocampus. Recently, LTP of the field excitatory postsynaptic potential (fEPSP) induced by exogenous ATP has been thoroughly explored. The present study demonstrates that cannabinoids inhibit ATP-induced LTP in hippocampal slices of rat. Administration of 10 μM of ATP led to strong inhibition of fEPSPs in CA1/CA3 hippocampal synapses. Within 40 min after ATP removal from bath solution, robust LTP was observed (fEPSP amplitude comprised 130.1 ± 3.8% of control, n = 10). This LTP never appeared when ATP was applied in addition to cannabinoid receptor agonist WIN55,212-2 (100 nM). Selective CB1 receptor antagonist, AM251 (500 nM), completely abolished this effect of WIN55,212-2. Our data indicate that like canonical LTP elicited by electrical stimulation, ATP-induced LTP is under control of CB1 receptors.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-012-9296-5) contains supplementary material, which is available to authorized users.
Keywords: LTP, ATP, Cannabinoids, WIN, Adenosine
Introduction
Apart from its role as “molecular unit of currency” of intracellular energy transfer, ATP is involved in intercellular signaling: when released, it acts as a neurotransmitter and powerful neuromodulator. Its role in neuronal plasticity mechanisms has been also disclosed [9–11].
To date, the sources of extracellular ATP are well determined. ATP is co-released with classical neurotransmitters at neuronal synapses, where it activates purinergic receptors and contributes to postsynaptic signals in these synapses [12–18]. Purinoreceptors, extracellular sensors of ATP, are abundantly expressed throughout the brain in glial cells and neurons [12, 19]. A number of studies indicate that the locus of modulatory action of ATP on synaptic transmission can be both post- and presynaptic [15, 20–23]. Although neurons have been traditionally considered as the sole source of ATP and adenosine in the CNS, glial cells have been recently shown to release ATP as well [24, 25].
Within hippocampal neuronal circuits, ATP is involved in numerous mechanisms of signal transduction, for example, in facilitation of excitatory transmission to stratum radiatum interneurons [22]. ATP mediates glutamatergic activity-dependent heterosynaptic suppression when released from astrocytes [26]. A significant part of ATP-evoked effects can be attributed to the hydrolysis products of this nucleotide.
ATP can act directly via P2X [27], P2Y [28] receptors and indirectly via its catabolites, ADP, AMP, cAMP, and adenosine produced by membrane-bound ecto-nucleotidases, ecto-protein kinases, and ecto-ATPases [17, 29, 30]. Adenosine is known to be a potent inhibitory neuromodulator, increasing postsynaptic K+ conductance and decreasing presynaptic Ca2+ conductance [18, 31–34].
In a series of studies [9, 10, 35–37], superfusion of hippocampal slices with ATP caused, after initial depression of evoked potentials, rebound facilitation of field excitatory postsynaptic potentials (fEPSP). The latter effect is observed after drug removal and persists in in vitro preparations for a relatively prolonged period, up to 1 h. This phenomenon has been classified as “ATP-induced long-term potentiation (LTP)” [10] and thus can be compared to electrically evoked LTP, originally described in the hippocampus by Bliss and Lomo [38]
Long-lasting changes in synaptic efficiency caused by ATP were first defined by Wieraszko [10]. Extensive analysis of ATP-induced plasticity has been carried out by Fujii group [9]. The phenomenon has been found to depend on NMDA receptor activity [8]. In further studies, phosphorylation of extracellular membrane domains (possibly those of NMDA receptors) by ecto-protein kinases has been suggested as a part of underlying mechanism of ATP-induced LTP [9]. Recent studies demonstrate that ATP does not modulate activity-dependent homosynaptic long-term depression (LTD) in rat CA1 hippocampal region. These studies suggest that the effects of ATP on the synaptic plasticity are selective, triggering LTP but not interfering with LTD [39].
The expression of cannabinoid receptors is relatively high in hippocampus, cerebellum, and basal ganglia [40]. Endogenous cannabinoids are intercellular signaling molecules. They are synthesized from postsynaptic membrane lipids in Ca2+-triggered manner and travel back through the postsynaptic membrane reaching presynaptic terminals [41]. As a result, these retrograde messengers bind to presynaptic CB1 receptors and depress synaptic transmission in hippocampus and cerebellum via the presynaptic mechanism. Typically, these relatively rapid changes in synaptic transmission involve depression of neurotransmitter release both in inhibitory and excitatory synapses [42]. Several studies indicate that tetanus-induced LTP at hippocampal excitatory synapses is impaired by cannabinoids [43–45]. Induction of LTP in CA1 area can trigger a group I mGluR-dependent LTD at inhibitory synapses (I-LTD) which is mediated by retrograde endocannabinoid signaling. I-LTD may, in turn, underlie changes of pyramidal cell excitability associated with LTP at excitatory synapses [46]. This is indicative of CB1 receptors playing a critical role for learning and memory formation in hippocampus (see also [1–7]). In this paper, we demonstrate that exogenous cannabinoids prevent ATP-induced LTP in CA1 area of rat hippocampus.
Materials and methods
Rat hippocampal slice preparation
All experiments were performed in accordance with the Guiding Principles for Care and Use of Animals in the Field of Physiological Sciences of the Physiological Society of Ukraine and approved by the local animal care Committee of the Bogomoletz Institute of Physiology. Wistar rats (19–20 days old) were anesthetized by diethyl ether and then decapitated. Hippocampi were gently removed and cut into transverse slices (400 μm) with a vibrating slice cutter (Campden Vibroslicer) in ice-cold artificial cerebrospinal fluid (ACSF). The cutting procedure was performed in ACSF that contained (in millimolar): 119 NaCl, 2.5 KCl, 1.3 MgSO4∙7H2O, 1 NaH2PO4, 26.2 NaHCO3, 2.5 CaCl2, and 11 glucose. ACSF was equilibrated with 95% O2/5% CO2 (310 mOsm/L; pH 7.4 when saturated with the gas mixture).The slices were incubated in the standard ACSF for 40–60 min at room temperature before the experiments. Just before recording, a slice was transferred to the recording chamber that was continuously perfused with ACSF which was delivered with gravity-fed perfusion system at a flow rate of 2.5–3 ml/min. The CA1 pyramidal cell layer and Schaffer collateral pathway were visually identified with the infrared differential interference contrast video microscope (Olympus); IR-1000 (DAGE-MTI, Michigan City) and captured with CoolSNAP ES2 (CCD ICX285). Recordings were made at temperature 30–32°C in the recording chamber.
Field postsynaptic potential recording (fEPSP) in the rat hippocampal slices
Standard electrophysiological techniques were used to record field potentials. Presynaptic stimulation was applied to the medial part of Shaffer collaterals using a bipolar tungsten electrode at 0.05 Hz frequency. fEPSPs were recorded in a pyramidal layer of CA1 area using a glass microelectrode. In all experiments, the amplitude of test fEPSP was adjusted to one third of a maximum. Recording electrodes were fabricated from borosilicate glass capillaries of 1.5 mm outer diameter (Model GD-1.5, Narishige Scientific Instruments Lab.) using programmable puller (P-97; Sutter Instruments).
Data acquisition and analysis
Recordings and preprocessing of data were made by custom software written by Dr. Grebenyuk in Labview 8.0. Data analysis was performed with p-CLAMP (Molecular Devices, CA, USA) and Origin 7. Values are the mean ± SE for n slices. Two-tailed Student’s t test for equal sample size and Welch's t test for unequal sample size were used for statistical comparison.
Drugs
The following drugs were purchased from Sigma (St Louis, MO, USA): adenosine 5′-triphosphate sodium salt (ATP), WIN55,212-2 mesylate, ®-(+)-[2,3-dihydro-5-methyl-3[(4-morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-benzoxazinyl]-(1-naphthalenyl)methanone mesylate salt, DPCPX (8-cyclopentyl-1,3-dipropylxanthine), and adenosine. AM251 (N-(Piperidin-1-yl)-5-(4-iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide) NF 279 (8,8′-[Carbonylbis(imino-4,1-phenylenecarbonylimino-4,1- phenylenecarbonylimino)]bis-1,3,5-naphthalenetrisulfonic acid hexasodium salt), A74003 (N-[1-[[(Cyanoamino)(5-quinolinylamino)methylene]amino]- 2,2-dimethylpropyl]-3,4-dimethoxybenzeneacetamide). Reactive Blue 2 and PPADS (pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid tetrasodium salt) were all purchased from Tocris Cookson (Bristol, UK). WIN55,212-2 and AM251 were dissolved in DMSO, and the other drugs were dissolved in deionized water. The drugs were stored as concentrated stock solutions at −20°C and dissolved in the extracellular solution right before recordings.
Results
Effect of cannabinoids on ATP-mediated plasticity in hippocampus
Bath application of ATP (10 μM, 10 min) caused strong inhibition of both population spike and fEPSP amplitude (up to 40% of control amplitude). After washout of ATP, prolonged enhancement of fEPSP (up to 140% for at least 40 min, see Fig.1a) was observed. These results are in concert with earlier observations by other groups [8, 10, 20, 35]. Interestingly, short application of ATP (10 μM, 5 min, Supplementary Fig. S1a, n = 5) as well as prolonged application (10 μM, 30 min, Supplementary Fig. S1b, n = 4) was not followed by potentiation.
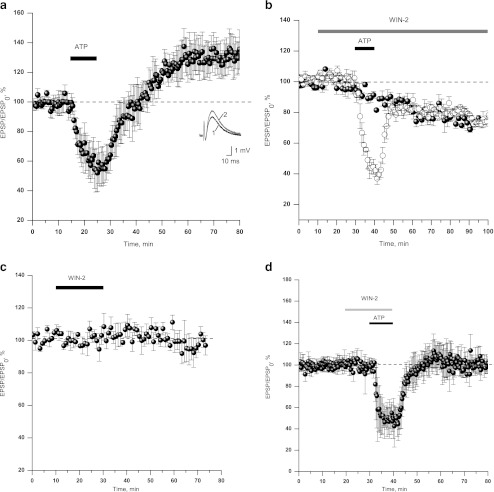
Fig. 1.
Cannabinoids prevent the induction of ATP-induced LTP in CA1 region of hippocampus. a ATP-induced long-term potentiation (130.1 ± 3.8%, n = 10). Time course plot represents averaged amplitudes of fEPSPs recorded in CA1 neurons in response to test stimuli delivered to Schaffer collaterals/commissural afferents at 0.05 Hz. Horizontal bar represents application protocol: ATP (10 μM, here and below). Inset: Examples of field fEPSP in control (1) and after LTP induction (2). b fEPSP amplitude declines in the continuous presence of WIN55,212-2 (WIN-2, below), 100nM (solid spheres); WIN-2 disables induction of ATP-induced LTP (n = 4, open circles). c Short application of WIN-2 (100 nM) did not affect fEPSP amplitude. d ATP does not induce LTP after short application of 100 nM of WIN-2
To test the hypothesis that cannabinoids affect ATP-mediated LTP processes in hippocampus, we applied 10 μM of ATP in addition to cannabinoid receptor agonist, WIN55,212-2 (WIN-2, below), 100 nM. In line with other study, Fig. 1b demonstrates a rundown of fEPSP amplitude in continuous presence of WIN-2 (100 nM, solid circles, n = 4). In another set of experiments, application of ATP (10 μM, 10 min, Fig. 1b, open circles, n = 4) during continuous WIN-2 application reproduced short-term drop in fEPSP amplitude with no sign of subsequent potentiation. We have succeeded in separation of inhibitory action of WIN-2 on synaptic transmission from its specific effect on the synaptic plasticity. Application of 100 nM WIN-2 for 20 min did not result in the rundown of synaptic transmission (Fig.1c, n = 5).
Application of 10 μM ATP in addition to WIN-2 produced inhibition of fEPSP amplitude. However, in contrast to ATP-only treated slices, the inhibition was not followed by fEPSP potentiation after drug washout. The final amplitude of fEPSP after the washout of both drugs comprised 97.4 ± 4.5% of control fEPSP amplitude (Fig.1d, n = 4). When applied after establishment of LTP, WIN-2 (100 nM, 20 min) did not alter the amplitude of fEPSP (Fig. S2, n = 2).
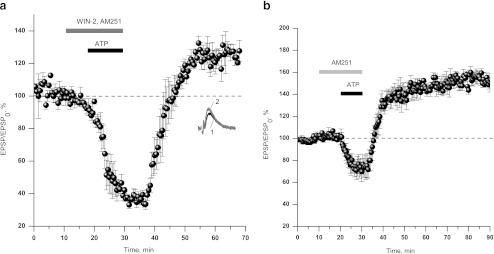
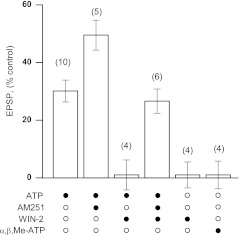
We verified whether the action of WIN-2 is mediated by CB1 receptors. Selective antagonist of CB1 receptors, AM251 (500 nM), enabled ATP-induced LTP in the presence of WIN-2. Application of the cocktail comprising 10 μM ATP, 500 nM AM251, and 2 μM WIN-2 resulted in transient depression and slow augmentation of fEPSP above control level upon cocktail washout (126.6 ± 4.2% of control fEPSP amplitude, n = 6, Fig.2a). AM251 itself did not affect synaptic transmission (data not shown). Application of ATP in addition to AM251 (Fig. 2b) resulted in significantly stronger LTP as compared to a result of sole ATP application (149.5 ± 5.2%, n = 5 vs. 130.1 ± 3.8%, n = 10, p < 0.01). This observation may indicate that CB1 receptors mediate tonic inhibition of ATP-mediated plasticity. The summary of data on the induction of LTP in various conditions is presented in Fig. 3.
Fig. 2.
Cannabinoid receptors are responsible for the suppression of ATP-induced LTP. a Selective CB1 receptor antagonist, AM251 (500 nM), allows the induction of ATP-induced LTP in the presence of high concentration of WIN-2 (2 μM, 126.6 ± 4.2%, n = 6). b AM251 (500 nM) does not alter synaptic transmission, but yields a stronger ATP-induced LTP as compared to control LTP (149.5 ± 5.2%, n = 5)
Fig. 3.
The summary of data on the induction of LTP in different combinations of ATP and cannabinoid receptor agonist/antagonist. Columns represent mean relative values of fEPSP amplitude after drug washout. The mean amplitude of fEPSP in the 10-min period before the application of any drug was defined as 100%. Solid circles denote the presence of corresponding drug in the experiment, whereas their absence in the application mixture is indicated by open circles
Effects of ATP analogs
ATP is known to degrade very rapidly to adenosine and other products of catabolism [29]. In order to check the possibility that observed phenomena result from the activity of ATP hydrolysis products, we used non-hydrolysable ATP analog, α,β,methylene-ATP, attempting to reproduce the effects of ATP.
α,β,methylene-ATP failed to reproduce either inhibition of fEPSP during the drug superfusion or potentiation of fEPSP upon drug removal (Fig. 3). Complete lack of α,β,methylene-ATP effect indicates that ATP hydrolysis is critical for induction of ATP-induced LTP. Thus, we confirm previous data, suggesting critical dependence of ATP-induced LTP on ATP dephosphorylation by ecto-protein kinases and concomitant phosphorylation of membrane proteins [9].
Rapid breakdown of ATP resulting from ecto-protein kinase and ecto-ATPase activity leads to local accumulation of adenosine in extracellular space. This can be a primary cause of fEPSP depression accompanying application of ATP.
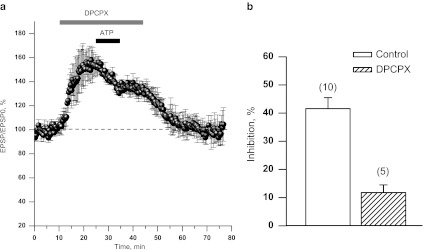
In order to examine the involvement of adenosine receptors in the observed effects, we used selective A1 receptor antagonist, DPCPX (200 nM). ATP was applied in addition to the A1 antagonist after 10 min of preincubation with the latter. DPCPX alone induced potentiation of fEPSP amplitude (up to 150–170%, Fig.4a). When applied in addition to DPCPX, ATP produced much smaller inhibition of fEPSC than in experiments given in Fig.1a (11.8 ± 2.7% vs. 41.6 ± 3.9%, Fig.4b) and did not result in LTP after washout.
Fig. 4.
DPCPX, selective A1 receptor antagonist prevents induction of ATP-induced LTP. a fEPSPs recorded from the slices perfused with 10 μM ATP in addition to 200 nM DPCPX. fEPSP is back to control after removal of both drugs. bOpen column: Magnitude of fEPSP inhibition by ATP in control (obtained in the experiments demonstrated in Fig. 1a). Solid column: a drop in fEPSP amplitude measured as the steady-state after ATP application a
Purinoreceptors
The data concerning the involvement of purinoreceptors in ATP-induced modulation of synaptic transmission are rather controversial [12, 16, 47]. We pursued this issue performing experiments with selective antagonists to various subtypes of P2X and P2Y receptors: PPADS (10 μM, nonselective P2X antagonist), NF279 (2 μM, P2X1 selective antagonist), A74003 (1 μM, P2X7-selective antagonist), and Reactive Blue 2 (10 μM, nonselective P2Y antagonist). Each of these antagonists was applied 10 min before ATP application; all the drugs were removed simultaneously with ATP. In all cases, ATP-induced depression of fEPSP was not markedly altered.
However, all these antagonists abolished or strongly inhibited LTP induction (Fig.5). Tentatively, we conclude that the inhibition of fEPSP during ATP application cannot be attributed to the activation of P2 receptors; however, these receptors may play a certain role in the initiation of ATP-induced LTP (see “Discussion” section).
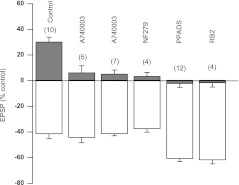
Fig. 5.
P2 receptor antagonists prevent ATP-induced LTP. The figure summarizes effects of various P2 antagonists on fEPSP amplitude. Each drug was applied 10 min before ATP and washed out simultaneously with ATP. Open columns represent maximal inhibition of fEPSP amplitude by ATP. Solid columns represent fEPSP amplitudes at the steady-state after drug washout. Numbers in brackets indicate the number of experiments
Discussion
Cannabinoid receptors have been shown to impair induction of tetanus-induced LTP in hippocampus [45, 48] and LTD in cerebellum [49]. We have tested a possibility of functional involvement of cannabinoid receptors into a novel type of synaptic plasticity in hippocampus, ATP-induced LTP. Our data demonstrate the ability of cannabinoid receptors to control the induction of this form of LTP in CA1 area of rat hippocampus: WIN-2 used in concentration of 100 nM completely abolished ATP-induced LTP.
There is diversity of reports in regard to the effects exhibited by cannabinoids on the synaptic transmission. Along with the cases where WIN-2 acted to inhibit synaptic transmission [50], cannabinoid-induced astrocyte-mediated potentiation of synaptic transmission has been shown [51]. The effect of cannabinoids was also principally dependent on the age of animals: in neonatal rats (10–13 days) WIN-2 inhibited synaptic transmission, but did not alter single fEPSPs in young adult rats (4–6 weeks) [52]. Even for a given neuron, cannabinoids inhibited vesicular release to a lesser extent at dendritic synapses as compared to perisomatic ones [53]. We have found that, when applied at 100-nM concentration (minimal concentration at which reproducible effects could be observed) for 20 min, WIN-2 did not alter fEPSP amplitude, but completely prevented ATP-induced LTP. When applied after induction of LTP by ATP, WIN did not affect fEPSP indicating that cannabinoid receptors affect induction rather than maintenance of ATP-induced LTP.
Recent data show the presence of postsynaptic CB2 cannabinoid receptors in hippocampus [54]. Although WIN-2 activates both CB1 and CB2 receptors, the effect of WIN was completely abolished by AM251, selective antagonist of CB1 receptors, which is indicative of CB1 receptors playing a regulatory role in ATP-induced LTP. Presynaptic localization of CB1 receptors prompts that the blockade of LTP by cannabinoid agonist results from a CB1 receptor-mediated decrease in the probability of glutamate release [55, 56]. However, used in our experiments in minimal concentration, WIN-2 did not result in apparent changes in the synaptic transmission while leading to the complete inhibition of ATP-induced LTP. These observations indicate the existence of a specific mechanism by which cannabinoids affect ATP-induced synaptic plasticity. The nature of such mechanism remains to be addressed.
The phenomenon of ATP-induced LTP has been studied by several groups [9–11], but exact mechanisms of the phenomenon remain obscure. According to Dunwiddie et al., ATP is rapidly converted to adenosine by membrane ecto-ATPases and ecto-protein kinases. The half-life of ATP in extracellular space comprises approximately 200 ms [29]. This fact suggests that some manifestations of ATP as a signaling agent can be related to adenosine. Indeed, our experiments indicate that A1 adenosine receptors are necessary for the development of ATP-induced LTP (Fig.4). On the other hand, experiments with non-hydrolysable analog of ATP, α,β,methylene-ATP, demonstrate that the hydrolysis of ATP is necessary for LTP to occur. Fujii and co-authors suggested NMDA receptors as the target for the phosphorylation [9] during ATP hydrolysis. In our study, blockade of almost any type of purinoreceptors led to failure in LTP induction (Fig. 5). In view of this observation, the role of P2X and P2Y receptors in this phenomenon seems highly probable. It should be noted though that there is a strong evidence on the nonspecific actions of P2 receptor antagonists [57, 58]. Thus, it has been shown that all P2X and P2Y antagonists also inhibit ecto-nucleotidases and ecto-ATPases in concentrations similar to those used to block purinoreceptors. One can hypothesize that ecto-protein kinases supposed to form a principal link in ATP-induced LTP [8, 9, 35, 36] may be also inhibited by P2-receptor antagonists, thus preventing the development of ATP-induced LTP.
Glial cells are established modulators of neuronal activity and memory processes [59]. Being a potent source of extracellular ATP, adenosine, and neurotransmitters in CNS, glial cells may play a role in ATP-induced LTP. Extracellular ATP directly targets astroglial and neuronal P2X receptors [60] stimulating ATP and glutamate release from astrocytes and modulating the function of postsynaptic receptors [61–64]. In addition, extracellular adenosine has been shown to induce release of glutamate and NO [65, 66] from glial cells. NO, in its turn, enhances vesicular release by stimulating cGMP synthesis at the presynaptic site and upregulates trafficking of AMPA receptors at the postsynaptic site [67]. Thereby, it may contribute to the induction of ATP-induced LTP like it has been demonstrated for tetanus-induced LTP [68–72]. The present study indicates that long-term synaptic plasticity induced by ATP is cannabinoid-dependent: ATP-induced LTP is inhibited by the activation CB1 receptors.
Electronic supplementary material
ATP exerts LTP in a certain time window. Application of ATP (10 μM) for 5 min (a) and for 30 min (b) does not elicit potentiation of fEPSP (JPEG 329 kb)
WIN-2 (100 nM) does not affect fEPSP after induction of LTP by ATP (JPEG 420 kb)
Acknowledgment
This work was supported by the grant SFFR F46.2/001 from The State Fund for Fundamental Research of Ukraine
References
- 1.Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. J Neurophysiol. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- 2.Domenici MR, Azad SC, Marsicano G, Schierloh A, Wotjak CT, Dodt HU, Zieglgansberger W, Lutz B, Rammes G. Cannabinoid receptor type 1 located on presynaptic terminals of principal neurons in the forebrain controls glutamatergic synaptic transmission. J Neurosci. 2006;26:5794–5799. doi: 10.1523/JNEUROSCI.0372-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirby MT, Hampson RE, Deadwyler SA. Cannabinoids selectively decrease paired-pulse facilitation of perforant path synaptic potentials in the dentate gyrus in vitro. Brain Res. 1995;688:114–120. doi: 10.1016/0006-8993(95)00521-Q. [DOI] [PubMed] [Google Scholar]
- 4.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 7.Wolff MC, Leander JD. SR141716A, a cannabinoid CB1 receptor antagonist, improves memory in a delayed radial maze task. Eur J Pharmacol. 2003;477:213–217. doi: 10.1016/j.ejphar.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Fujii S. ATP- and adenosine-mediated signaling in the central nervous system: the role of extracellular ATP in hippocampal long-term potentiation. J Pharmacol Sci. 2004;94:103–106. doi: 10.1254/jphs.94.103. [DOI] [PubMed] [Google Scholar]
- 9.Fujii S, Kato H, Furuse H, Ito K, Osada H, Hamaguchi T, Kuroda Y. The mechanism of ATP-induced long-term potentiation involves extracellular phosphorylation of membrane proteins in guinea-pig hippocampal CA1 neurons. Neurosci Lett. 1995;187:130–132. doi: 10.1016/0304-3940(95)11347-9. [DOI] [PubMed] [Google Scholar]
- 10.Wieraszko A, Seyfried TN. ATP-induced synaptic potentiation in hippocampal slices. Brain Res. 1989;491:356–359. doi: 10.1016/0006-8993(89)90070-X. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki Y, Kaneko K, Fujii S, Kato H, Ito KI. Long-term potentiation and long-term depression induced by local application of ATP to hippocampal CA1 neurons of the guinea pig. Hippocampus. 2003;13:81–92. doi: 10.1002/hipo.7999. [DOI] [PubMed] [Google Scholar]
- 12.Pankratov Y, Lalo U, Krishtal OA, Verkhratsky A. P2X receptors and synaptic plasticity. Neuroscience. 2009;158(1):137–148. doi: 10.1016/j.neuroscience.2008.03.076. [DOI] [PubMed] [Google Scholar]
- 13.Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurons of the rat hippocampus. Eur J Neurosci. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 14.Pankratov Y, Lalo U, Krishtal O, Verkhratsky A. Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J Physiol. 2002;542:529–536. doi: 10.1113/jphysiol.2002.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pankratov Y, Lalo U, Krishtal O, Verkhratsky A. P2X receptor-mediated excitatory synaptic currents in somatosensory cortex. Mol Cell Neurosci. 2003;24:842–849. doi: 10.1016/S1044-7431(03)00233-1. [DOI] [PubMed] [Google Scholar]
- 16.Pankratov Y, Lalo U, Verkhratsky A, North RA. Vesicular release of ATP at central synapses. Pflugers Arch. 2006;452:589–597. doi: 10.1007/s00424-006-0061-x. [DOI] [PubMed] [Google Scholar]
- 17.Cunha RA, Vizi ES, Ribeiro JA, Sebastiao AM. Preferential release of ATP and its extracellular catabolism as a source of adenosine upon high- but not low-frequency stimulation of rat hippocampal slices. J Neurochem. 1996;67:2180–2187. doi: 10.1046/j.1471-4159.1996.67052180.x. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 19.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 20.Cunha RA, Ribeiro JA. ATP as a presynaptic modulator. Life Sciences. 2000;68:119–137. doi: 10.1016/S0024-3205(00)00923-1. [DOI] [PubMed] [Google Scholar]
- 21.Coppi E, Pugliese AM, Stephan H, Muller CE, Pedata F. Role of P2 purinergic receptors in synaptic transmission under normoxic and ischaemic conditions in the CA1 region of rat hippocampal slices. Purinergic Signal. 2007;3:203–219. doi: 10.1007/s11302-006-9049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khakh BS, Gittermann D, Cockayne DA, Jones A. ATP modulation of excitatory synapses onto interneurons. J Neurosci. 2003;23:7426–7437. doi: 10.1523/JNEUROSCI.23-19-07426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khakh BS. ATP-gated P2X receptors on excitatory nerve terminals onto interneurons initiate a form of asynchronous glutamate release. Neuropharmacology. 2009;56:216–222. doi: 10.1016/j.neuropharm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Haydon PG, Yeung ES. Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Anal Chem. 2000;72:2001–2007. doi: 10.1021/ac9912146. [DOI] [PubMed] [Google Scholar]
- 25.Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem. 2004;88:246–256. doi: 10.1111/j.1471-4159.2004.02204.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, Wu CP, Poo MM, Duan S. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–982. doi: 10.1016/S0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues RJ, Almeida T, Richardson PJ, Oliveira CR, Cunha RA. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J Neurosci. 2005;25:6286–6295. doi: 10.1523/JNEUROSCI.0628-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendoza-Fern + бndez V, Andrew RD, Barajas-L + ¦pez C. ATP inhibits glutamate synaptic release by acting at P2Y receptors in pyramidal neurons of hippocampal slices. J Pharmacol Exp Ther. 2000;293:172–179. [PubMed] [Google Scholar]
- 29.Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci. 1997;17:7673–7682. doi: 10.1523/JNEUROSCI.17-20-07673.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masino SA, Diao L, Illes P, Zahniser NR, Larson GA, Br J, Fredholm BB, Dunwiddie TV. Modulation of hippocampal glutamatergic transmission by ATP is dependent on adenosine A1 receptors. J Pharmacol Exp Ther. 2002;303:356–363. doi: 10.1124/jpet.102.036731. [DOI] [PubMed] [Google Scholar]
- 31.Cunha RA. Adenosine as a neuromodulator and as a homeostatic regulator in the nervous system: different roles, different sources and different receptors. Neurochem Int. 2001;38:107–125. doi: 10.1016/S0197-0186(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 32.Greene RW, Haas HL. The electrophysiology of adenosine in the mammalian central nervous system. Prog Neurobiol. 1991;36:329–341. doi: 10.1016/0301-0082(91)90005-L. [DOI] [PubMed] [Google Scholar]
- 33.Salter MW, De KY, Henry JL. ATP-sensitive K + channels mediate an IPSP in dorsal horn neurones elicited by sensory stimulation. Synapse. 1992;11:214–220. doi: 10.1002/syn.890110306. [DOI] [PubMed] [Google Scholar]
- 34.Trussell LO, Jackson MB. Adenosine-activated potassium conductance in cultured striatal neurons. Proc Natl Acad Sci U S A. 1985;82:4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujii S, Kato H, Kuroda Y. Extracellular adenosine 5'-triphosphate plus activation of glutamatergic receptors induces long-term potentiation in CA1 neurons of guinea pig hippocampal slices. Neurosci Lett. 1999;276:21–24. doi: 10.1016/S0304-3940(99)00776-4. [DOI] [PubMed] [Google Scholar]
- 36.Fujii S, Kato H, Kuroda Y. Cooperativity between extracellular adenosine 5'-triphosphate and activation of N-methyl-D-aspartate receptors in long-term potentiation induction in hippocampal CA1 neurons. Neuroscience. 2002;113:617–628. doi: 10.1016/S0306-4522(02)00190-2. [DOI] [PubMed] [Google Scholar]
- 37.O'Kane EM, Stone TW. Characterisation of ATP-induced facilitation of transmission in rat hippocampus. Eur J Pharmacol. 2000;409:159–166. doi: 10.1016/S0014-2999(00)00785-8. [DOI] [PubMed] [Google Scholar]
- 38.Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva BM, Mendonça A, Ribeiro JA. Long-term depression is not modulated by ATP receptors in the rat CA1 hippocampal region. Neurosci Lett. 2005;383:345–349. doi: 10.1016/j.neulet.2005.04.051. [DOI] [PubMed] [Google Scholar]
- 40.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/S0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 41.Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68:247–286. doi: 10.1016/S0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- 42.Lafourcade CA. Presynaptic mechanisms of endocannabinoid-mediated long-term depression in the hippocampus. J Neurophysiol. 2009;102:2009–2012. doi: 10.1152/jn.00441.2009. [DOI] [PubMed] [Google Scholar]
- 43.Collins DR, Pertwee RG, Davies SN. Prevention by the cannabinoid antagonist, SR141716A, of cannabinoid-mediated blockade of long-term potentiation in the rat hippocampal slice. Br J Pharmacol. 1995;115:869–870. doi: 10.1111/j.1476-5381.1995.tb15889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davies SN, Pertwee RG, Riedel G. Functions of cannabinoid receptors in the hippocampus. Neuropharmacology. 2002;42:993–1007. doi: 10.1016/S0028-3908(02)00060-6. [DOI] [PubMed] [Google Scholar]
- 45.Terranova JP, Michaud JC, Le FG, Soubrie P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212-2: reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- 46.Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/S0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- 47.Pankratov YV, Lalo UV, Krishtal OA. Role for P2X receptors in long-term potentiation. J Neurosci. 2002;22:8363–8369. doi: 10.1523/JNEUROSCI.22-19-08363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nowicky AV, Teyler TJ, Vardaris RM. The modulation of long-term potentiation by delta-9-tetrahydrocannabinol in the rat hippocampus, in vitro. Brain Res Bull. 1987;19:663–672. doi: 10.1016/0361-9230(87)90052-9. [DOI] [PubMed] [Google Scholar]
- 49.Levenes C, Daniel H, Soubrie P, Crepel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. J Physiol. 1998;510(Pt 3):867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serpa A, Ribeiro JA, Sebastiao AM. Cannabinoid CB(1) and adenosine A(1) receptors independently inhibit hippocampal synaptic transmission. Eur J Pharmacol. 2009;623:41–46. doi: 10.1016/j.ejphar.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 51.Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–126. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 52.Al-Hayani A, Davies SN. Cannabinoid receptor mediated inhibition of excitatory synaptic transmission in the rat hippocampal slice is developmentally regulated. Br J Pharmacol. 2000;131:663–665. doi: 10.1038/sj.bjp.0703642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee SH, Foldy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010;30:7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brusco A, Tagliaferro P, Saez T, Onaivi ES. Postsynaptic localization of CB2 cannabinoid receptors in the rat hippocampus. Synapse. 2008;62:944–949. doi: 10.1002/syn.20569. [DOI] [PubMed] [Google Scholar]
- 55.Diana MA, Marty A. Characterization of depolarization-induced suppression of inhibition using paired interneuron–Purkinje cell recordings. J Neurosci. 2003;23:5906–5918. doi: 10.1523/JNEUROSCI.23-13-05906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 57.Chen BC, Lee CM, Lin WW. Inhibition of ecto-ATPase by PPADS, suramin and reactive blue in endothelial cells, C6 glioma cells and RAW 264.7 macrophages. Br J Pharmacol. 1996;119:1628–1634. doi: 10.1111/j.1476-5381.1996.tb16082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziganshin AU, Ziganshina LE, Bodin P, Bailey D, Burnstock G. Effects of P2-purinoceptor antagonists on ecto-nucleotidase activity of guinea-pig vas deferens cultured smooth muscle cells. Biochem Mol Biol Int. 1995;36:863–869. [PubMed] [Google Scholar]
- 59.Gibbs ME, Hutchinson D, Hertz L. Astrocytic involvement in learning and memory consolidation. Neurosci Biobehav Rev. 2008;32:927–944. doi: 10.1016/j.neubiorev.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Palygin O, Lalo U, Verkhratsky A, Pankratov Y. Ionotropic NMDA and P2X1/5 receptors mediate synaptically induced Ca2+ signalling in cortical astrocytes. Cell Calcium. 2010;48:225–231. doi: 10.1016/j.ceca.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Lalo U, Andrew J, Palygin O, Pankratov Y. Ca2+-dependent modulation of GABAA and NMDA receptors by extracellular ATP: implication for function of tripartite synapse. Biochem Soc Trans. 2009;37:1407–1411. doi: 10.1042/BST0371407. [DOI] [PubMed] [Google Scholar]
- 62.Grebenyuk SE, Lozovaya NA, Tsintsadze TS, Krishtal OA. Post-synaptic N-methyl-d-aspartate signalling in hippocampal neurons of rat: spillover increases the impact of each spike in a short burst discharge. Neurosci Lett. 2004;361:60–63. doi: 10.1016/j.neulet.2004.02.040. [DOI] [PubMed] [Google Scholar]
- 63.Kochlamazashvili G, Senkov O, Grebenyuk S, Robinson C, Xiao MF, Stummeyer K, Gerardy-Schahn R, Engel AK, Feig L, Semyanov A, Suppiramaniam V, Schachner M, Dityatev A. Neural cell adhesion molecule-associated polysialic acid regulates synaptic plasticity and learning by restraining the signaling through GluN2B-containing NMDA receptors. J Neurosci. 2010;30:4171–4183. doi: 10.1523/JNEUROSCI.5806-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lozovaya NA, Grebenyuk SE, Tsintsadze TS, Feng B, Monaghan DT, Krishtal OA. Extrasynaptic NR2B and NR2D subunits of NMDA receptors shape 'superslow' afterburst EPSC in rat hippocampus. J Physiol. 2004;558:451–463. doi: 10.1113/jphysiol.2004.063792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nishizaki T, Nagai K, Nomura T, Tada H, Kanno T, Tozaki H, Li XX, Kondoh T, Kodama N, Takahashi E, Sakai N, Tanaka K, Saito N. A new neuromodulatory pathway with a glial contribution mediated via A2a adenosine receptors. Glia. 2002;39:133–147. doi: 10.1002/glia.10100. [DOI] [PubMed] [Google Scholar]
- 66.Janigro D, Wender R, Ransom G, Tinklepaugh DL, Winn HR. Adenosine-induced release of nitric oxide from cortical astrocytes. Neuroreport. 1996;7(10):1640–1644. doi: 10.1097/00001756-199607080-00023. [DOI] [PubMed] [Google Scholar]
- 67.Serulle Y, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff E. A role for cGMP-dependent protein kinase II in AMPA receptor trafficking and synaptic plasticity. BMC Pharmacology. 2009;9:S44. doi: 10.1186/1471-2210-9-S1-S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bon C, Bohme GA, Doble A, Stutzmann JM, Blanchard JC. A role for nitric oxide in long-term potentiation. Eur J Neurosci. 1992;4:420–424. doi: 10.1111/j.1460-9568.1992.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 69.Grassi S, Pettorossi VE. Role of nitric oxide in long-term potentiation of the rat medial vestibular nuclei. Neuroscience. 2000;101:157–164. doi: 10.1016/S0306-4522(00)00334-1. [DOI] [PubMed] [Google Scholar]
- 70.Hawkins RD, Zhuo M, Arancio O. Nitric oxide and carbon monoxide as possible retrograde messengers in hippocampal long-term potentiation. J Neurobiol. 1994;25:652–665. doi: 10.1002/neu.480250607. [DOI] [PubMed] [Google Scholar]
- 71.Ko GY, Kelly PT. Nitric oxide acts as a postsynaptic signaling molecule in calcium/calmodulin-induced synaptic potentiation in hippocampal CA1 pyramidal neurons. J Neurosci. 1999;19:6784–6794. doi: 10.1523/JNEUROSCI.19-16-06784.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ATP exerts LTP in a certain time window. Application of ATP (10 μM) for 5 min (a) and for 30 min (b) does not elicit potentiation of fEPSP (JPEG 329 kb)
WIN-2 (100 nM) does not affect fEPSP after induction of LTP by ATP (JPEG 420 kb)