Abstract
Adenosine and its metabolite, inosine, have been described as molecules that participate in regulation of inflammatory response. The aim of this study was to investigate the effect of adenosine and inosine in a mouse model of carrageenan-induced pleurisy as well as the participation of adenosine receptors in this response. Injection of carrageenan into the pleural cavity induced an acute inflammatory response characterized by leukocyte migration, pleural exudation, and increased release of interleukin-1β and tumor necrosis factor-α in pleural exudates. The treatment with adenosine (0.3–100 mg/kg, i.p.) and inosine (0.1–300 mg/kg, i.p.) 30 min before carrageenan injection reduced significantly all these parameters analyzed. Our results also demonstrated that A2A and A2B receptors seem to mediate the adenosine and inosine effects observed, since pretreatment with selective antagonists of adenosine A2A (ZM241385) and A2B (alloxazine) receptors, reverted the inhibitory effects of adenosine and inosine in pleural inflammation. The involvement of A2 receptors was reinforced with adenosine receptor agonist CGS21680 treatment, since its anti-inflammatory effects were reversed completely and partially with ZM241385 and alloxazine injection, respectively. Moreover, the combined treatment with subeffective dose of adenosine (0.3 mg/kg) and inosine (1.0 mg/kg) induced a synergistic anti-inflammatory effect. Thus, based on these findings, we propose that inosine contributes with adenosine to exert anti-inflammatory effects in pleural inflammation, reinforcing the notion that endogenous nucleosides play an important role in controlling inflammatory diseases. This effect is likely mediated by the activation of adenosine A2 subtype receptors and inhibition of production or release of pro-inflammatory cytokines.
Keywords: Adenosine, Inosine, Carrageenan, Pleurisy, Adenosine receptors
Introduction
Purine nucleosides, such as adenosine and its primary metabolite inosine, are low molecular weight molecules that participate in a wide variety of intracellular biochemical processes [1]. Adenosine is one of the breakdown products from adenosine triphosphate (ATP) by the action of enzymes such as apyrase and nucleotidase [2, 3]. At present, it is known that following adenosine release from cells or after its extracellular formation, adenosine diffuses to the surroundings where it binds to adenosine receptors [4, 5]; through which several studies showed that adenosine elicits its anti-inflammatory action on cells [6]. These receptors are classified in four types named A1, A2A, A2B, and A3—all of which are members of G protein-coupled family of receptors [5, 7]. Indeed, of all known nucleosides, adenosine is the best characterized immunomodulator, showing anti-inflammatory effects [8] that are related to the inhibition of neutrophil migration and oxygen metabolite production [9–11]. Protective effects of adenosine and adenosine receptor agonists have also been shown in different models of inflammatory disease including asthma [12], inflammatory bowel disease [13], endotoxin-mediated shock [14], rheumatoid arthritis [15], sepsis [16], and wound healing [17].
Events such as inflammation, hypoxia, and tissue injury account for adenosine degradation and generation of its metabolite, inosine [18], which was generally believed to be an inactive breakdown product [1]. Only recently, it has been recognized that inosine can also bind to adenosine receptors and initiate intracellular signaling events [1]. In agreement, studies had shown that like adenosine, inosine also presents anti-inflammatory effects related with the activation of adenosine receptors, mainly the A2A and A3, that include reduction of pro-inflammatory cytokine production, protective tissue effects from endotoxin-induced [19, 20], and 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced inflammation [21].
There is a growing interest in elucidating the mechanisms by which adenosine and inosine modulate the immune system, since the inhibitory adenosine receptors and their downstream signaling pathways are promising targets for new anti-inflammatory therapies [22]. In addition, there is a need to better understand the adenosine metabolism during an acute inflammatory process and whether its metabolite inosine acts in synergism with adenosine or alone to modulate inflammation. Accordingly, this study was undertaken to evaluate: (1) the anti-inflammatory effects of adenosine and inosine in an acute model of inflammation; (2) the adenosine A2 receptor subtype involved in mediating the adenosine- and inosine-induced effects; and (3) the possible synergic effects between adenosine and inosine.
Material and methods
Animals
Female Swiss mice (18–35 g) were housed at 22 ± 2 °C under a 12-h light/12-h dark cycle (lights on at 06:00 p.m.) and with access to food and water ad libitum. Animals were acclimatized to the laboratory for at least 1 day before the experiments. The experiments were performed after the approval of ethics committee from the Institute of Biological Sciences of the Universidade Federal do Paraná, with approval protocol number 320/2008 and were carried out in accordance with current ethical guidelines for the care and use of laboratory animals.
Experimental procedures
Induction of pleurisy
Pleurisy was induced in anesthetized animals by an intrapleural injection of carrageenan (1 %) or a sterile saline solution (0.9 % NaCl) into the right pleural space through the chest skin (final volume 0.1 ml) [23, 24]. On the day of the experiments, animals were challenged with a solution of Evans blue dye (25 mg/kg, 0.2 ml, i.v.) in order to evaluate further the degree of exudation in the pleural space [23, 24]. The animals were killed 4 h after injection of phlogistic agent and the pleural cavity was washed with 1 ml of sterile saline solution plus heparin (20 IU/ml) and the volume was collected with automatic pipettes. Total leukocyte counts were performed in Neubauer chambers by means of an optical microscope after diluting the pleural fluid with Türk solution (1:20). Cellular smears were prepared with another aliquot of pleural washing and stained with May–Grünwald–Giemsa for differential analysis, which was performed under immersion objective. A sample of the collected fluid (300 μl) from the pleural space was separated and stored in a freezer (−20 °C) to determine the concentration of Evans blue dye. The amount of dye was evaluated by obtaining the absorbance values at 600 nm with a spectrophotometer, by interpolation from the standard curve of Evans blue dye in the range of 1–500 μg/ml.
Drug treatment
The mice were treated intraperitoneally with adenosine (0.3–100 mg/kg) or inosine (1.0–300 mg/kg) 30 min prior to carrageenan injection. To study the involvement of A2 receptors, the selective adenosine A2A receptor antagonist, ZM241385 (1.5 mg/kg, i.p.), and selective adenosine A2B receptor antagonist, alloxazine (5 mg/kg, i.p.), were administrated alone or 30 min before adenosine, inosine, or the selective A2A receptor agonist, CGS21680 (0.1 mg/kg, i.p.), treatment. The choice of the doses of each drug was based on preliminary experiments carried out in our laboratory.
To study whether inosine and adenosine have a synergic effect, we injected intraperitoneally adenosine (0.3 mg/kg) and in the contralateral side, inosine (1.0 mg/kg, i.p). Our results demonstrated that these doses did not exert any anti-inflammatory effects, and hence, they were chosen to study the synergic effect.
Adenosine, inosine, and all the adenosine receptor agonist or antagonists were diluted in Tween 80 plus sterile NaCl (0.9 %) solution. The final concentration of Tween 80 did not exceed 5 % and did not cause any per se effect.
Determination of IL-1β and TNF-α levels in mice pleural lavage
Pleural exudates were collected with 1 ml of sterile NaCl (0.9 %) solution containing heparin (20 IU/ml). The pleural fluid samples were centrifuged at 1,300×g and 4 °C for 10 min [25]. The supernatant was rapidly frozen and stored at −70 °C for later measurement of interleukin (IL)-1β and tumor necrosis factor (TNF)-α levels using specific enzyme-linked immunosorbent assay kits according to manufacturer's instructions.
Statistical analysis
The results are presented as mean ± standard error of the mean (S.E.M.), except the ID50 values (i.e., the dose of adenosine or inosine that reduce the inflammatory response by 50 % relative to the control value), which are reported as geometric means accompanied by their respective 95 % confidence limits. The ID50 value was determined by linear regression from individual experiments using linear regression GraphPad Software (GraphPad Software, San Diego, CA, USA). The statistical significance of differences between groups was detected by ANOVA followed by Newman–Keuls test. P values less than 0.05 (P < 0.05) were considered as indicative of significance.
Results
Effects of adenosine and inosine treatment in acute pleural inflammation
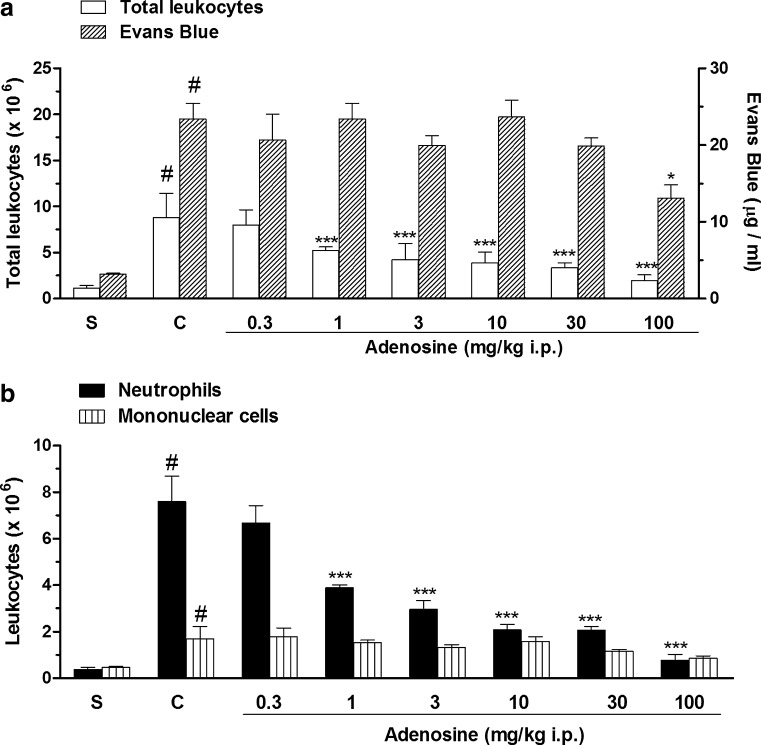
Our results showed that the intrapleural injection of carrageenan produced an acute inflammation characterized by plasma leakage and migration of a large amount of leukocytes represented mainly by neutrophils (Fig. 1a, b). The treatment with adenosine (0.3–100 mg/kg, i.p.) given 30 min prior to carrageenan produced a significant decrease in total cell count and neutrophils with a mean ID50 value of 12.24 (6.75–22.80) mg/kg and 3.90 (2.66–5.70) mg/kg and inhibitions of 75 ± 3 and 89 ± 4 %, respectively (Fig. 1a, b). However, at the same doses, adenosine reduced less extensively the exudation and the influx of mononuclear cells with inhibitions of 55 ± 5 % and 54 ± 6 %, respectively (Fig. 1a, b).
Fig. 1.
Effects of adenosine on total leukocyte count and pleural leakage (a) and on neutrophil and mononuclear cell count (b), in pleural exudates. S represents the group injected with saline 0.9 %; C indicates the group treated with carrageenan and the vehicle used to dilute adenosine; the doses of 0.3–100 mg/kg correspond to the group treated with adenosine and injected with carrageenan. The difference between groups was determined by ANOVA followed by Newman–Keuls multiple comparison test. *P < 0.05, ***P < 0.001 when compared with carrageenan-treated group and #P < 0.001 when compared to the saline group (S)
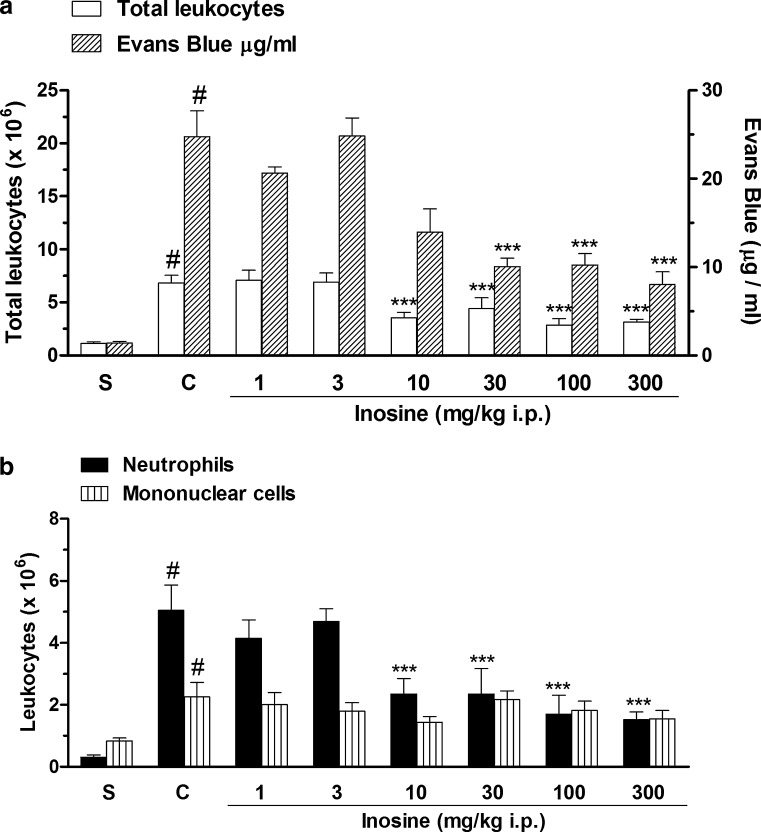
In addition, we also observed that intraperitoneal treatment with the adenosine metabolite, inosine (1.0–300 mg/kg), significantly reduced the total cell influx in acute pleural inflammation, with inhibition of 58 ± 9 % at a dose of 100 mg/kg (Fig. 2a). Inosine treatment significantly reduced the neutrophil migration and the degree of exudation into pleural cavity with mean ID50 values of 6.40 (3.32–12.64) mg/kg and 38.17 (17.45–83.50) mg/kg and with inhibitions of 70 ± 5 and 66 ± 6 %, respectively, at the dose of 300 mg/kg, i.p. (Fig. 2a, b). In contrast, inosine administration did not reduce mononuclear cell count (Fig. 2b).
Fig. 2.
Effects of inosine on total leukocyte count and pleural leakage (a) and on neutrophil and mononuclear cell count (b), in pleural exudates. S represents the group injected with saline 0.9 %; C indicates the group treated with carrageenan and the vehicle used to dilute inosine; the doses of 0.1–300 mg/kg correspond to the group treated with inosine and injected with carrageenan. The difference between groups was determined by ANOVA followed by Newman–Keuls multiple comparison test. ***P < 0.001 when compared with carrageenan-treated group and #P < 0.001 when compared to the saline group (S)
Adenosine receptors and adenosine and inosine effects on acute pleural inflammation
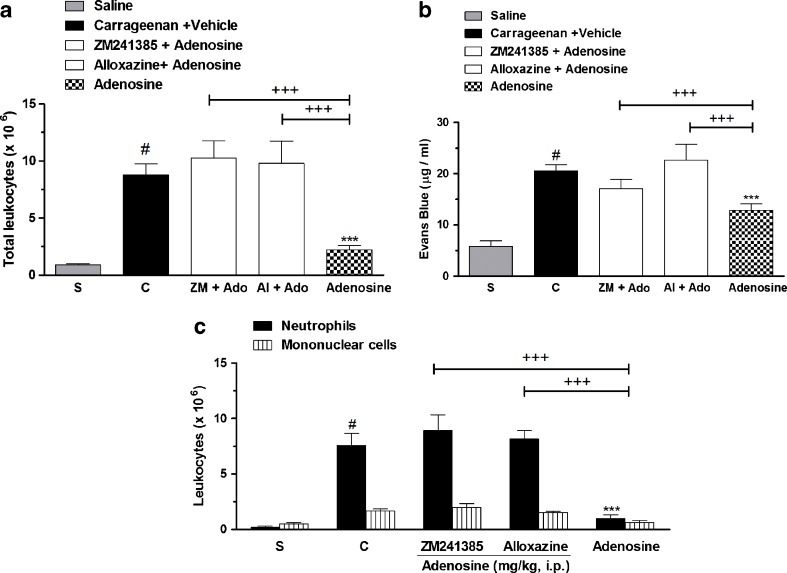
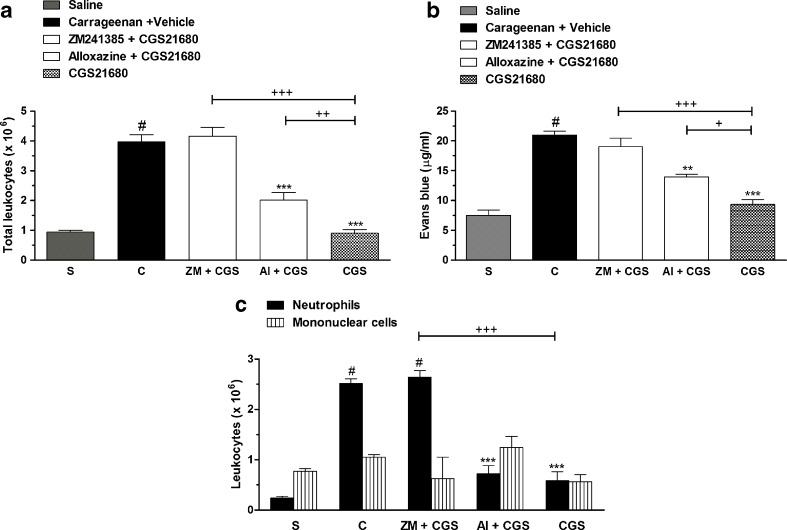
Figure 3 shows that pretreatment with ZM241385, an adenosine A2A receptor antagonist, and alloxazine, an adenosine A2B receptor antagonist, given 30 min before adenosine treatment (100 mg/kg, i.p.) reverted completely (100 %) the effects of adenosine on total, neutrophil, and mononuclear cell counts and exudation (Fig. 3a, b). Interestingly, the pretreatment with ZM241685 and alloxazine completely reverted the adenosine effects against mononuclear cell count (Fig. 3c). It is important to mention that the doses of antagonists used in our study were standardized in a separate series of experiments and did not show any per se effect (Table 1).
Fig. 3.
Adenosine receptor antagonists and adenosine effects on (a) total leucocytes, (b) pleural leakage, and (c) neutrophils and mononuclear cells, in pleural exudates. S represents the group injected with saline 0.9 %; C indicates the group treated with carrageenan and vehicle used to dilute adenosine or antagonists. The difference between groups was determined by ANOVA followed by Newman–Keuls multiple comparison test. *P < 0.05, ***P < 0.001 when compared with carrageenan-treated group; #P < 0.001 when compared to saline group (S); +P < 0.05, +++P < 0.001, when compared with adenosine-treated group
Table 1.
Effects of adenosine receptor antagonists in carrageenan-induced pleural inflammation
| Leukocyte count and pleural exudation | Totals (× 106/ml) | Neutrophils (× 106/ml) | Mononclear cells (× 106/ml) | Evans blue (μg/ml) |
|---|---|---|---|---|
| Control groups | ||||
| Saline | 0.82 ± 0.12 | 0.10 ± 0.03 | 0.70 ± 0.09 | 1.05 ± 0.25 |
| Carrageenan (1 %) | 4.97 ± 0.24* | 3.48 ± 0.21* | 1.47 ± 0.10* | 9.85 ± 0.92* |
| Vehicle (Tween 5 %) | 4.96 ± 0.26* | 3.57 ± 0.28* | 1.45 ± 0.07* | 9.83 ± 0.81* |
| Groups treated with antagonists | ||||
| ZM241385 (1.5 mg/kg, i.p.) | 4.96 ± 0.22 | 3.13 ± 0.15 | 1.68 ± 0.09 | 8.89 ± 1.12 |
| Alloxazine (5.0 mg/kg, i.p.) | 4.53 ± 0.42 | 3.13 ± 0.36 | 1.65 ± 0.30 | 8.58 ± 0.78 |
The difference between groups was determined by ANOVA followed by Newman–Keuls multiple comparison test
*P < 0.001 when compared to saline group
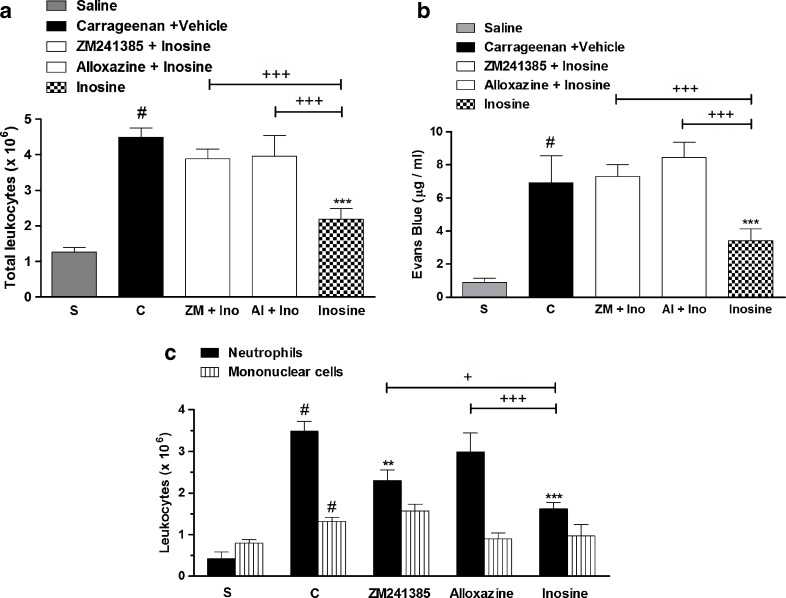
Our results showed that pretreatment with the adenosine receptor antagonists, ZM241385 and alloxazine, reverted by 70 ± 4 and 73 ± 9 %, respectively, the inosine (10 mg/kg, i.p.) effects against total cell count and by 63 ± 6 and 85 ± 13 % the inosine effects against differential neutrophil count (Fig. 4a–c). Furthermore, all the adenosine receptors antagonists used in this study reverted completely (100 %) the inosine effects against exudation (Fig. 4b). The dose of 10 mg/kg was chosen to study the involvement of adenosine receptors because our results showed that inosine did not present a dose–response curve, and besides, the effect of inosine at this dose, on the inflammatory parameters analyzed above, did not differ statistically from the higher doses used.
Fig. 4.
Adenosine receptor antagonists and inosine effects on (a) total leucocytes, (b) pleural leakage, and (c) neutrophils and mononuclear cells, in pleural exudates. S represents the group injected with saline 0.9 %; C indicates the group treated with carrageenan and vehicle used to dilute inosine or antagonists. The difference between groups was determined by ANOVA followed by Newman–Keuls multiple comparison test. **P < 0.01, ***P < 0.001 when compared with carrageenan-treated group; #P < 0.001 when compared to saline group (S); +P < 0.05, +++P < 0.001, when compared with inosine-treated group
Additional experiments were conducted with CGS21680, a selective adenosine A2 receptor agonist, with selectivity for A2A receptor. Our results showed that CGS21680 reduced significantly the total leukocyte, neutrophil differential cell count, and pleural leakage with inhibitions of 95 ± 3, 85 ± 9, and 64 ± 9 %, respectively. The pretreatment with ZM241385 reverted by 90 ± 6 % of CGS21680 effects against pleural leakage and completely abolished the agonist effects against total leukocytes and neutrophils (Fig. 5a–c). Interestingly, the pretreatment with alloxazine reverted the total leukocyte cell count and the pleural exudation by 45 ± 6 and 43 ± 6 %, respectively. The doses of ZM241385 or alloxazine used were similarly administered to evaluate the involvement of adenosine A2A receptors in adenosine or inosine effects. These results strengthen our hypothesis that the A2A and A2B receptors participate in the modulation of acute pleural inflammation.
Fig. 5.
Adenosine antagonists and CGS21680 effects on (a) total leucocytes, (b) pleural leakage, and (c) neutrophils and mononuclear cells, in pleural exudates. S represents the group injected with saline 0.9 %; C indicates the group treated with carrageenan and vehicle used to dilute CGS21680 or antagonists. The difference between groups was determined by ANOVA followed by Newman–Keuls multiple comparison test. **P < 0.01, ***P < 0.001 when compared with carrageenan-treated group; #P < 0.001 when compared to saline group (S); +P < 0.05, ++P < 0.01, +++P < 0.001, when compared with CGS21680-treated group
Involvement of adenosine receptors and adenosine and inosine effects on cytokine levels in pleural exudates
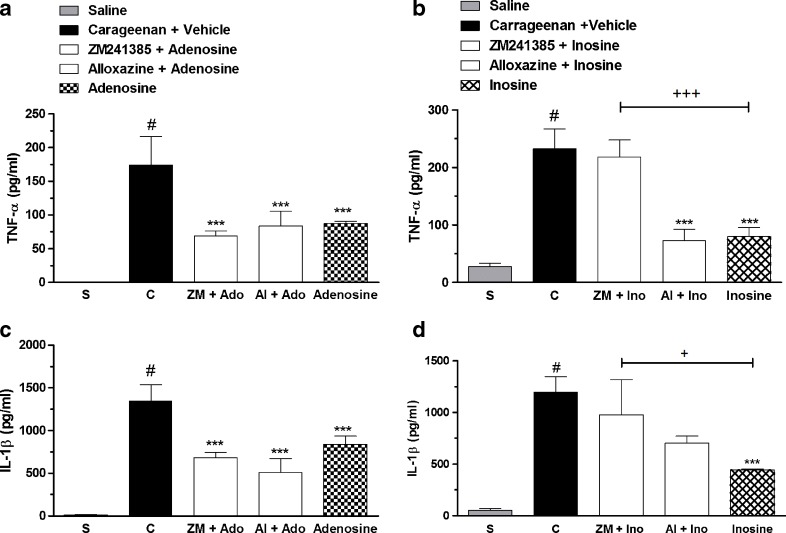
Our results showed that the TNF-α and IL-1β levels were significantly increased in pleural exudates collected from carageenan-injected mice (Fig. 6a–d). Adenosine treatment (100 mg/kg, i.p.) reduced 50 ± 2 and 38 ± 6 %, the TNF-α and IL-1β levels in exudates, respectively, and the pretreatment with adenosine antagonist receptors did not revert the adenosine effects (Fig. 6a, c). Moreover, inosine reduced 51 ± 12 and 63 ± 1 % of the TNF-α and IL-1β levels in pleural samples, respectively (Fig. 6b, d). The pretreatment with ZM241385 reverted 93 ± 7 and 63 ± 15 %, respectively, inosine effects in TNF-α and IL-1β assay, suggesting the participation of adenosine A2A receptors in inosine effects (Fig. 6b, d).
Fig. 6.
Levels of TNF-α and IL-1β on pleural exudates from mice pretreated with adenosine receptors antagonists and treated with adenosine (100 mg/kg, i.p.) or inosine (10 mg/kg, i.p.). The results were expressed as mean ± S.E.M. (n = 6). Dotted column represents saline control group; closed column represents the control group injected with carrageenan and vehicle (saline 0.9 % plus Tween 5 %); open column group pretreated with the antagonists of adenosine receptors and treated with adenosine or inosine; chess column represents the group treated only with adenosine (100 mg/kg, i.p.) or inosine (10 mg/kg, i.p.). The difference between groups was determined by ANOVA followed by Newman–Keuls multiple comparison test. *P < 0.05, ***P < 0.001 when compared with the group treated only with the phlogistic (CAR plus vehicle); #P < 0.001 when compared to saline group; and +P < 0.05, +++P < 0.001, when compared with adenosine- or inosine-treated group
Study of adenosine metabolism into inosine in carrageenan-induced pleurisy
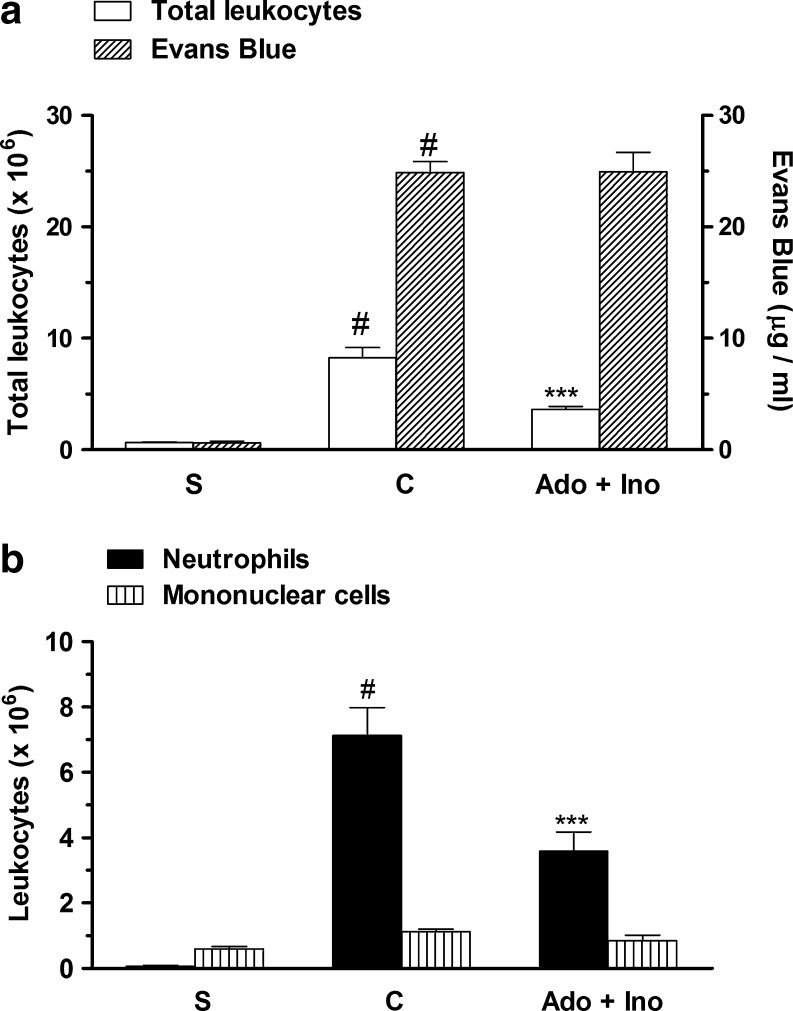
In order to verify if inosine contributes with adenosine effects on acute pleural inflammation, mice were pretreated with adenosine (0.3 mg/kg, i.p.) and inosine (1.0 mg/kg, i.p.) at subeffective doses. The results presented in Fig. 7a, b show a synergistic effect that was seem after the treatment with adenosine and inosine. This treatment reduced significantly the total leukocyte and the neutrophil count in exudates, with inhibitions of 56 ± 6 and 56 ± 4 %, respectively, without affecting mononuclear cell count and exudation (Fig. 7a, b).
Fig. 7.
Synergic effect of pretreatment with adenosine and inosine, in pleural exudates. S represents the group injected with saline 0.9 %; C indicates the group treated with carrageenan and vehicle used to dilute inosine or adenosine; Ado + Ino represents the group co-treated with adenosine (0.3 mg/kg, i.p.) and inosine (1.0 mg/kg, i.p.). The difference between groups was determined by ANOVA followed by Newman–Keuls multiple comparison test. ***P < 0.001 when compared with carrageenan-treated group and #P < 0.001 when compared to saline group (S)
Discussion
The inflammatory response is a complex process. It involves selective and temporal migration of specific cell types to the affected site, fluid exudation, and usually some form of resolution. These events are orchestrated by a plethora of mediators that act sequentially to give rise to the ever-changing pattern of inflammation [26]. There are several models of inflammation that serve to investigate anti-inflammatory mechanisms and to test novel anti-inflammatory therapies that may be applicable to humans [26]. A useful model to assess the contribution of mediators involved in vascular changes and leukocyte migration associated with acute inflammation is carrageenan-induced acute pleural inflammation [24, 27, 28].
It has been shown that mouse pleural inflammatory response induced by carrageenan elicits the release of chemical mediators such as histamine, bradykinin, substance P, and prostaglandins that is followed by exudation, release of cytokines such as TNF-α and IL-1β, and neutrophil infiltration into inflammatory site, which peaked 4 h after pleurisy induction [24, 28, 29]. Here, we confirm these observations and demonstrated that pretreatment of mice with adenosine or inosine reduced the main features of acute inflammation including exudation and the number of leukocytes, characterized mainly by a reduction in neutrophil differential cell count, into pleural cavity, suggesting that both have a critical role in the control of acute inflammatory events.
Our results are in agreement with literature that describes adenosine and inosine as critical molecules involved in the regulation of inflammatory and immune processes [20, 30–32]. Adenosine accumulates in the extracellular space in response to metabolic stress and cell damage, and elevations in extracellular adenosine are found in conditions of ischemia, hypoxia, inflammation, and trauma [33, 34]. It is believed that this increase represents tissue injury and can be viewed as organ protective [33]. Accordingly, during inflammation and hypoxia, adenosine suppresses adaptative immune responses, promoting resolution of inflammation in pathologic events such as bowel disease and acute lung injury [35]. A similar role for adenosine was observed in ischemia and reperfusion, where adenosine signaling dampens inflammation and also promotes the resolution of injury through the activation of A2A and A2B adenosine receptors expressed on inflammatory cells and tissue-resident cells, respectively [36]. Moreover, together with adenosine, inosine was described to reduce cellular injury in a model of renal ischemia, suggesting that inosine can participate in tissue protection during ischemic situations [37].
Several studies demonstrate that adenosine during the inflammatory process is quickly converted into inosine by the enzyme adenosine deaminase expressed in activated leukocytes and on vascular endothelia [38, 39]. A recent study has described that during inflammation and hypoxia, there is a transcriptional increase in functional surface apyrase (CD39) and nucleotidase (CD73). In addition, following adenosine generation and receptor activation, adenosine diffuses away from the receptor and is rapidly transported from the extracellular toward the intracellular space mainly via equilibrative nucleoside transporters (ENT-1 and ENT-2) [40]. Having reached the intracellular space, adenosine can either undergo deamination by adenosine deaminase to inosine or conversion to AMP by adenosine kinase (AK) [37]. Current studies showed that time-dependent exposure of tissue to inflammation and hypoxia change adenosine metabolism, and this change is related to hypoxia-inducible factor (HIF)-1α transcriptional repression of ENT-2 and AK, resulting in decreased capacity to transport extracellular adenosine and intracellular adenosine accumulation, increasing adenosine levels and its signaling, with additional protecting effects to vascular endothelia and tissue [39, 40].
During inflammation and hypoxia, an increase in activity and expression of adenosine deaminase and in its complexing protein CD26 was also demonstrated [41]. This increase promotes adenosine conversion into inosine within seconds, terminates adenosine signaling, and thus, can initiate inosine signaling.
Taking together these current studies, the fact that adenosine and inosine have a quick metabolism and the agreement between the literature data and our results that describe inosine as an anti-inflammatory molecule, we hypothesize a possible interaction of effects between adenosine and inosine. Therefore, we perform an administration of subeffective dose of adenosine and inosine to study a synergic effect. Interestingly, our results demonstrated that treatment of animals with the combination of adenosine and inosine, at individual doses with no anti-inflammatory effects, results in a synergistic effect, reducing the inflammatory parameters analyzed in pleural exudates. This is the first evidence that these purines can act together to reduce de inflammatory acute response. However, additional experiments are needed to strengthen this hypothesis.
Inosine has been believed to be an inert metabolite, without biologic actions [34], but recent evidences indicate that like adenosine, inosine can exert many immunomodulatory actions through occupancy of G protein-coupled purinergic receptors of the P1 type, namely, A1, A2A, A2B, and A3 receptors. A1 and A3 predominantly coupled to Gi/o protein, lowering the intracellular levels of AMPc, whereas A2A and A2B coupled to Gs, therefore elevating of intracellular AMPc [5, 7].
In addition, studies had shown that extracellular adenosine A2A receptor activation downregulates inflammation in vivo [20] and is required for the effects of adenosine and inosine, both in vivo and in vitro heightening the interest in naturally occurring purines [20, 21, 32]. The activation of adenosine A2A receptors by adenosine, during inflammation or ischemic stimuli, promotes inhibitory effects on polymorphonuclear cells, reducing superoxide anion generation [42], adhesion, and phagocytosis [9, 43, 44].
Inosine also is known to exert wide raging anti-inflammatory effects that include inhibition of pro-inflammatory cytokine, chemokine production, protection in septic shock, colitis, and acute lung injury [31, 45, 46]. Some studies have shown that inosine can stimulate adenosine A2A receptors and is protective in models of Concanavalin A-induced liver damage, endotoxin-induced sepsis [20, 47], and TNBS-induced colitis [21].
Our results corroborate with literature data and suggest that adenosine or inosine can directly or indirectly modulates carrageenan-induced pleural inflammation through adenosine A2A receptors, since blocking this receptor with ZM241385, reverted the effects of both adenosine and inosine against leukocyte migration and vascular leakage. In addition, the CGS21680 treatment reduced the inflammatory parameters analyzed—an effect reverted with ZM241385 treatment, reinforcing the evidence that A2A is involved in the modulation of acute pleural inflammation. In agreement, it has recently been demonstrated that treatment with CGS21680, an agonist of adenosine A2A receptor, reduced pleural inflammation and lung injury induced by carrageenan in mice [48].
Some studies have shown that CGS21680 has high affinity for A2A and low affinity for A2B receptors [4, 49]. Accordingly, this can explain the partial reversion of CGS21680 effects observed, in our results, after alloxazine administration.
Our results showed that treatment with alloxazine, a selective antagonist of adenosine A2B receptor, reverted adenosine and inosine effects. In addition, the anti-inflammatory effects of A2B have already been described in literature. Yang and colleagues reported that A2B null mice present augmentation of pro-inflammatory cytokine levels, such as TNF-α, upregulation of vascular adhesion proteins, and leukocyte migration in response to lipopolysaccharide (LPS)-induced acute inflammation [50]. Thus, our study provides the first evidence that adenosine and inosine can activate adenosine A2A and A2B receptors in acute pleural inflammation, and therefore, being important regulators of this process.
It has been described that ATP is released by activated neutrophils and its phosphohydrolysis to adenosine promotes the barrier protection of endothelium by the activation of surface endothelial adenosine A2B receptor [3]. Indeed, a study conducted by Eckle and colleagues showed that vascular permeability was significantly increased in vascular organs of adenosine A−2B/− receptor—mice subjected to ambient hypoxia. In this same study, the pivotal role of the A2B in attenuating hypoxia-induced increases in vascular leak, mainly in the lungs, was also observed with selective agonists or antagonists of adenosine A2B receptor in mice or in vitro treatment with siRNA-targeting adenosine A2B receptor. Furthermore, additional studies suggested a critical role of HIF-1 in the transcriptional induction of the adenosine A2B receptor during hypoxia [51].
Recently, an interesting study conducted by the same group extended the data described above and revealed an adenosine A2B receptor-dependent lung protection to ventilation-induced lung injury and LPS-induced lung injury, related to a decrease in pulmonary inflammation, increase in alveolar fluid clearance, reduction in pulmonary edema, and maintenance of capillary–alveolar barrier—a mechanism that involves adenosine A2B receptor expression with elevation of pulmonary cAMP levels [52].
Given the above-mentioned studies and the fact that carrageenan-induced pleural inflammation is a well-established model of acute lung injury [48, 53, 54], it is tempting to speculate that adenosine and inosine acting through adenosine A2B receptors increase the endothelial barrier protection, reducing the loss of fluid to pleural cavity, consequently reducing pleural exudation.
In this study we also demonstrated that adenosine and inosine significantly reduced the IL-1β and TNF-α level in pleural exudates, an effect that seems to be dependent of adenosine A2A receptor activation only in animals treated with inosine. Indeed these cytokines have a critical modulatory action in the acute pleural inflammatory reaction caused by carrageenan [55] promoting the recruitment of leukocytes and expression of adhesion molecules at the site of inflammation [56, 57]. Accordingly, literature has also shown that adenosine A2A receptor is the predominant receptor subtype responsible for inhibiting TNF-α production [58]. Regarding the quick metabolism of adenosine into inosine and it synergic effects showed here, we can hypothesize that in acute pleural inflammation the adenosine effects in reducing cytokine levels are due to adenosine conversion into inosine mediated via A2A receptors.
The results presented here are relevant, since they have demonstrated that administration of subeffective dose of adenosine and inosine presents synergistic anti-inflammatory effect in an acute model of pleural inflammation. Thus, these findings extent the literature data and may be important in the understanding adenosinergic pathway and adenosine receptors role in the acute inflammation. Furthermore, continuing focus on research to study endogenous inhibitors of inflammation is expected to improve the design and development of novel inflammation-inhibiting drugs [59].
Conclusions
The present work demonstrates that adenosine and inosine exert a great anti-inflammatory effect in carrageenan-induced pleurisy. This effect is related to the activation of A2 adenosine receptors and inhibition of production or release of inflammatory cytokines. New studies using these and other models of inflammation need to be conducted to better determine the important role of adenosine and inosine and their receptors in the acute inflammatory response. Collectively, our results extend the literature data and provide experimental evidence for adenosine and inosine anti-inflammatory action and strengthen the importance of the purine nucleotides as a potential therapeutic target that could be successfully used to treat inflammatory diseases.
Acknowledgments
This work was supported by grants from Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior and Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil.
Conflict of interest
None
Contributor Information
Fernanda da Rocha Lapa, Email: fernandarlapa@yahoo.com.br.
Morgana Duarte da Silva, Email: morganafisioterapia@yahoo.com.br.
Daniela de Almeida Cabrini, Email: cabrini@ufpr.br.
Adair R. S. Santos, Phone: +55-48-37219352, FAX: +55-048-37219672, Email: arssantos@ccb.ufsc.br, Email: adairrs.santos@gmail.com
References
- 1.Hasko G, Sitkovsky MV, Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci. 2004;25(3):152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Livingston M, Heaney LG, Ennis M. Adenosine, inflammation and asthma—a review. Inflamm Res. 2004;53(5):171–178. doi: 10.1007/s00011-004-1248-2. [DOI] [PubMed] [Google Scholar]
- 3.Eltzschig HK, Ibla JC, Furuta GT, Leonard MO, Jacobson KA, Enjyoji K, Robson SC, Colgan SP. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198(5):783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50(3):413–492. [PubMed] [Google Scholar]
- 5.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 6.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116(8):2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bours MJ, Swennen EL, Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Schneider L, Pietschmann M, Hartwig W, Marcos SS, Hackert T, Gebhard MM, Uhl W, Buchler MW, Werner J. Inosine reduces microcirculatory disturbance and inflammatory organ damage in experimental acute pancreatitis in rats. Am J Surg. 2006;191(4):510–514. doi: 10.1016/j.amjsurg.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Cronstein BN. Adenosine, an endogenous anti-inflammatory agent. J Appl Physiol. 1994;76(1):5–13. doi: 10.1152/jappl.1994.76.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Wakai A, Wang JH, Winter DC, Street JT, O’Sullivan RG, Redmond HP. Adenosine inhibits neutrophil vascular endothelial growth factor release and transendothelial migration via A2B receptor activation. Shock. 2001;15(4):297–301. doi: 10.1097/00024382-200115040-00008. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann I, Hoelzl A, Schliephake F, Hummel T, Chouker A, Lysenko L, Peter K, Thiel M. Effects of adenosine on functions of polymorphonuclear leukocytes from patients with septic shock. Shock. 2007;27(1):25–31. doi: 10.1097/01.shk.0000238066.00074.90. [DOI] [PubMed] [Google Scholar]
- 12.El-Hashim AZ, Abduo HT, Rachid OM, Luqmani YA, Al Ayadhy BY, Alkhaledi GM. Intranasal administration of NECA can induce both anti-inflammatory and pro-inflammatory effects in BALB/c mice: evidence for A 2A receptor sub-type mediation of NECA-induced anti-inflammatory effects. Pulm Pharmacol Ther. 2009;22(3):243–252. doi: 10.1016/j.pupt.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 13.Antonioli L, Fornai M, Colucci R, Ghisu N, Settimo F, Natale G, Kastsiuchenka O, Duranti E, Virdis A, Vassalle C, Motta C, Mugnaini L, Breschi MC, Blandizzi C, Taca M. Inhibition of adenosine deaminase attenuates inflammation in experimental colitis. J Pharmacol Exp Ther. 2007;322(2):435–442. doi: 10.1124/jpet.107.122762. [DOI] [PubMed] [Google Scholar]
- 14.Hasko G, Nemeth ZH, Vizi ES, Salzman AL, Szabo C. An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur J Pharmacol. 1998;358(3):261–268. doi: 10.1016/S0014-2999(98)00619-0. [DOI] [PubMed] [Google Scholar]
- 15.Szabo C, Scott GS, Virag L, Egnaczyk G, Salzman AL, Shanley TP, Hasko G. Suppression of macrophage inflammatory protein (MIP)-1alpha production and collagen-induced arthritis by adenosine receptor agonists. Br J Pharmacol. 1998;125(2):379–387. doi: 10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law WR. Adenosine receptors in the response to sepsis: what do receptor-specific knockouts tell us? Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R957–R958. doi: 10.1152/ajpregu.00412.2006. [DOI] [PubMed] [Google Scholar]
- 17.Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186(9):1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eltzschig HK. Adenosine: an old drug newly discovered. Anesthesiology. 2009;111(4):904–915. doi: 10.1097/ALN.0b013e3181b060f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL, Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164(2):1013–1019. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- 20.Gomez G, Sitkovsky MV. Differential requirement for A2a and A3 adenosine receptors for the protective effect of inosine in vivo. Blood. 2003;102(13):4472–4478. doi: 10.1182/blood-2002-11-3624. [DOI] [PubMed] [Google Scholar]
- 21.Rahimian R, Fakhfouri G, Daneshmand A, Mohammadi H, Bahremand A, Rasouli MR, Mousavizadeh K, Dehpour AR. Adenosine A(2A) receptors and uric acid mediate protective effects of inosine against TNBS-induced colitis in rats. Eur J Pharmacol. 2010;649(1–3):376–381. doi: 10.1016/j.ejphar.2010.09.044. [DOI] [PubMed] [Google Scholar]
- 22.Linden J. New insights into the regulation of inflammation by adenosine. J Clin Invest. 2006;116(7):1835–1837. doi: 10.1172/JCI29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriques MG, Rae GA, Cordeiro RS, Williams TJ. Endothelin-1 inhibits PAF-induced paw oedema and pleurisy in the mouse. Br J Pharmacol. 1992;106(3):579–582. doi: 10.1111/j.1476-5381.1992.tb14378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleh TS, Calixto JB, Medeiros YS. Anti-inflammatory effects of theophylline, cromolyn and salbutamol in a murine model of pleurisy. Br J Pharmacol. 1996;118(3):811–819. doi: 10.1111/j.1476-5381.1996.tb15472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moroney MA, Alcaraz MJ, Forder RA, Carey F, Hoult JR. Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J Pharm Pharmacol. 1988;40(11):787–792. doi: 10.1111/j.2042-7158.1988.tb05173.x. [DOI] [PubMed] [Google Scholar]
- 26.Moore AR. Pleural models of inflammation: immune and nonimmune. Methods Mol Biol. 2003;225:123–128. doi: 10.1385/1-59259-374-7:123. [DOI] [PubMed] [Google Scholar]
- 27.Lo TN, Almeida AP, Beaven MA. Dextran and carrageenan evoke different inflammatory responses in rat with respect to composition of infiltrates and effect of indomethacin. J Pharmacol Exp Ther. 1982;221(1):261–267. [PubMed] [Google Scholar]
- 28.Menegazzi M, Paola R, Mazzon E, Genovese T, Crisafulli C, Dal Bosco M, Zou Z, Suzuki H, Cuzzocrea S. Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol Res. 2008;58(1):22–31. doi: 10.1016/j.phrs.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Tomlinson A, Appleton I, Moore AR, Gilroy DW, Willis D, Mitchell JA, Willoughby DA. Cyclo-oxygenase and nitric oxide synthase isoforms in rat carrageenin-induced pleurisy. Br J Pharmacol. 1994;113(3):693–698. doi: 10.1111/j.1476-5381.1994.tb17048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A. Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br J Pharmacol. 2008;153(Suppl 1):S457–S464. doi: 10.1038/bjp.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liaudet L, Pacher P, Mabley JG, Virag L, Soriano FG, Hasko G, Szabo C. Activation of poly(ADP-Ribose) polymerase-1 is a central mechanism of lipopolysaccharide-induced acute lung inflammation. Am J Respir Crit Care Med. 2002;165(3):372–377. doi: 10.1164/ajrccm.165.3.2106050. [DOI] [PubMed] [Google Scholar]
- 32.Bouma MG, Stad RK, Wildenberg FA, Buurman WA. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J Immunol. 1994;153(9):4159–4168. [PubMed] [Google Scholar]
- 33.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Linden J. Molecular approach to adenosine receptors: receptor-mediated mechanisms of tissue protection. Annu Rev Pharmacol Toxicol. 2001;41:775–787. doi: 10.1146/annurev.pharmtox.41.1.775. [DOI] [PubMed] [Google Scholar]
- 35.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17(11):1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modis K, Gero D, Nagy N, Szoleczky P, Toth ZD, Szabo C. Cytoprotective effects of adenosine and inosine in an in vitro model of acute tubular necrosis. Br J Pharmacol. 2009;158(6):1565–1578. doi: 10.1111/j.1476-5381.2009.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trams EG, Lauter CJ. On the sidedness of plasma membrane enzymes. Biochim Biophys Acta. 1974;345(2):180–197. doi: 10.1016/0005-2736(74)90257-0. [DOI] [PubMed] [Google Scholar]
- 39.Spicuzza L, Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol. 2006;533(1–3):77–88. doi: 10.1016/j.ejphar.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 40.Loffler M, Morote-Garcia JC, Eltzschig SA, Coe IR, Eltzschig HK. Physiological roles of vascular nucleoside transporters. Arterioscler Thromb Vasc Biol. 2007;27(5):1004–1013. doi: 10.1161/ATVBAHA.106.126714. [DOI] [PubMed] [Google Scholar]
- 41.Eltzschig HK, Faigle M, Knapp S, Karhausen J, Ibla J, Rosenberger P, Odegard KC, Laussen PC, Thompson LF, Colgan SP. Endothelial catabolism of extracellular adenosine during hypoxia: the role of surface adenosine deaminase and CD26. Blood. 2006;108(5):1602–1610. doi: 10.1182/blood-2006-02-001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Revan S, Montesinos MC, Naime D, Landau S, Cronstein BN. Adenosine A2 receptor occupancy regulates stimulated neutrophil function via activation of a serine/threonine protein phosphatase. J Biol Chem. 1996;271(29):17114–17118. doi: 10.1074/jbc.271.29.17114. [DOI] [PubMed] [Google Scholar]
- 43.Lappas CM, Sullivan GW, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Investig Drugs. 2005;14(7):797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- 44.Murphree LJ, Sullivan GW, Marshall MA, Linden J. Lipopolysaccharide rapidly modifies adenosine receptor transcripts in murine and human macrophages: role of NF-kappaB in A(2A) adenosine receptor induction. Biochem J. 2005;391(Pt 3):575–580. doi: 10.1042/BJ20050888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liaudet L, Mabley JG, Soriano FG, Pacher P, Marton A, Hasko G, Szabo C. Inosine reduces systemic inflammation and improves survival in septic shock induced by cecal ligation and puncture. Am J Respir Crit Care Med. 2001;164(7):1213–1220. doi: 10.1164/ajrccm.164.7.2101013. [DOI] [PubMed] [Google Scholar]
- 46.Mabley JG, Pacher P, Liaudet L, Soriano FG, Hasko G, Marton A, Szabo C, Salzman AL. Inosine reduces inflammation and improves survival in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284(1):G138–G144. doi: 10.1152/ajpgi.00060.2002. [DOI] [PubMed] [Google Scholar]
- 47.Luscinskas FW, Ma S, Nusrat A, Parkos CA, Shaw SK. The role of endothelial cell lateral junctions during leukocyte trafficking. Immunol Rev. 2002;186:57–67. doi: 10.1034/j.1600-065X.2002.18606.x. [DOI] [PubMed] [Google Scholar]
- 48.Impellizzeri D, Di Paola R, Esposito E, Mazzon E, Paterniti I, Melani A, Bramanti P, Pedata F, Cuzzocrea S (2011) CGS 21680, an agonist of the adenosine (A(2A)) receptor, decreases acute lung inflammation. Eur J Pharmacol. doi:10.1016/j.ejphar.2011.06.049 [DOI] [PubMed]
- 49.Fredholm BB, Ijzerman AP, Jacobson KA, Linden J, Muller CE. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev. 2011;63(1):1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116(7):1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111(4):2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118(10):3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kroegel C, Antony VB. Immunobiology of pleural inflammation: potential implications for pathogenesis, diagnosis and therapy. Eur Respir J Off J Eur Soc Clin Respir Physiol. 1997;10(10):2411–2418. doi: 10.1183/09031936.97.10102411. [DOI] [PubMed] [Google Scholar]
- 54.Ianaro A, Ialenti A, Maffia P, Sautebin L, Rombola L, Carnuccio R, Iuvone T, D’Acquisto F, Rosa M. Anti-inflammatory activity of macrolide antibiotics. J Pharmacol Exp Ther. 2000;292(1):156–163. [PubMed] [Google Scholar]
- 55.Frode TS, Souza GE, Calixto JB. The modulatory role played by TNF-alpha and IL-1 beta in the inflammatory responses induced by carrageenan in the mouse model of pleurisy. Cytokine. 2001;13(3):162–168. doi: 10.1006/cyto.2000.0816. [DOI] [PubMed] [Google Scholar]
- 56.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6(4):232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 58.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317(1):172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 59.Sitkovsky MV. Use of the A(2A) adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem Pharmacol. 2003;65(4):493–501. doi: 10.1016/S0006-2952(02)01548-4. [DOI] [PubMed] [Google Scholar]