Abstract
In the nucleus tractus solitarii (NTS) of rats, blockade of extracellular ATP breakdown to adenosine reduces arterial blood pressure (AP) increases that follow stimulation of the hypothalamic defense area (HDA). The effects of ATP on NTS P2 receptors, during stimulation of the HDA, are still unclear. The aim of this study was to determine whether activation of P2 receptors in the NTS mediates cardiovascular responses to HDA stimulation. Further investigation was taken to establish if changes in hindlimb vascular conductance (HVC) elicited by electrical stimulation of the HDA, or activation of P2 receptors in the NTS, are relayed in the rostral ventrolateral medulla (RVLM); and if those responses depend on glutamate release by ATP acting on presynaptic terminals. In anesthetized and paralyzed rats, electrical stimulation of the HDA increased AP and HVC. Blockade of P2 or glutamate receptors in the NTS, with bilateral microinjections of suramin (10 mM) or kynurenate (50 mM) reduced only the evoked increase in HVC by 75 % or more. Similar results were obtained with the blockade combining both antagonists. Blockade of P2 and glutamate receptors in the RVLM also reduced the increases in HVC to stimulation of the HDA by up to 75 %. Bilateral microinjections of kynurenate in the RVLM abolished changes in AP and HVC to injections of the P2 receptor agonist α,β-methylene ATP (20 mM) into the NTS. The findings suggest that HDA-NTS-RVLM pathways in control of HVC are mediated by activation of P2 and glutamate receptors in the brainstem in alerting-defense reactions.
Keywords: Hypothalamic defense area, RVLM, NTS, Blood flow, ATP
Introduction
Alerting-defense reactions prepare the body to life-threatening situations and are artificially replicated by electrical stimulation of the hypothalamus [1]. Activation of the hypothalamic defense area (HDA), corresponding to the perifornical hypothalamus [2], increases arterial pressure (AP), heart rate (HR), and vascular conductance to the hindlimb (HVC) in cat [3] and rat [4–6]. The increase in vascular conductance is mediated by withdrawal of sympathetic tone and release of epinephrine by the adrenal medulla [5, 6]. Identified projections from HDA neurons to the rostral ventrolateral medulla (RVLM) and nucleus tractus solitarius (NTS) suggest that cardiovascular responses elicited by hypothalamic stimulation are mediated in the medulla [7–9]. Stimulation of the HDA produces excitatory post-synaptic potentials in neurons at the intermediate NTS of cat [10, 11]; suggesting that the resulting rise in peripheral HVC can derive from activation of sympathoinhibitory pathways involved in the baroreflex. In rat, electrical stimulation of the HDA increases the firing rate of neurons in the RVLM that are barosensitive and bulbospinal [12], presumably resulting in decrease of peripheral HVC. Due to such contrast, it is likely that selective pathways from the HDA to the medulla operate in parallel during alerting defense responses. Notably, antagonism of glutamate receptors in the RVLM exclusively reduces the increase in HVC (i.e., hindlimb vasodilation) evoked electrical stimulation of the HDA [13].
P2 receptors, for ATP, are expressed in the NTS [14, 15] and in the RVLM [15]. In anesthetized rats, stimulation of P2 receptors in the subpostremal NTS by microinjecting the P2x1-3 agonist α,β-methylene ATP elicits hindlimb vasodilation (increase in HVC) associated with reductions in AP and HR [16]. In the RVLM, iontophoretic injections of different P2 agonists increases the firing rate of cardiovascular bulbospinal neurons [17]. It is demonstrated that adenosine in the RVLM [18] and NTS [18–20] of rats derives from extracellular ATP and underlies the rise in AP elicited by stimulation of the HDA [18]. However, blockade of ATP breakdown into adenosine in either NTS or RVLM does not completely block the evoked increase of HVC, in response to stimulation of the HDA. It seems that adenosine is not the major mediator of the increases in HVC in response to HDA stimulation. In addition, the role of medullary P2 receptors in the NTS and RVLM on cardiovascular responses to stimulation of the HDA still remains to be established. In the NTS, ATP apparently acts as a neuromodulator interacts with terminals promoting glutamate release [21], thus altering sympathetic nerve discharge. In fact, in vitro activation of purinergic P2 receptors located in the presynaptic membrane produces release of glutamate in the neuron terminal observed [22, 23].
In the present study, we hypothesized that stimulation of the HDA increases the concentration of ATP in the NTS and, by binding to P2 receptors, affects the vasodilation of the hindlimb. Additionally, due to existence of direct projections from the NTS to the RVLM [24], we speculated that neurons in this area mediate responses to activation of the NTS. Therefore, first we aimed to determine whether or not activation of P2 receptors in the NTS mediate the increase in HVC evoked by stimulation of the HDA. Secondly, we sought to determine if purinergic and glutamate synapses to RVLM, presumably from the NTS, mediate the hindlimb vasodilation elicited by pharmacological stimulation of P2 receptors into the NTS or by electrical stimulation of the HDA.
Methods
All experiments were performed in male Wistar rats (300–350 g). Experiments were conducted according to the Brazilian State Code for Animal Protection and were approved by the Universidade Federal de São Paulo Ethics Committee (protocol number: 02146/08).
General procedures
Anesthesia was induced in by inhalation of halothane (3 % in 100 % O2). Following cannulation of the left jugular vein, urethane (0.6 g/kg in 0.6 g/ml) and α-chloralose (50 mg/kg in 50 mg/ml) were injected intravenously. Corneal reflex and paw pinch were tested during experiment and anesthesia was supplemented by infusing 0.1 ml of the mixture if necessary to maintain a depth of anesthesia at which hindpaw pinch evoked no motor reflexes. After institution of neuromuscular blockade, anesthetic depth was maintained at a level such that paw pinch evoked minimal autonomic reflexes (<10 mmHg change in blood pressure). Temperature was monitored with a rectal thermometer and kept at 37 ± 0.5°C with an infrared lamp. The right common carotid artery was cannulated to measure arterial pressure (AP) and heart rate (HR). The tracheostomy was performed and neuromuscular blockade was established with d-tubocurarine (0.5 mg/kg, i.v.). Animals were artificially ventilated using oxygen-enriched room air.
Transit time perivascular flow probes placement
Hindlimb blood flow was recorded by the transit time method [25] using perivascular probes. A midline abdominal incision was performed to expose the abdominal aorta just above the bifurcation of the iliac arteries. Connective tissue was cleaned from the aorta, which was separated from the vena cava. An ultrasound probe was implanted, attached to the artery, filled with ultrasound gel and exteriorized through the transverse abdominal muscle and skin. Probes were connected to a T206 blood flow meter (Transonic Systems, Inc., Ithaca, NY, USA) and recorded in milliliters per minute using a 0.1-Hz low pass filter. The vasodilation was observed by recording increases in hindlimb vascular conductance (HVC) and was evoked either by electrical stimulation of the HDA or by chemical stimulation of NTS neurons. Hindlimb vascular conductance (HVC) baseline was calculated online by the ratio aortic hindlimb blood flow (milliliters) divided per mean arterial pressure (MAP; millimeter of mercury) and converted to microliter by ×1,000, expressed in microliter per millimeter of mercury per min.
Data analysis
Hindlimb vascular conductance (HVC) baseline was expressed in absolute values (μl/min*mmHg) and variations were normalized as a percent of baseline adopting 100 % as the maximum response during control stimulation. Statistical comparison of maximum peak responses before and after microinjections were conducted using Student's paired t test and are presented as mean ± SEM. Comparison of different groups were done by unpaired Student's t test or two-way ANOVA and are presented as mean ± SEM; differences were considered statistically significant where P < 0.05.
Stereotaxic surgery
Animals were mounted in a stereotaxic frame and the head was positioned in the horizontal position relative to Bregma and Lambda (−3.4 mm). A dental drill was used to create a squared window in the skull between Bregma and Lambda and the dura was removed from the cerebral cortex on both sides of the superior sagittal sinus. A concentric electrode was lowered into the HDA, based on the stereotaxic coordinates by Paxinos and Watson [2]: 2.8–3.5 mm caudal to Bregma, 2.0–2.5 mm lateral to sagittal sinus and 8.0–9.0 mm ventral to cortex surface. The position of the electrode remained unchanged during the whole experiment. Electrical stimulation of the HDA was achieved using the following parameters: 150–200 μA, 0.6 ms, 100 Hz for 5 s. The HDA was characterized functionally by cardiovascular changes based on the descriptions of Yardley and Hilton [5].
The dorsal surface of the medulla was exposed by occipital craniotomy, followed by removal of the dura and arachnoid membranes. Drug injections were made via a single-barrelled borosilicate micropipette vertically positioned by an extra stereotaxic arm. Coordinates for the subpostremal NTS were 0.5 mm rostral and 0.5 mm lateral to calamus scriptorus and 0.5 mm ventral to the dorsal surface. The RVLM was accessed by the following coordinates: 3.5–4.0 mm caudal and 2.0 mm lateral from the Lambda and 2.5 mm ventral from the dorsal surface. All microinjections were made with a volume of 50 nl. In order to microinject a different drug or dye, the dorsal-ventral coordinate was annotated and the pipette was withdrawn from the brainstem without changing the antero-posterior and medio-lateral coordinates. For multiple injections (e.g., suramin, kynurenate, dye), the pipette was flushed with saline 0.9 %, refilled and positioned in the same dorsal-ventral coordinate. If RVLM injection sites fell more than 300 μm caudal to the caudal pole of the facial nucleus, animals were excluded from further analysis. If NTS injections were more than 500 μm ventral from the dorsal surface, then they were excluded from further analysis (see “Histology” section).
Histology
At the end of the experiments Evans blue 2 % was injected to enable histological confirmation of injection sites. HDA stimulated sites were marked by an electrolytic lesion (DC mode, 0.5 mA for 30 s; Fig. 2e). Rats received an intravenous injection of sodium pentobarbital (100 mg/kg) and were then perfused with 250 mL of 0.9 % NaCl followed by 300 ml of formalin 10 %. Brains were removed and post-fixed in 10 % formalin for 48 h. Coronal sections of brain (40 μm) were cut using a freezing microtome (Leica Wetzlar) and mounted in series of 120 μm distance between slices on glass slides. The tissue was stained with 1 % neutral red and analyzed by light microscopy (Nikon-Optshot-2).
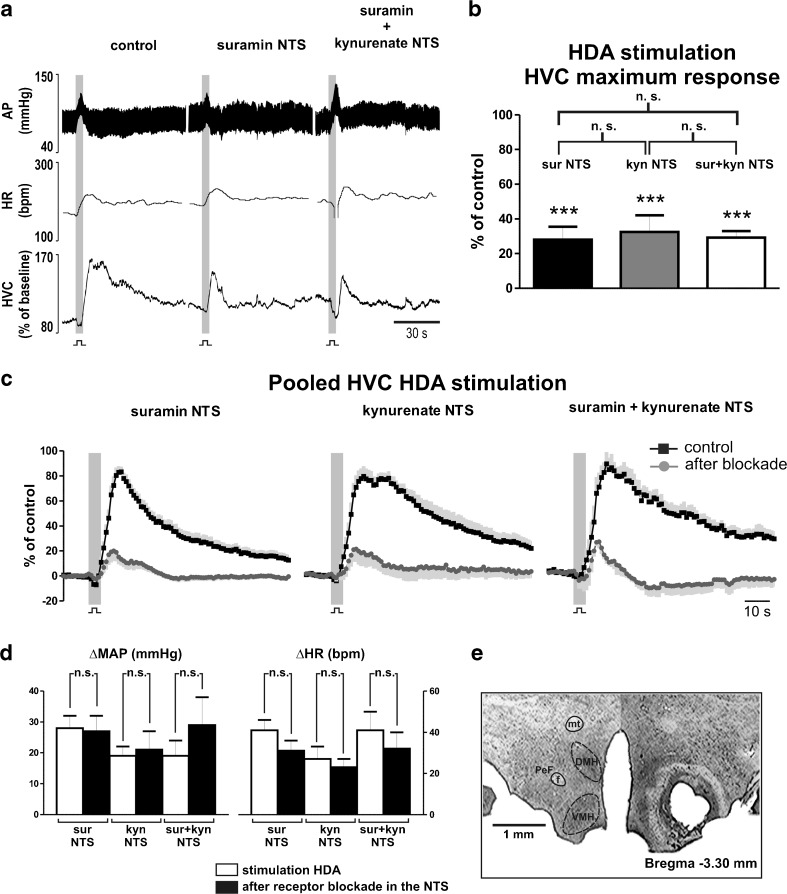
Fig. 2.
Effects of P2 and glutamate receptors blockade in the NTS on cardiovascular responses elicited by stimulation of the HDA. a Representative recording of one animal in response to stimulation of the hypothalamic defense area (HDA; 150–200 μA, 0.6 ms, 100 Hz for 5 s) before and after bilateral microinjections of suramin (10 mM) and kynurenate (50 mM) in the nucleus tractus solitarii (NTS). AP arterial blood pressure; HR heart rate; HVC hindlimb vascular conductance. b Group data showing the maximum response generated by hypothalamic stimulation after antagonism of P2 (sur NTS), glutamate (kyn NTS) and both (sur + kyn) receptors in the NTS. c Pooled HVC responses elicited by stimulation of the HDA during control, after blockade of P2 receptors, glutamate receptors, and combined P2 and glutamate receptors in the NTS. Black squares, averaged curve during control; dark graycircles, averaged curve after blockade; in light gray, standard error of the mean. d Grouped data of increases elicited in mean arterial pressure (MAP) and HR by hypothalamic stimulation before and after after antagonism of P2 (sur NTS), glutamate (kyn NTS), and both (sur + kyn) receptors in the NTS. e Photomicrograph of a coronal section showing electrolytic lesion of the hypothalamic defense area, corresponding to the perifornical hypothalamus (PeF). mt mammillothalamic tract; f fornix; DMH dorsomedial hypothalamus; VMH ventromedial hypothalamus. ***P < 0.001 difference to control; n.s., P > 0.05
Experimental protocols
In order to achieve complete blockade of P2 and glutamate receptors in the NTS and RVLM, drugs were injected bilaterally. Previous anatomical studies [7, 8] showed bilateral projections, predominantly ipsilateral, from the perifornical hypothalamus to the NTS and ventrolateral medulla.
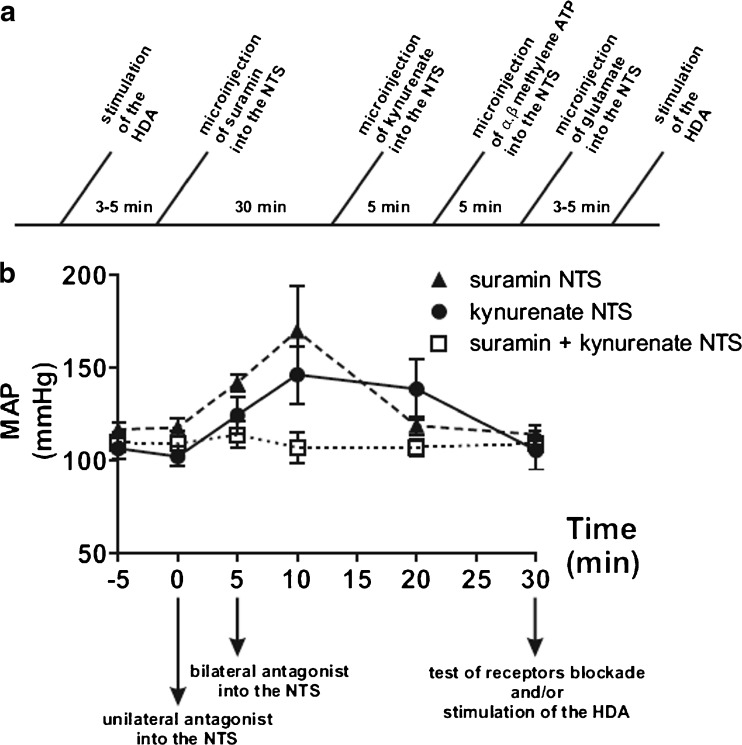
Doses of drugs for microinjections were chosen based on previous studies in order to compare with previously reported effects. The dose of suramin injected into the NTS was based on previous studies performed in whole animal [26, 27]. This allowed comparing the effects in HVC to previous findings in lumbar sympathetic discharge. It was demonstrated that 10 mM completely blocks cardiovascular changes caused by microinjections of 20 mM α,β-methylene ATP into the subpostremal NTS. A tenfold lower dose of suramin causes non-significant blockade [27], whereas a tenfold higher dose produces unspecific blockade [17]. Because high doses of suramin are capable of non-selective antagonism of glutamate receptors [28, 29], glutamate was injected following suramin to test the specificity of the pharmacological blockade. All cardiovascular variables were measured in resting condition, in response to stimulation of the HDA or pharmacological activation of NTS or RVLM neurons. An example of the time line can be seen in Fig. 1a. Following microinjection of drugs, variables were measured after stabilization of the cardiovascular parameters (Fig. 1b). In order to confirm the blockade of receptors, respective agonists were microinjected. Integrity of neural structures after microinjections was verified in some experiments by recovery of responses 90 min after injection of antagonists.
Fig. 1.
Effects of suramin and kynurenate microinjections into the NTS on mean arterial pressure (MAP). a Representative time-line of an experimental protocol involving blockade of both P2 and glutamate receptors in the NTS and stimulation of the HDA. b Bilateral microinjections of suramin (10 mM) or kynurenate (50 mM) into the NTS elicited a transitory increase in MAP baseline (N = 5). The values of MAP returned to in resting condition after ∼30 min, for suramin, and kynurenate. No further increase in MAP was elicited by kynurenate, after previous blockade with suramin
The experimental protocols adopted in the present study are as follows:
First, increases in AP, HR, and HVC to HDA stimulation were compared before and after bilateral microinjections of suramin (10 mM) or kynurenate (50 mM) into the NTS. Results were further compared after combined blockade of P2 and glutamate receptors. Following suramin (10 mM), kynurenate (50 mM) was microinjected into the NTS and stimulation of HDA was repeated. The volume of injections were 50 nl.
In a different group, HVC responses to HDA stimulation were compared before and after bilateral microinjections of PPADS (5 mM) into the NTS, an alternative antagonist for P2 receptors.
Blockade of P2 and glutamate receptors was tested by unilateral microinjection of the respective agonists: α,β-methylene ATP (20 mM) and l-glutamate (50 mM) before and 5 min after stabilization of cardiovascular parameters (∼20–30 min; Fig. 1), elicited by bilateral microinjections of respective antagonists suramin (10 mM) and kynurenate (50 mM) into the NTS.
New series of experiments were conducted to determine if P2 and glutamate receptors in RVLM neurons participate or not in cardiovascular responses produced by HDA stimulation. Responses to HDA stimulation were compared before and after bilateral microinjections of suramin (10 mM) and kynurenate (50 mM) in the RVLM. Finally, to determine the participation of RVLM glutamate synapses on cardiovascular responses to activation of P2 receptors in NTS neurons, α,β-methylene ATP (20 mM) was injected into the NTS before and after bilateral microinjections of kynurenate (50 mM) in the RVLM.
Relationship of NTS P2 receptors with peripheral mechanisms in control of vascular conductance was investigated by bilateral microinjections of suramin into the NTS following blockade of α adrenoceptors. Responses to HDA stimulation were compared before and after intravenous infusion of phentolamine (1 mg/kg) and subsequent bilateral microinjections of suramin (10 mM) into the NTS.
Drugs used for microinjections in this study were: l-glutamate (50 mM), α,β-methylene-adenosine-5′-triphosphate (α,β-methylene ATP; 20 mM,), suramin (10 mM), pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS; 5 mM), kynurenate (50 mM) and Evans blue (2 % in 0.9 % NaCl) for histological verification of injection sites. The dose of intravenous infusion of phentolamine was 1 mg/kg in 1 mg/ml solution (∼0.3 ml/rat). All drugs were obtained from Sigma Aldrich, St Louis—USA. All microinjections had a volume of 50 nl; intravenous injections had a volume of 0.1 ml/100 g of body weight.
Results
P2 and glutamate receptors blockade in the NTS reduce the hindlimb vasodilation to HDA stimulation
High frequency electrical stimulation (150–200 μA, 0.6 ms, 100 Hz for 5 s) of the HDA elicited an increase in AP, HR, and HVC (Fig. 2a). Bilateral microinjections of suramin (10 mM), a P2 receptor antagonist, into the NTS reduced the rise in HVC (100 % vs. 28 ± 7 %; P < 0.001) evoked by HDA stimulation (Fig. 2a–c). The magnitude of evoked increases in AP and HR did not change (AP, 28 ± 4 vs. 27 ± 5 mmHg; HR: 41 ± 5 vs. 31 ± 5 bpm; P > 0.05) after blockade of P2 receptors in the NTS (Fig. 2d). Microinjection of suramin elicited a transitory increase in AP, which returned to baseline after 20 min (Fig. 1b). Following stabilization, baseline levels of AP, HR, and HVC did not change in comparison to values prior to microinjections of suramin into the NTS (Table 1). Similar effects in HVC responses to HDA stimulation (32 ± 10 %; P < 0.001) were seen in a different group (N = 11), following glutamate receptor blockade with bilateral microinjections of kynurenate (50 mM) into the NTS (Fig. 2b, c). The blockade of glutamate receptors in the NTS did not alter (P > 0.05) the evoked changes in AP (19 ± 3 vs. 21 ± 6 mmHg) and HR (27 ± 6 vs. 23 ± 4 bpm) by stimulation of the HDA (Fig. 2d). Kynurenate into the NTS augmented AP that reverted to resting levels after ∼30 min (Fig. 1b). After the transient cardiovascular effects, AP, HR, and HVC baselines were similar to values prior to microinjections of kynurenate (Table 1). The combined blockade of P2 and glutamate receptors in the NTS produced no further reduction in the HVC response (29 ± 4 %; P > 0.05; N = 5) elicited by HDA stimulation (Fig. 2b, c). Additionally, the corresponding evoked rises in AP (19 ± 5 vs. 29 ± 9 mmHg) and HR (41 ± 9 vs. 32 ± 8 bpm) remained unchanged (Fig. 2d). Following bilateral microinjections of suramin into the NTS, subsequent bilateral microinjections of kynurenate did not elicit an additional increase in resting AP (110 ± 4 mmHg vs. 105 ± 8 mmHg, Fig. 1b), in contrast to injections of either antagonists isolated. The baseline levels of HR, and HVC also did not change after combined microinjections of suramin and kynurenate into the NTS (Table 1).
Table 1.
Baseline levels prior and after blockade of P2 and glutamate receptors in the NTS and RVLM
| Suramin + kynurenate NTS | Kynurenate NTS | Suramin + kynurenate RVLM | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | Sur NTS | Sur + kyn NTS | Control | Kyn NTS | Control | Sur RVLM | Sur + kyn RVLM | |
| MAP (mmHg) | 110 ± 4 | 109 ± 8 | 105 ± 8 | 100 ± 10 | 108 ± 6 | 115 ± 1 | 122 ± 7 | 114 ± 5 |
| HR (bpm) | 362 ± 15 | 329 ± 16 | 301 ± 27 | 347 ± 18 | 369 ± 21 | 364 ± 13 | 359 ± 16 | 299 ± 5 * |
| HVC (μl/mmHg*min) | 100 ± 11 | 112 ± 16 | 91 ± 11 | 96 ± 8 | 102 ± 15 | 85 ± 13 | 64 ± 7 | 68 ± 11 |
*P < 0.05 in comparison to control level
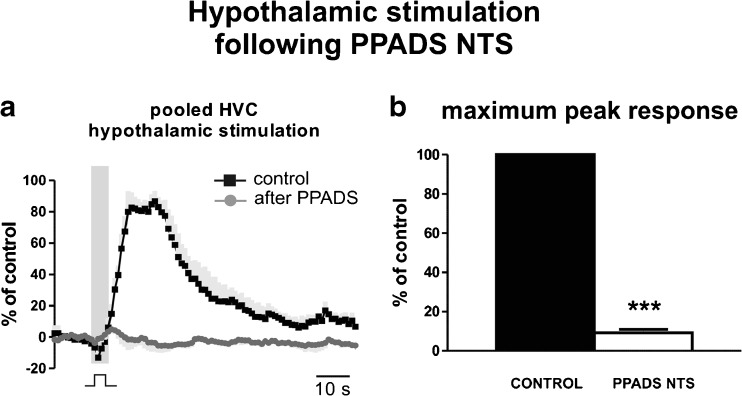
Bilateral microinjection of PPADS (5 mM), an alternative antagonist for P2 receptors, into the NTS also diminished the changes in HVC elicited by HDA stimulation (Fig. 3a). In the NTS, PPADS blocked the increase in HVC (100 % vs. 9 ± 2 %, N = 4; P < 0.001) elicited by electrical stimulation of the HDA (Fig. 3b).
Fig. 3.
Effect of PPADS on hindlimb vasodilation evoked by HDA stimulation. a Pooled hindlimb vascular conductance (HVC) curves constructed from responses generated by stimulation of the HDA (150–200 μA, 0.6 ms, 100 Hz for 5 s) during control and after PPADS (5 mM; Sigma Aldrich, St Louis—USA) in the nucleus tractus solitarii (NTS). Blockade of P2 receptors with bilateral microinjections of PPADS into the NTS reduced the elicited vasodilation compared to control. b Group data (N = 4) showing reduction of maximum responses generated by hypothalamic stimulation after antagonism of P2 receptors with PPADS (PPADS NTS). ***P < 0.001
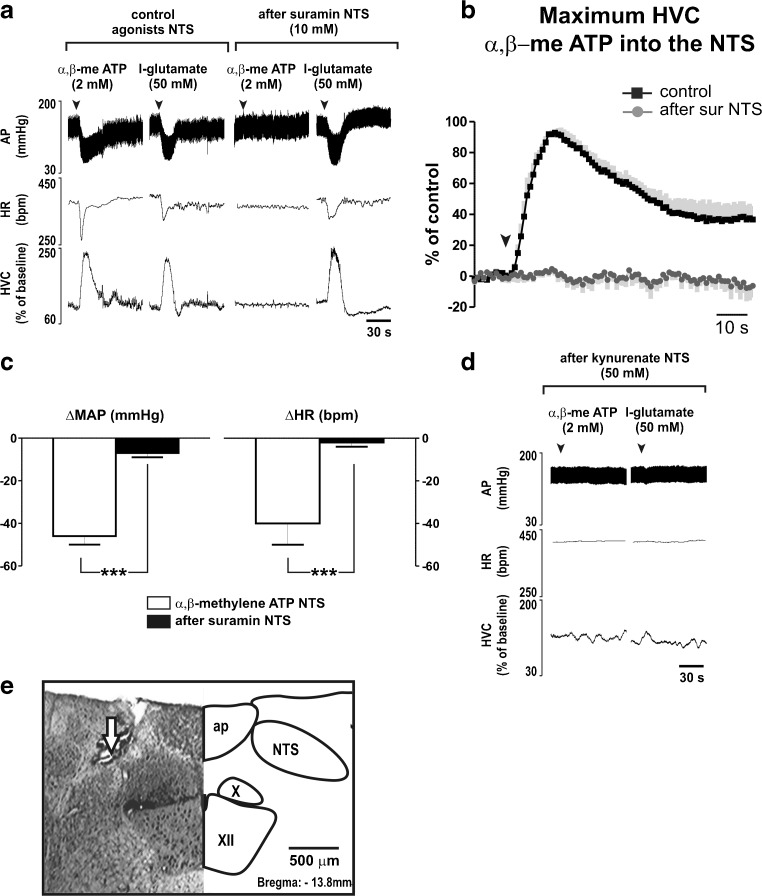
The efficiency of blockade of P2 and glutamate receptors in NTS neurons (Fig. 4e) was tested by microinjections of the respective agonists: α,β-methylene ATP (2 mM) and l-glutamate (50 mM). Bilateral microinjections of suramin (10 mM; N = 5) into the NTS completely abolished the maximum evoked changes in AP (−46 ± 4 vs. −7 ± 2 mmHg; P < 0.001), HR (−40 ± 10 vs. −2 ± 2 bpm; P < 0.001) and HVC (100 % vs. 8 ± 2 %; P < 0.001) caused by injection of α,β-methylene ATP (Fig. 4a–c). Cardiovascular responses to l-glutamate into the NTS, however, were unaffected (Fig. 4a). On the other hand, glutamate antagonism with kynurenate (50 mM) injected in the same sites abolished cardiovascular responses to local injection of either α,β-methylene ATP or l-glutamate (Fig. 4d). Responses in AP, HR, and HVC to α,β-methylene ATP into the NTS were recovered 90 min after microinjections of suramin.
Fig. 4.
Selectivity of suramin into the NTS in response of α,β-methylene ATP and glutamate. a Representative cardiovascular recordings of one animal in response to microinjections of α,β-methylene ATP (α,β-me ATP; 2 mM) or l-glutamate (50 mM) in the nucleus tractus solitarii (NTS) before and after bilateral microinjections of suramin (10 mM) into the NTS. b Grouped responses in HVC induced by microinjection of α,β-me ATP into the NTS before and after blockade of P2 receptors. Black squares, averaged curve during control; dark graycircles, averaged curve after blockade; in light gray, standard error of the mean. c Grouped data of decreases in mean arterial pressure (MAP) and heart rate (HR) elicited by microinjection of α,β-methylene ATP into the NTS before and after microinjections of suramin (10 mM) into the NTS. d Representative cardiovascular recordings of one animal in response to microinjections of α,β-methylene ATP (α,β-me ATP; 2 mM) or l-glutamate (50 mM) in the nucleus tractus solitarii (NTS) after bilateral microinjections of kynurenate (50 mM) into the NTS. e Photomicrograph of a coronal bulbar section representative of the group, confirming injection site into the NTS. ap area postrema; NTS nucleus tractus solitarii; X dorsal motor vagus nuclei; XII nucleus hypoglossus. ***P < 0.001 difference to control
Hindlimb vasodilation to activation of HDA or NTS neurons is relayed in the RVLM
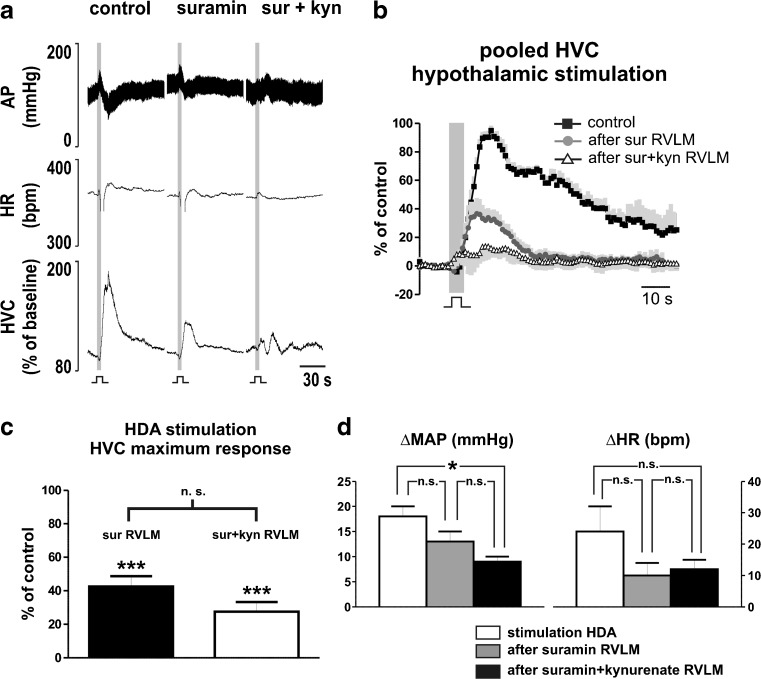
Bilateral microinjections of suramin into the RVLM reduced the response in HVC to stimulation of the HDA (100 % vs. 43 ± 7 %; P < 0.001; N = 4; Fig. 5a–c). Increases in AP (18 ± 2 vs. 13 ± 2 mmHg; P > 0.05) and HR (24 ± 8 vs. 10 ± 4 bpm; P > 0.05) evoked by hypothalamic stimulation were not affected by injection of suramin into the NTS (Fig. 5d). The combined antagonism of P2 and glutamate receptors in the RVLM with suramin (10 mM) and kynurenate (50 mM) produced no significant further reduction in the HVC response (28 ± 6 %) to HDA stimulation in comparison to blockade with suramin only (P > 0.05; Fig. 5a–c). In contrast, the combined blockade of P2 and glutamate receptors in the RVLM reduced the increase in AP (18 ± 2 vs. 9 ± 1 mmHg; P < 0.05), but not in HR (24 ± 8 vs. 12 ± 3 bpm; P > 0.05), elicited by hypothalamic stimulation (Fig. 5d). Baseline levels of AP, HR, and HVC remained unaffected after bilateral microinjections of suramin, but HR baseline was reduced (364 ± 13 vs. 299 ± 5 bpm; P < 0.05) after the combined microinjections of suramin and kynurenate (Table 1).
Fig. 5.
Involvement of P2 and glutamate receptors in RVLM neurons on cardiovascular changes to HDA stimulation. a Representative recording of one animal in response to stimulation of the hypothalamic defense area (HDA; 150–200 μA, 0.6 ms, 100 Hz for 5 s) before and after microinjections of suramin (10 mM) and combined blockade of suramin (10 mM) and kynurenate (50 mM) in the rostral ventrolateral medulla (RVLM). b Avereged curve of HVC responses produced by stimulation of the HDA during control, after antagonism of P2 receptors (sur) and after combined blockade of P2 and glutamate receptors (sur + kyn) in the RVLM. Black squares, averaged curve during control; dark graycircles, averaged curve after first blockade; open triangles, averaged curve after combined blockade; in light gray, standard error of the mean. c Group data showing the maximum response generated by hypothalamic stimulation after antagonism of P2 (sur RVLM), and subsequent combined blockade of P2 and glutamate receptors (sur + kyn RVLM) receptors in the ventrolateral medulla. ***P < 0.001 difference to control; n.s., nonsignificant. d Group data of increases in mean arterial pressure (MAP) and heart rate (HR) elicited by HDA stimulation before and after microinjections of suramin (10 mM), and suramin (10 mM) + kynurenate (50 mM) into the RVLM. *P < 0.05; ***P < 0.001; n.s. non-significant
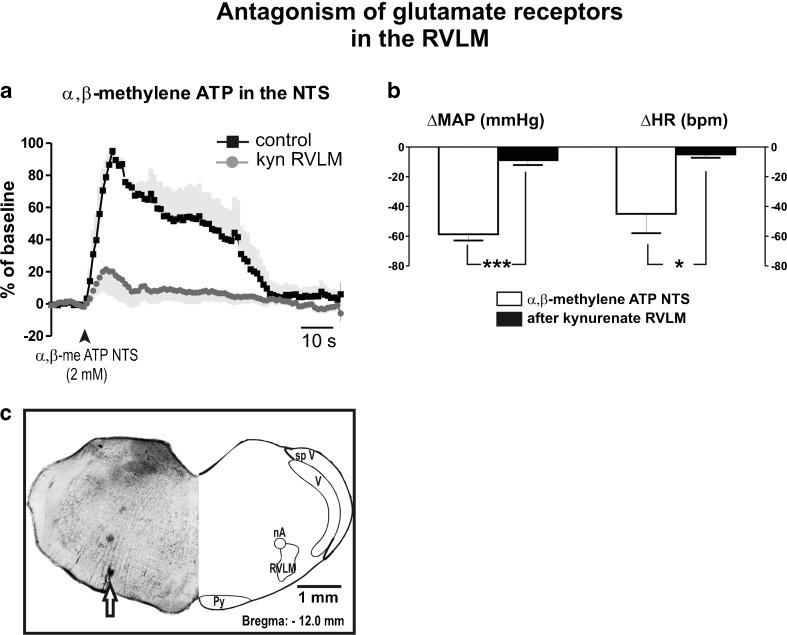
Unilateral microinjections of α,β-methylene ATP (2 mM) into the NTS increased HVC that was partially reduced (100 % vs 33 ± 14 %; P < 0.01; N = 6) after bilateral microinjections of kynurenate (50 mM) into the RVLM (Fig. 6a). Antagonism of glutamate receptors in the RVLM also reduced responses in AP (−59 ± 4 vs −9 ± 3 mmHg; P < 0.001) and HR (−45 ± 12 vs −5 ± 2 bpm; P < 0.05), elicited by microinjection of α,β-methylene ATP into the NTS (Fig. 6b). All cardiovascular responses to activation of P2 receptors in NTS neurons were recovered 90 min after kynurenate microinjections.
Fig. 6.
Glutamate transmission in the RVLM mediates cardiovascular responses to microinjection of α,β-methylene ATP into the NTS. a Averaged curve of responses in hindlimb vascular conductance (HVC) induced by microinjection of α,β-methylene ATP (α,β-me ATP; 2 mM) in the nucleus tractus solitarii (NTS) before and after blockade of glutamate receptors in the rostral ventrolateral medulla (RVLM) with kynurenate (50 mM). Black squares, averaged curve during control; in dark graycircles, averaged curve after blockade; in light gray, standard error of the mean. b Pooled changes in arterial blood pressure and heart rate evoked by α,β-me ATP into the NTS before (white) and after (black) microinjections of kynurenate into the RVLM. c Photomicrograph of a coronal bulbar section representative of the group, showing verification of the injection site into the RVLM. nA nucleus ambiguus; Py pyramidal tract; V trigeminal nucleus; sp V spinal trigeminal tract; RVLM rostral ventrolateral medulla
P2 receptors in the NTS mediate hindlimb vasodilation to activation of HDA independent from changes in the sympathetic vasoconstrictor tone
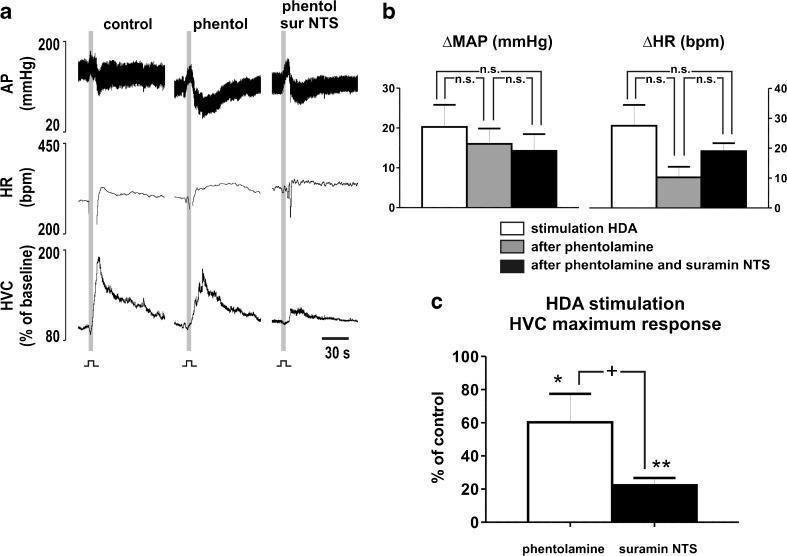
Intravenous infusion of phentolamine (1 mg/kg) reduced the baseline level of AP (115 ± 5 vs. 84 ± 3 mmHg; N = 4; P < 0.05), but the resting levels of HR (380 ± 11vs. 391 ± 39 bpm) and HVC (72 ± 18 vs. 61 ± 9 μl*mmHg−1*min−1) remained unchanged (P > 0.05). Following phentolamine, stimulation of HDA evoked responses similar to control (P > 0.05; Fig. 7a–b) on AP (20 ± 6 vs. 16 ± 4 mmHg) and HR (28 ± 7 vs. 10 ± 4), but the magnitude of response in HVC was reduced (100 % vs. 60 ± 17 %; P > 0.05; Fig. 7a, c). Subsequent bilateral microinjections of suramin into the NTS produced no further changes on cardiovascular baseline levels (AP, 87 ± 9 mmHg, HR, 374 ± 37 bpm, HVC, 101 ± 27 μl*mmHg−1*min−1; P > 0.05). The evoked rises in AP (16 ± 4 vs. 14 ± 4 mmHg) and HR (10 ± 4 vs. 19 ± 3 bpm) to HDA stimulation also remained unchanged (P > 0.05; Fig. 7b). In contrast, the evoked increase in HVC suffered an additional reduction (60 ± 17 % vs. 22 ± 4 %; P < 0.05) after suramin into the NTS (Fig. 7a).
Fig. 7.
P2 receptors in NTS neurons mediating sympathetic withdrawal and adrenaline release in response to HDA stimulation. a Representative recording of one animal in response to stimulation of the hypothalamic defense area (HDA; 150–200 μA, 0.6 ms, 100 Hz for 5 s) before and after intravenous infusion of phentolamine (1 mg/kg), and after following microinjections of suramin (10 mM) into the nucleus tractus solitarii (NTS). AP arterial blood pressure; HR heart rate; HVC hindlimb vascular conductance. b Grouped data of increases elicited in mean arterial pressure (MAP) and HR by hypothalamic stimulation before and after intravenous infusion of phentolamine, and after following microinjections of suram ininto the NTS. c Maximum responses in HVC generated by hypothalamic stimulation after intravenous infusion of phentolamine, and after following microinjections of suramin into the NTS. n.s. non-significant; *P < 0.05 to control; **P < 0.01 to control; +P < 0.05 to phenolamine
Discussion and Conclusions
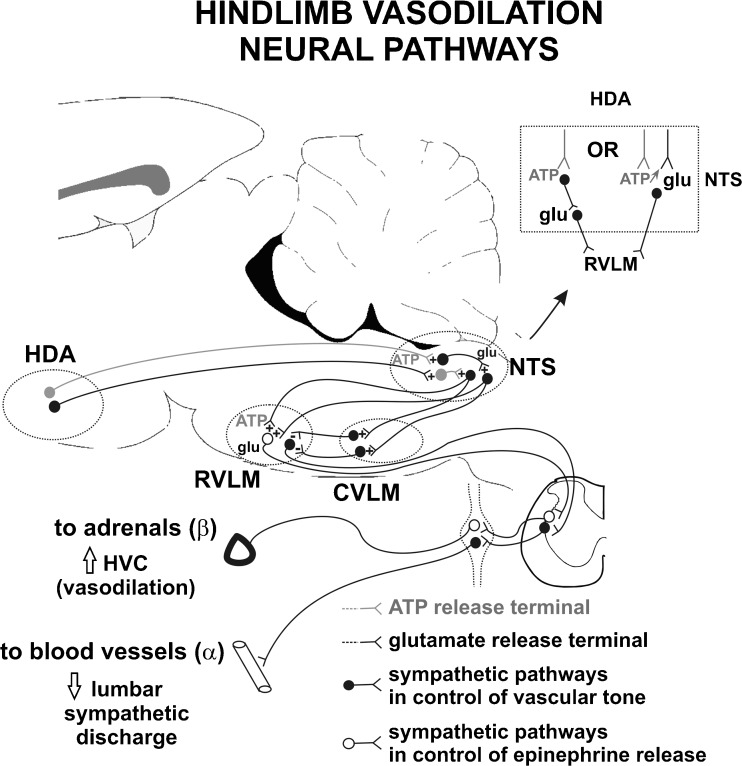
The principal findings of this study are as follows: first, an increase in the HVC of anesthetized rats in response to hypothalamic activation is mediated by release of ATP and glutamate in the NTS. Secondly, combined blockade of glutamate and P2 receptors in the RVLM reduced the evoked increase in HVC, similarly to the reduction observed after the combined blockade of NTS neurons. Thirdly, increases in HVC elicited by excitation of P2 receptors in NTS neurons, with microinjection of α,β-methylene ATP, was abolished after blockade of glutamate synapses in the RVLM. Fourthly, the reduction observed in the HVC response to stimulation of the HDA following antagonism of P2 receptors in the NTS was independent of the postganglionic activity to blood vessels. The vasodilation to the hindlimb elicited by activation of the hypothalamic defense area seems to involve ATP release in projections to the NTS, and ATP-glutamate by NTS neurons projecting to the RVLM. The purinergic transmission is likely related to networks in control of catecholamine release by the adrenal gland in alerting defense responses (Fig. 8).
Fig. 8.
Scheme of neural purinergic and glutamatergic pathways involved in the vasodilation of the hindlimb in alerting defense reactions. Summary of descending projections from the hypothalamic defense area (HDA) to the brainstem and neuromodulators involved in the control of cardiovascular responses. Alerting-defense reactions increase the ATP release in the NTS that activates parallel pathways, relayed in the RVLM, involved in the withdrawal of sympathetic tone to blood vessels and epinephrine release by the adrenal gland
All experiments in this study were conducted using single barreled pipettes. Although the injection sites were confirmed by histology, the interpretation of the data should consider the limits imposed by the use of single barreled pipettes. Nevertheless, the injection of the same volume of dye has been used extensively and is considered a reliable marker of the location of the drug injection. Due to the density of the extracellular milieu, viscosity of the fluid, and microinjection volume, we suggested that neurons located approximately 400 μm from the center of injections were affected.[30]. It is conceivable that in experiments where various microinjections were aimed at the same site, all drugs were injected within this 400 μm range affecting the similar groups of neurons.
Hindlimb vasodilation in alerting-defense responses is mediated by ATP in the NTS
In this study, activation of neurons in the HDA elicited hindlimb vasodilation associated with rapid rise in AP and HR. The cardiovascular responses to HDA stimulation were similar to previous descriptions by Yardley and Hilton [5, 6]. Blockade of P2 or glutamate receptors in the NTS greatly reduced the increase in HVC evoked by HDA stimulation. In rat, electrical stimulation of the HDA rapidly increases the concentration of adenosine in the NTS [20], which is likely to be occurring as a result of degradation of ATP [18]. However, it seems that the ATP released in the NTS following HDA stimulation additionally acts on local P2 receptors, either inducing a release of glutamate by acting on receptors in the presynaptic terminals [22, 23] or being co-released with glutamate in the presynaptic membrane [31–34]. Interestingly, both antagonists suramin and kynurenate injected in to the NTS did not reduce the responses in AP and HR evoked by HDA stimulation. A possible explanation is that the increase in AP was presumably due to vasoconstriction in mesenteric arteries [4, 13] but also partly in consequence of a rise in cardiac output (tachycardia). The increase in AP baseline following suramin or kynurenate microinjected into the NTS was similar to reports in previous studies [35–38]. Although the cardiovascular changes reverted to resting values prior to injections after ∼20–30 min, the receptors remained blocked by the antagonists that were tested by respective agonists.
Bilateral microinjections of suramin into the NTS completely blocked the cardiovascular responses to microinjection of α,β-methylene ATP into the same site. Increases in HVC to pharmacological stimulation of P2 receptors in the NTS were associated with transitory hypotension and bradycardia [16, 39], in opposition to rises in AP and HR in response to stimulation of the HDA. This paradox is possibly explained on the basis of differential mechanisms in control of HVC independent from regulation of HR and AP. First, there is anatomical evidence for direct projections from the hypothalamus to sympathetic premotor neurons in the spinal cord that bypass the brainstem [40]. Secondly, multiple direct pathways connect the HDA to the NTS. For instance, in cat, stimulation of the HDA elicits monosynaptic excitatory [11] and inhibitory [10] postsynaptic potentials in NTS neurons that receive inputs from baroreceptors. Thirdly, HDA stimulation elicits rapid adenosine release in the NTS derived from extracellular ATP [20], presumably eliciting a β-adrenergic iliac vasodilation that is overridden by a vasopressinergic vasoconstriction [41, 42]. Nonetheless, subunits of P2 receptors are expressed in NTS neurons of rat [14, 15]; and the reductions in HVC responses seen here can be explained by blockade of input signals from the HDA to the NTS [7, 8], independent of the pathways in control of AP and HR increases.
Although in high doses suramin may cause unspecific blockade of glutamate receptors [17, 29], pharmacological controls in the current study show that the dose of suramin exerted specific antagonism of P2 receptors. Microinjections of kynurenate into the NTS blocked the cardiovascular changes elicited by either α,β-methylene ATP or glutamate, but suramin selectively blocked the cardiovascular effects to α,β-methylene ATP into the NTS. In fact, cardiovascular sympathetic responses to activation of P2 receptors in NTS neurons apparently depend on glutamatergic mechanisms within the NTS [21]. The blockade of glutamate receptors in NTS neurons presumably reduced the sympathoinhibition in response to activation of P2x receptors in the NTS by α,β-methylene ATP [21]. Microinjections of kynurenate into the NTS following suramin, on the other hand, did not cause further reduction in the evoked vasodilation. It may be suggested that increase of ATP in the NTS, in response to activation of neurons in the HDA, activate subsequent sympathetic pathways by glutamate release (Fig. 8).
Cardiovascular effects to activation of P2 receptors in the NTS are relayed in the RVLM
Combined blockade of P2 and glutamate receptors in the RVLM reduced the increases in AP and HVC elicited by HDA stimulation. This finding differs from a previous study from our group [13] in which bilateral microinjections of kynurenate blocked only the increase in HVC. The reduction in the magnitude of the pressor responses to HDA stimulation after kynurenate and suramin in the RVLM is possibly due to ATP acting in cotrasmission with a different neuromodulator rather than glutamate. For instance, ATP can be co-released with acetylcholine, noradrenaline, 5-hyroxytryptamine, and dopamine in the central nervous system [31, 32]. The fact that antagonism of P2 and glutamate receptors in the RVLM reduces the evoked increase in HVC and AP suggests involvement of sympathetic premotor neurons in the RVLM in control of blood flow to the hindlimb of rats. Activation of hypothalamic neurons increases the firing rate of RVLM sympathetic premotor neurons [12], represented by the rise in AP seen in this study. The reduction in HVC and AP responses to HDA stimulation by combined microinjections of suramin and kynurenate into the RVLM possibly results from blockade of excitatory projections to sympathetic premotor neurons. The antagonism of P2 [17] and glutamate [43] receptors supposedly blocks excitatory inputs to RVLM sympathetic premotor cells. In cat, there is evidence of neuron subpopulations of RVLM controlling different vascular beds that are topographically distributed [44]. It can be speculated that kynurenate and suramin exerted a decrease in the activity of RVLM sympathetic premotor neurons, elicited by HDA stimulation; presumably reducing the excitation of sympathetic premotor neurons in control of catecholamine release and in control of blood vessels.
Bilateral microinjections of kynurenate in the RVLM also reduced the increase in HVC in response to microinjections of α,β-methylene ATP into the NTS. By contrast, it is known that the increase in HVC following activation of P2 receptors in NTS neurons supposedly results from a reduction in sympathetic nerve activity [21, 45]. This discrepancy can be explained partly by excitatory projections from the NTS to inhibitory interneurons in the RVLM. Direct excitatory projections from NTS neurons to the RVLM exist [24, 46], and many of these NTS neurons are positive for immunoreactions to VGluT1-3 [47, 48]. Inhibitory neurons projecting to RVLM cells are located mostly in the caudal ventrolateral medulla [49] but are also found in rostral segments that include the RVLM [50]. Thus, we speculate that interneurons within the RVLM may inhibit the sympathoexcitatory neurons. Nevertheless, direct [24] or indirect [51] inhibitory projections from the NTS to the RVLM also exist. We believe that the remaining vasodilation after kynurenate into the RVLM, elicited by activation of NTS P2 receptors, results from activation of the mentioned pathways.
P2 receptors in the NTS mediate the hindlimb vasodilation to stimulation of the HDA by promoting withdrawal of sympathetic tone and release of catecholamine by the adrenal glands
Bilateral injections of suramin into the NTS elicited a noteworthy further reduction in the vasodilation to HDA stimulation after blockade of α adrenoceptors in the vasculature with phentolamine. We and others [26] have observed that microinjections of α,β-methylene ATP into the NTS produce transitory hypotension, bradycardia, and hindlimb vasodilation, presumably due to sympathoinhibition. The hindlimb vasodilation seen in response to stimulation of the HDA [6] apparently involve withdrawal of sympathetic activity to blood vessels, via norepinephrine acting in α adrenoceptors [52, 53] and epinephrine release by the adrenal glands acting on β2 receptors in blood vessels [5, 6]. The activation of P2 receptors in the NTS following stimulation of the HDA in this study seems to intermediate both the withdrawal in sympathetic tone and the release of epinephrine. Although it was previously shown that activation of A2a receptors induces release of catecholamines by the adrenal medulla [45, 54], we believe that P2 receptors also partly contribute to epinephrine release in response to activation of the HDA. The findings here differ from previous evidence of sympathoinhibition to the adrenal gland [21, 45]. The discrepancy can be explained by the fact that adrenal sympathetic nerve activity regulates secretion of catecholamines related both to cardiovascular control and glucose metabolism [55]. Thus, decrease in adrenal sympathetic nerve activity to stimulation of P2 receptors in the NTS may correspond to inhibition of neurons in control of blood sugar levels.
Due to the fact that withdrawal of sympathetic drive was blocked in our experiments by phentolamine, the additional decrease in the evoked HVC response was likely caused by a reduction in epinephrine secretion. Sympathetic premotor neurons in the RVLM are activated by hypothalamic stimulation [12] and differentially project to sympathetic preganglionic neurons [56], segregated in two subgroups according to control of epinephrine and norepinephrine secretion by cromaffin cells [55]. We propose that HDA-NTS-RVLM pathways are mediated by ATP/glutamate and act in control of hindlimb vasodilation by eliciting both epinephrine secretion and withdrawal of sympathetic tone (Fig. 8).
Acknowledgements
Willian Seiji Korim is supported by a Macquarie Research Excellence Scholarship and a Coordenadoria de Aperfeiçoamento em Pesquisa (CAPES-DS) Scholarship. Work in the Author's laboratories is supported by the National Health and Medical Research Council of Australia (457069, 457080 and 604002), Australian Research Council (DP110102110), the Garnett Passe and Rodney Williams Memorial Foundation, Macquarie University and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; #477832/2010-5).
References
- 1.Abrahams VC, Hilton SM, Malcolm JL. Sensory connexions to the hypothalamus and mid-brain, and their role in the reflex activation of the defence reaction. J Physiol (Lond) 1962;164:1–16. doi: 10.1113/jphysiol.1962.sp006998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic Press, Inc; 1998. [Google Scholar]
- 3.Coote JH, Hilton SM, Perez-Gonzalez JF. Inhibition of the baroreceptor reflex on stimulation in the brain stem defence centre. J Physiol (Lond) 1979;288:549–560. [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira-Neto ML, Possas OS, Lopes OU, Cravo SL. Evidence for a role of nitric oxide in hindlimb vasodilation induced by hypothalamic stimulation in anesthetized rats. An Acad Bras Cienc. 2005;77:245–257. doi: 10.1590/S0001-37652005000200005. [DOI] [PubMed] [Google Scholar]
- 5.Yardley CP, Hilton SM. The hypothalamic and brainstem areas from which the cardiovascular and behavioural components of the defence reaction are elicited in the rat. J Auton Nerv Syst. 1986;15:227–244. doi: 10.1016/0165-1838(86)90066-4. [DOI] [PubMed] [Google Scholar]
- 6.Yardley CP, Hilton SM. Vasodilatation in hind-limb skeletal muscle evoked as part of the defence reaction in the rat. J Auton Nerv Syst. 1987;19:127–136. doi: 10.1016/0165-1838(87)90006-3. [DOI] [PubMed] [Google Scholar]
- 7.Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area: I. Descending projections. J Comp Neurol. 1992;315:313–332. doi: 10.1002/cne.903150307. [DOI] [PubMed] [Google Scholar]
- 8.Ciriello J, McMurray JC, Babic T, Oliveira CVR. Collateral axonal projections from hypothalamic hypocretin neurons to cardiovascular sites in nucleus ambiguus and nucleus tractus solitarius. Brain Res. 2003;991:133–141. doi: 10.1016/j.brainres.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mifflin SW, Spyer KM, Withington-Wray DJ. Baroreceptor inputs to the nucleus tractus solitarius in the cat: modulation by the hypothalamus. J Physiol (Lond) 1988;399:369–387. doi: 10.1113/jphysiol.1988.sp017086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva-Carvalho L, Dawid-Milner MS, Spyer KM. The pattern of excitatory inputs to the nucleus tractus solitarii evoked on stimulation in the hypothalamic defence area in the cat. J Physiol (Lond) 1995;487:727–737. doi: 10.1113/jphysiol.1995.sp020913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res. 1985;56:359–369. doi: 10.1161/01.RES.56.3.359. [DOI] [PubMed] [Google Scholar]
- 13.Cravo SL, Possas OS, Ferreira-Neto ML. Rostral ventrolateral medulla: an integrative site for muscle vasodilation during defense-alerting reactions. Cell Mol Neurobiol. 2003;23:579–595. doi: 10.1023/A:1025076130854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao ST, Barden JA, Lawrence AJ. On the immunohistochemical distribution of ionotropic P2X receptors in the nucleus tractus solitarius of the rat. Neuroscience. 2001;108:673–685. doi: 10.1016/S0306-4522(01)00438-9. [DOI] [PubMed] [Google Scholar]
- 15.Kanjhan R, Housley GD, Burton LD, Christie DL, Kippenberger A, et al. Distribution of the P2X2 receptor subunit of the ATP-gated ion channels in the rat central nervous system. J Comp Neurol. 1999;407:11–32. doi: 10.1002/(SICI)1096-9861(19990428)407:1<11::AID-CNE2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 16.Barraco RA, O'Leary DS, Ergene E, Scislo TJ. Activation of purinergic receptor subtypes in the nucleus tractus solitarius elicits specific regional vascular response patterns. J Auton Nerv Syst. 1996;59:113–124. doi: 10.1016/0165-1838(96)00014-8. [DOI] [PubMed] [Google Scholar]
- 17.Ralevic V, Thomas T, Burnstock G, Spyer KM. Characterization of P2 receptors modulating neural activity in rat rostral ventrolateral medulla. Neuroscience. 1999;94:867–878. doi: 10.1016/S0306-4522(99)00376-0. [DOI] [PubMed] [Google Scholar]
- 18.St Lambert JH, Thomas T, Burnstock G, Spyer KM. A source of adenosine involved in cardiovascular responses to defense area stimulation. Am J Physiol Regul Integr Comp Physiol. 1997;272:R195–R200. doi: 10.1152/ajpregu.1997.272.1.R195. [DOI] [PubMed] [Google Scholar]
- 19.Kato F, Shigetomi E. Distinct modulation of evoked and spontaneous EPSCs by purinoceptors in the nucleus tractus solitarii of the rat. J Physiol (Lond) 2001;530:469–486. doi: 10.1111/j.1469-7793.2001.0469k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dale N, Gourine AV, Llaudet E, Bulmer D, Thomas T, et al. Rapid adenosine release in the nucleus tractus solitarii during defense response in rats: real-time measurement in vivo. J Physiol (Lond) 2002;544:149–160. doi: 10.1113/jphysiol.2002.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scislo TJ, O'Leary DS. Differential role of ionotropic glutamatergic mechanisms in responses to NTS P(2x) and A(2a) receptor stimulation. Am J Physiol Heart Circ Physiol. 2000;278:H2057–H2068. doi: 10.1152/ajpheart.2000.278.6.H2057. [DOI] [PubMed] [Google Scholar]
- 22.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 23.Shigetomi E, Kato F. Action potential-independent release of glutamate by Ca2+ entry through presynaptic P2X receptors elicits postsynaptic firing in the brainstem autonomic network. J Neurosci. 2004;24:3125–3135. doi: 10.1523/JNEUROSCI.0090-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aicher SA, Saravay RH, Cravo S, Jeske I, Morrison SF, et al. Monosynaptic projections from the nucleus tractus solitarii to C1 adrenergic neurons in the rostral ventrolateral medulla: comparison with input from the caudal ventrolateral medulla. J Comp Neurol. 1996;373:62–75. doi: 10.1002/(SICI)1096-9861(19960909)373:1<62::AID-CNE6>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Welch WJ, Deng X, Snellen H, Wilcox CS. Validation of miniature ultrasonic transit-time flow probes for measurement of renal blood flow in rats. Am J Physiol. 1994;268:F175–F178. doi: 10.1152/ajprenal.1995.268.1.F175. [DOI] [PubMed] [Google Scholar]
- 26.Scislo TJ, Augustyniak RA, Barraco RA, Woodbury DJ, O'Leary DS. Activation of P(2x)-purinoceptors in the nucleus tractus solitarius elicits differential inhibition of lumbar and renal sympathetic nerve activity. J Auton Nerv Syst. 1997;62:103–110. doi: 10.1016/S0165-1838(96)00116-6. [DOI] [PubMed] [Google Scholar]
- 27.Ergene E, Dunbar JC, O'Leary DS, Barraco RA. Activation of P2-purinoceptors in the nucleus tractus solitarius mediate depressor responses. Neurosci Lett. 1994;174:188–192. doi: 10.1016/0304-3940(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 28.Nakazawa K, Inoue K, Ito K, Koizumi S. Inhibition by suramin and reactive blue 2 of GABA and glutamate receptor channels in rat hippocampal neurons. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:202–208. doi: 10.1007/BF00169334. [DOI] [PubMed] [Google Scholar]
- 29.Gu JG, Bardoni R, Magherini PC, MacDermott AB. Effects of the P2 purinoceptor antagonists suramin and pyridoxal- phosphate-6-azophenyl-2′,4′-disulfonic acid on glutamatergic synaptic transmission in rat dorsal horn neurons of the spinal cord. Neurosci Lett. 1998;253:167–170. doi: 10.1016/S0304-3940(98)00632-6. [DOI] [PubMed] [Google Scholar]
- 30.Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- 31.Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G. Purinergic cotransmission. Exp Physiol. 2009;94:20–24. doi: 10.1113/expphysiol.2008.043620. [DOI] [PubMed] [Google Scholar]
- 33.Illes P, Wirkner K, Nörenberg W, Masino SA, Dunwiddie TV. Interaction between the transmitters ATP and glutamate in the central nervous system. Drug Dev Res. 2001;52:76–82. doi: 10.1002/ddr.1100. [DOI] [Google Scholar]
- 34.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Leone C, Gordon FJ. Is l-glutamate a neurotransmitter of baroreceptor information in the nucleus of the tractus solitarius? J Pharmacol Exp Ther. 1989;250:953–962. [PubMed] [Google Scholar]
- 36.Pawloski-Dahm C, Gordon FJ. Evidence for a kynurenate-insensitive glutamate receptor in nucleus tractus solitarii. Am J Physiol Heart Circ Physiol. 1992;262:H1611–H1615. doi: 10.1152/ajpheart.1992.262.5.H1611. [DOI] [PubMed] [Google Scholar]
- 37.Scislo TJ, O'Leary DS. Mechanisms mediating regional sympathoactivatory responses to stimulation of NTS A(1) adenosine receptors. Am J Physiol Heart Circ Physiol. 2002;283:H1588–H1599. doi: 10.1152/ajpheart.00897.2001. [DOI] [PubMed] [Google Scholar]
- 38.Talman WT. Kynurenic acid microinjected into the nucleus tractus solitarius of rat blocks the arterial baroreflex but not responses to glutamate. Neurosci Lett. 1989;102:247–252. doi: 10.1016/0304-3940(89)90086-4. [DOI] [PubMed] [Google Scholar]
- 39.Kitchen AM, O'Leary DS, Scislo TJ. Sympathetic and parasympathetic component of bradycardia triggered by stimulation of NTS P2X receptors. Am J Physiol Heart Circ Physiol. 2006;290:H807–H812. doi: 10.1152/ajpheart.00889.2005. [DOI] [PubMed] [Google Scholar]
- 40.Saper CB, Loewy AD, Swanson LW, Cowan WM. Direct hypothalamo-autonomic connections. Brain Res. 1976;117:305–312. doi: 10.1016/0006-8993(76)90738-1. [DOI] [PubMed] [Google Scholar]
- 41.McClure JM, Rossi NF, Chen H, O'Leary DS, Scislo TJ. Vasopressin is a major vasoconstrictor involved in hindlimb vascular responses to stimulation of adenosine A(1) receptors in the nucleus of the solitary tract. Am J Physiol Heart Circ Physiol. 2009;297:H1661–H1672. doi: 10.1152/ajpheart.00432.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McClure JM, O'Leary DS, Scislo TJ. Neural and humoral control of regional vascular beds via A1 adenosine receptors located in the nucleus tractus solitarii. Am J Physiol Regul Integr Comp Physiol. 2011;300:R744–R755. doi: 10.1152/ajpregu.00565.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun MK, Hackett JT, Guyenet PG. Sympathoexcitatory neurons of rostral ventrolateral medulla exhibit pacemaker properties in the presence of a glutamate-receptor antagonist. Brain Res. 1988;438:23–40. doi: 10.1016/0006-8993(88)91320-0. [DOI] [PubMed] [Google Scholar]
- 44.Dampney RA, McAllen RM. Differential control of sympathetic fibres supplying hindlimb skin and muscle by subretrofacial neurones in the cat. J Physiol (Lond) 1988;395:41–56. doi: 10.1113/jphysiol.1988.sp016907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scislo TJ, O'Leary DS. Differential control of renal vs. adrenal sympathetic nerve activity by NTS A(2a) and P(2x) purinoceptors. Am J Physiol Heart Circ Physiol. 1998;275:H2130–H2139. doi: 10.1152/ajpheart.1998.275.6.H2130. [DOI] [PubMed] [Google Scholar]
- 46.Somogyi P, Minson JB, Morilak D, Llewellyn-Smith I, McIlhinney JR, et al. Evidence for an excitatory amino acid pathway in the brainstem and for its involvement in cardiovascular control. Brain Res. 1989;496:401–407. doi: 10.1016/0006-8993(89)91097-4. [DOI] [PubMed] [Google Scholar]
- 47.Lin LH, Edwards RH, Fremeau RTJ, Fujiyama F, Kaneko T, et al. Localization of vesicular glutamate transporters and neuronal nitric oxide synthase in rat nucleus tractus solitarii. Neuroscience. 2004;123:247–255. doi: 10.1016/j.neuroscience.2003.08.063. [DOI] [PubMed] [Google Scholar]
- 48.Lin LH, Talman WT. Nitroxidergic neurons in rat nucleus tractus solitarii express vesicular glutamate transporter 3. J Chem Neuroanat. 2005;29:179–191. doi: 10.1016/j.jchemneu.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Cravo SL, Morrison SF, Reis DJ. Differentiation of two cardiovascular regions within caudal ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1991;261:R985–R994. doi: 10.1152/ajpregu.1991.261.4.R985. [DOI] [PubMed] [Google Scholar]
- 50.Stornetta RL, McQuiston TJ, Guyenet PG. GABAergic and glycinergic presympathetic neurons of rat medulla oblongata identified by retrograde transport of pseudorabies virus and in situ hybridization. J Comp Neurol. 2004;479:257–270. doi: 10.1002/cne.20332. [DOI] [PubMed] [Google Scholar]
- 51.Aicher SA, Kurucz OS, Reis DJ, Milner TA. Nucleus tractus solitarius efferent terminals synapse on neurons in the caudal ventrolateral medulla that project to the rostral ventrolateral medulla. Brain Res. 1995;693:51–63. doi: 10.1016/0006-8993(95)00660-I. [DOI] [PubMed] [Google Scholar]
- 52.Hotta H, Nishijo K, Sato A, Sato Y, Tanzawa S. Stimulation of lumbar sympathetic trunk produces vasoconstriction of the vasa nervorum in the sciatic nerve via α-adrenergic receptors in rats. Neurosci Lett. 1991;133:249–252. doi: 10.1016/0304-3940(91)90581-D. [DOI] [PubMed] [Google Scholar]
- 53.Vanhoutte PM, Verbeuren TJ, Webb RC. Local modulation of adrenergic neuroeffector interaction in the blood vessel well. Physiol Rev. 1981;61:151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]
- 54.Kitchen AM, Scislo TJ, O'Leary DS. NTS A2a purinoceptor activation elicits hindlimb vasodilation primarily via a β-adrenergic mechanism. Am J Physiol Heart Circ Physiol. 2000;278:H1775–H1782. doi: 10.1152/ajpheart.2000.278.6.H1775. [DOI] [PubMed] [Google Scholar]
- 55.Morrison SF, Cao WH. Different adrenal sympathetic preganglionic neurons regulate epinephrine and norepinephrine secretion. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1763–R1775. doi: 10.1152/ajpregu.2000.279.5.R1763. [DOI] [PubMed] [Google Scholar]
- 56.Strack AM, Sawyer WB, Platt KB, Loewy AD. CNS cell groups regulating the sympathetic outflow to adrenal gland as revealed by transneuronal cell body labeling with pseudorabies virus. Brain Res. 1989;491:274–296. doi: 10.1016/0006-8993(89)90063-2. [DOI] [PubMed] [Google Scholar]