TEXT
How do changes in the environment trigger selective internalization of membrane proteins? This question is of broad biological importance since efficient endocytosis impacts many facets of cell physiology. In this issue of Molecular and Cellular Biology, Merhi and André (11) provide a compelling answer. They define a molecular pathway that leads from nutrient uptake through intracellular signaling to internalization of a specific nutrient permease. Their findings elegantly connect a cast of regulatory characters, including the nutrient-regulated TORC1 complex, a protein kinase (Npr1) that is a TORC1 target, and the 14-3-3 phosphoserine-binding proteins (11). Importantly, they demonstrate that two members of the α-arrestin or arrestin-related trafficking adaptor (ART) family, Bul1 and Bul2, serve as linchpins connecting nutrient signaling to protein trafficking (11). The pathway that they describe bears striking similarity to the recently reported control of two other α-arrestins (1, 10), suggesting that a conserved mechanism regulates arrestin-mediated trafficking.
A Gap in our knowledge.
In Saccharomyces cerevisiae, the general amino acid permease Gap1 is a nonspecific transporter of all l-amino acids and many amino acid analogs (5). Since studies of Gap1 began over 40 years ago in the Grenson lab (5), the trafficking of Gap1 has served as a model to identify basic features of signal-induced endocytosis. Subsequent work from many groups, notably the Kaiser and André labs, has demonstrated that Rsp5, now known to be a protein-ubiquitin ligase (the mammalian ortholog is Nedd4) (14), and Npr1, now known to be a protein kinase (as reviewed in reference 9), antagonistically control Gap1 delivery to the plasma membrane in response to changes in the available nitrogen source (6, 17). When cells are grown on a nonpreferred (or poor) nitrogen source, like proline, Gap1 localizes to the plasma membrane, where its ability to transport a broad spectrum of amino acids assists in nitrogen scavenging. Under these conditions, Gap1 is stabilized at the cell surface by active Npr1 kinase (3, 16). Npr1 is negatively regulated by TORC1, which is active when amino acids are abundant (9, 15). Conversely, growth on a preferred (or good) nitrogen source like ammonium promotes Rsp5-mediated ubiquitylation of Gap1 and stimulates its endocytosis (17). Like many membrane proteins, Gap1 lacks the sequence motifs (consensus PPXY or LPXY [14]) needed to bind Rsp5 directly. Instead, Bul1 and Bul2, which have these motifs, likely recruit Rsp5 to stimulate Gap1 ubiquitylation and internalization (6, 13, 17). However, a detailed molecular mechanism connecting nutrient supply to Gap1 trafficking remained elusive. It was not clear how nitrogen quality regulated Gap1 localization or what controlled Bul-mediated ubiquitylation of Gap1.
Ammonium ion uptake leads the way.
In this work, Merhi and André (11) make extensive use of the well-established nitrogen regulation of Gap1 trafficking: they grow cells on a nonpreferred nitrogen source (proline), where Gap1 is localized to the plasma membrane, and then add NH4+, a preferred nitrogen source, which induces Gap1 ubiquitylation and internalization. They demonstrate, first, that NH4+-induced Gap1 ubiquitylation and endocytosis require uptake through the ammonium ion permeases Mep1, Mep2, and Mep3 and production of glutamate, mainly by glutamate dehydrogenase Gdh1 during growth on glucose (Gdh3 does so under nonfermentative conditions). Although Gdh2 is thought to have mainly a catabolic role (converting glutamate to α-ketoglutarate), Mehri and André found that when NH4+ is plentiful, it can also contribute to glutamate synthesis (11). Glutamate, in turn, is the nitrogen donor for synthesis of many amino acids. Thus, the authors hypothesize that when NH4+ is added to proline-grown cells, it may increase amino acid levels and activate TORC1, which promotes robust growth and proliferation under nutrient-replete conditions. How intracellular amino acids activate TORC1 is not fully understood. Recent work demonstrates that leucine bound to its leucyl-tRNA synthetase (LeuRS) interacts with the Rag GTPase in the yeast EGO complex, which in turn activates TORC1 (2). Perhaps, addition of NH4+ and its conversion to glutamate increase leucine levels and/or or other amino acid levels to stimulate TORC1 via a similar mechanism, but this remains to be determined. Active TORC1 destabilizes Gap1 by stimulating endocytosis of the existing permease (as reviewed in reference 9); TORC1 inhibits Npr1 in a switch-like manner by directly phosphorylating negative regulatory sites in Npr1 and concomitantly preventing dephosphorylation of those sites by sequestering the phosphatase needed to dephosphorylate Npr1, Sit4 (as reviewed in reference 9). It was known that loss of Npr1 function causes enhanced ubiquitylation and internalization of Gap1 (3, 16). Here, the authors show that Npr1 remains dephosphorylated, even after NH4+ addition, if (i) TORC1 is pharmacologically inhibited with rapamycin or (ii) NH4+ uptake is prevented (in mep1Δ mep2Δ mep3Δ triple mutant cells) (11). Thus, the authors begin to reveal Npr1 regulation: NH4+ internalization and conversion to glutamate and perhaps other amino acids activate TORC1 through an undefined mechanism (which may be similar to the tRNA-synthetase/EGO activation pathway [2]), and this in turn inhibits Npr1 and promotes Gap1 internalization (Fig. 1). It will be interesting to see in future studies if components of this same signaling pathway are important for Gap1 endocytosis when it is induced by amino acids transported by the permease itself. Since Gap1 is not a direct substrate of Npr1, the path between nutrient regulation of the Npr1 protein kinase and Gap1 sorting was still unclear.
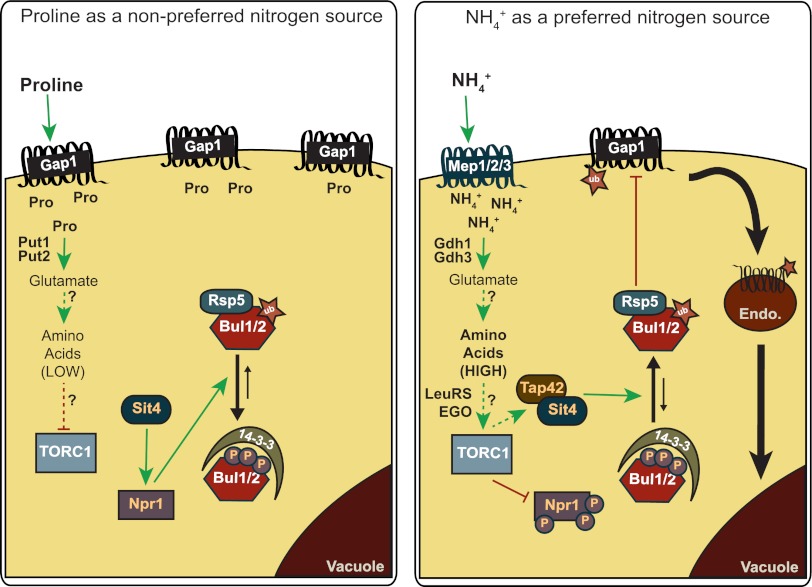
Fig 1.
Model of the molecular mechanism governing Bul-mediated trafficking of Gap1. In cells grown on a nonpreferred nitrogen source (left panel), like proline, Gap1 is stabilized at the plasma membrane where it scavenges nitrogen. Proline taken up through Gap1 is converted to glutamate by Put1 and Put2, the proline oxidase and pyrroline carboxylate dehydrogenase found in the mitochondria. However, proline-to-glutamate conversion is not efficient and may result in a smaller intracellular pool of glutamate and other amino acids that is unable to robustly activate TORC1. Gap1 stabilization at the cell surface is due to TORC1 inhibition. Npr1 becomes an active kinase as it is no longer phosphorylated in a TORC1-dependent manner and is dephosphorylated in a Sit4-dependent manner. It should be noted that Sit4 phosphatase does not associate with Tap42 when TORC1 is inhibited, altering Sit4 substrate specificity; the Bul proteins are not dephosphorylated. These conditions lead to Npr1-dependent phosphorylation of Bul1 and Bul2, which promotes Bul association with 14-3-3 proteins and prevents Bul-mediated ubiquitylation and endocytosis of Gap1. In cells where the preferred nitrogen source ammonium (NH4+) is added to proline-grown cells (right panel), the ammonium permeases (Mep1, -2, and -3) are needed for Gap1 endocytosis. Internalized ammonium is converted to glutamate by condensation with α-ketoglutarate, which is catalyzed by the glutamate dehydrogenases Gdh1 and Gdh3 (the latter used in the presence of a nonfermentable carbon source). Though it is typically associated with catabolism, Merhi and André suggest that Gdh2 may also synthesize glutamate. Glutamate is a nitrogen donor for the biosynthesis of many amino acids, and rapid addition of ammonium to proline-grown cells may increase glutamate levels and subsequently increase the pool of other amino acids. Elevated intracellular amino acid levels activate TORC1; however, a precise mechanism for how each amino acid contributes to this remains to be defined. Leucine specifically has been shown to bind leucyl-tRNA synthetase (LeuRS), which activates the Rag GTPase in the EGO complex, to in turn activate TORC1. A similar mechanism may operate here as a result of increased leucine or other amino acids; however, this remains to be experimentally determined. Active TORC1 kinase phosphorylates and inhibits Npr1, so that it no longer maintains Bul1 and Bul2 in a phosphorylated state. Active Sit4 phosphatase further promotes Bul1 and Bul2 dephosphorylation. Association of Sit4 with Tap42 directs its substrate specificity and may promote its targeting to Bul1 and Bul2. Dephosphorylation of Buls prevents 14-3-3 binding and promotes their ubiquitylation. Dephosphorylated and ubiquitylated Buls promote Rsp5-mediated ubiquitylation of Gap1 at the cell surface, triggering Gap1 endocytosis. Internalized Gap1 is trafficked to and degraded in the vacuole (yeast lysosome equivalent). Green arrows indicate activation. Red T-bars indicate inhibition. Dashed lines indicate where direct regulation has not yet been demonstrated and suggest that additional regulators may be important. Question marks denote aspects of the model that require further experimental support. Thick black lines indicate protein trafficking. Thin black arrows indicate the phosphoswitch on Bul1 and Bul2. Phosphorylation is denoted by purple circles with “P” inside, and ubiquitylation is denoted by a star with “ub” inside.
Arrestin' developments.
The link between nutrient signal-regulated Npr1 and Gap1 trafficking is the α-arrestins. The authors show, first, that Bul1 and Bul2 are bona fide members of the α-arrestin family. Mutation of either the Bul1 Rsp5-binding PPXY motif or residues in its conserved arrestin domain prevents Bul1-mediated ubiquitylation and endocytosis of Gap1 (11). These findings are important: Bul1 and Bul2 were only recently recognized as bearing resemblance to other members of the α-arrestin family, and this work is the first demonstration that elements needed for the function of other α-arrestins are also critical for Bul function, solidifying their status as α-arrestins.
The authors then tie nitrogen regulation to Bul function by demonstrating that Bul1 and Bul2 are dephosphorylated, and concomitantly ubiquitylated by Rsp5, in response to NH4+ addition (11). Furthermore, in the absence of functional Npr1, Bul1 dephosphorylation and partial ubiquitylation cause sorting of Gap1 to the vacuole, even on a nonpreferred nitrogen source. Although the authors do not show direct Npr1-mediated phosphorylation of Bul1 in this study, other α-arrestins are established Npr1 substrates (10, 12). Thus, Npr1 may phosphorylate Buls directly. Consistent with this, Bul1 is shown here to undergo an Npr1-dependent phosphoswitch. In proline-grown cells where Npr1 is highly active, Bul1 phosphorylation, as judged by mobility shift, is observed and Bul1-dependent Gap1 endocytosis is impaired (11). When ammonium is added and Npr1 is inactive, Bul1 dephosphorylation occurs and Gap1 internalization is stimulated (Fig. 1). What, then, controls Bul1 dephosphorylation?
Interestingly, Merhi and André show that Bul1 dephosphorylation depends on the phosphatase Sit4 (11). As suggested by prior work and confirmed here, the absence of Sit4 results in hyperphosphorylated, constitutively inactive Npr1 (15). Thus, the authors expected to find that in the absence of Sit4, phosphoinhibition of Npr1 would result in Gap1 removal from the cell surface and its localization to the vacuole. Surprisingly, in cells lacking Sit4, Gap1 localizes to the plasma membrane, strong evidence that Sit4 plays an additional role in the Gap1 regulatory circuit (11). Indeed, in sit4Δ cells, Bul1 is no longer dephosphorylated when NH4+ is added, and Gap1 remains at the plasma membrane. Although not addressed in this work, one interpretation of these findings is that the Buls themselves are direct substrates of Sit4, and NH4+-induced and Sit4-dependent dephosphorylation of Buls is required for Bul-stimulated Gap1 ubiquitylation and endocytosis (Fig. 1). However, this model leaves Sit4 with two seemingly contradictory roles: (i) promoting Gap1 stability at the cell surface by dephosphorylating and activating Npr1 and (ii) promoting Gap1 endocytosis by dephosphorylating Bul1. This conundrum may be resolved by nitrogen-regulated association of Sit4 with proteins like Tap41 and Tap42 (9), which help target Sit4 to specific substrates. This interesting Sit4-regulatory dichotomy and assessment of direct regulation of Buls by Npr1 and/or Sit4 are worthy of future study.
14-3-3 proteins corral the Buls.
Merhi and André next sought to understand how phosphorylation inhibits Bul function. Since other yeast α-arrestins bind 14-3-3 proteins, Bmh1 and Bmh2, in a phosphorylation-dependent manner (7), they explored the possibility that 14-3-3 proteins might be important phosphodependent negative regulators of Bul function. Indeed, they show that in proline-grown cells, phosphorylated Bul1 copurifies Bmh2, whereas in NH4+-grown cells (where Buls are dephosphorylated) Bmh2 no longer associates with Bul1 (11). Although others have observed that S. cerevisiae cells lacking both Bmh1 and Bmh2 are inviable, Mehri and André were able to generate bmh1Δ bmh2Δ double mutants in their strain background. In their Bmh1- and Bmh2-deficient cells, Gap1 trafficked to the vacuole even when proline was the nitrogen source. Thus, Bmh1 and Bmh2 are negative regulators of Bul function (11). Presumably, Npr1 phosphorylation of the Buls allows 14-3-3 binding, which precludes Bul-mediated downregulation of Gap1. Conversely, dephosphorylation of Buls alleviates 14-3-3-mediated repression and allows the Buls to promote Gap1 ubiquitylation and endocytosis (Fig. 1).
Phosphoregulation of arrestins—an emerging theme.
Two other recent studies report a strikingly similar phosphoinhibition of α-arrestin-mediated endocytosis. Becuwe et al. (1) show that, in lactate-grown cells, phosphorylation of α-arrestin Rod1 (Art4) promotes 14-3-3 binding and prevents Rod1-dependent ubiquitylation and endocytosis of the lactic acid permease Jen1. Conversely, in the presence of glucose, Rod1 is dephosphorylated, no longer binds14-3-3 proteins, becomes ubiquitylated, and triggers Jen1 endocytosis. Although Rod1 interacts with the Rsp5 ubiquitin ligase under all conditions tested, Becuwe et al. propose that phosphorylation and 14-3-3 binding preclude Rsp5-mediated ubiquitylation of Rod1 and also presumably Jen1 (1). Thus, phosphorylation and 14-3-3 binding inhibit both Rod1- and Bul1-mediated endocytosis. However, a key difference between Bul1 and Rod1 is the link between α-arrestin dephosphorylation and ubiquitylation. Bul1 becomes ubiquitylated in response to a preferred nitrogen source even when its dephosphorylation is prevented (by loss of Sit4). Under these conditions, since Bul1 is still phosphorylated, 14-3-3 binding should be maintained, and therefore, 14-3-3 binding must not prevent Bul-mediated endocytosis by blocking Bul1 ubiquitylation. Thus, alternative roles for phosphorylation- and/or 14-3-3-mediated processes may be involved in controlling α-arrestin function.
In support of this, α-arrestin Ldb19 (Art1) is ubiquitylated even in the absence of cycloheximide, a stimulus known to prevent phosphorylation of Art1 and stimulate Art1-mediated endocytosis of the arginine permease Can1 (8, 10). Thus, ubiquitylation and phosphorylation appear to be uncoupled in Art1. A role for 14-3-3 proteins in regulating Art1-mediated endocytosis has not been explored; however, like Bul1 and Rod1, phosphorylation inhibits Art1-mediated endocytosis. Moreover, as with Bul1 and Bul2, nutrients, TORC1, and Npr1 all control Art1 phosphorylation (10). Npr1 phosphorylates Art1 in vitro, and Art1 phosphorylation inhibits its localization to the plasma membrane and its ability to internalize Can1 in vivo (10). The impact of phosphorylation on Bul1 and Bul2 localization has not yet been tested; however, it seems likely that phosphorylation and/or 14-3-3 binding may prevent Bul1 localization to the cell surface and inhibit its endocytic function.
Similar phosphoinhibition of the β-arrestins, dedicated adaptors involved in G-protein-coupled receptor internalization, has been observed. Dephosphorylation of mammalian or dipteran β-arrestins promotes their association with the plasma membrane, clathrin, and AP-2 and is important for receptor endocytosis (as reviewed in reference 4). Also, β-arrestins interact with 14-3-3 proteins, and this association is reduced upon receptor stimulation by agonist (18), concomitant with β-arrestin dephosphorylation. Thus, the paradigm that has emerged from this study for phosphoregulation of α-arrestins in yeast may be broadly applicable to both α- and β-arrestins across all eukaryotes.
ACKNOWLEDGMENTS
I am grateful to Alexander Sorkin, Adam Kwiatkowski, and Jeremy Thorner for critical reading of the manuscript and editorial suggestions.
My related research was supported by NIH R01 grants GM-48728 and GM-21841 awarded to Martha S. Cyert and Jeremy Thorner, respectively, and is now supported by developmental funds from the Department of Cell Biology (Pittsburgh).
Footnotes
Published ahead of print 1 October 2012
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1. Becuwe M, et al. 2012. A molecular switch on an arrestin-like protein relays glucose signaling to transporter endocytosis. J. Cell Biol. 196:247–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonfils G, et al. 2012. Leucyl-tRNA synthetase controls TORC1 via the EGO complex. Mol. Cell 46:105–110 [DOI] [PubMed] [Google Scholar]
- 3. De Craene JO, Soetens O, Andre B. 2001. The Npr1 kinase controls biosynthetic and endocytic sorting of the yeast Gap1 permease. J. Biol. Chem. 276:43939–43948 [DOI] [PubMed] [Google Scholar]
- 4. Delom F, Fessart D. 2011. Role of phosphorylation in the control of clathrin-mediated internalization of GPCR. Int. J. Cell Biol. 2011:246954 doi:10.1155/2011/246954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Grenson M, Hou C, Crabeel M. 1970. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J. Bacteriol. 103:770–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Helliwell SB, Losko S, Kaiser CA. 2001. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol. 153:649–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kakiuchi K, et al. 2007. Proteomic analysis of in vivo 14-3-3 interactions in the yeast Saccharomyces cerevisiae. Biochemistry 46:7781–7792 [DOI] [PubMed] [Google Scholar]
- 8. Lin CH, MacGurn JA, Chu T, Stefan CJ, Emr SD. 2008. Arrestin-related ubiquitin-ligase adaptors regulate endocytosis and protein turnover at the cell surface. Cell 135:714–725 [DOI] [PubMed] [Google Scholar]
- 9. Loewith R, Hall MN. 2011. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 189:1177–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. MacGurn JA, Hsu PC, Smolka MB, Emr SD. 2011. TORC1 regulates endocytosis via Npr1-mediated phosphoinhibition of a ubiquitin ligase adaptor. Cell 147:1104–1117 [DOI] [PubMed] [Google Scholar]
- 11. Merhi A, André B. 2012. Internal amino acids promote Gap1 permease ubiquitylation via TORC/Npr1/14-3-3-dependent control of the Bul arrestin-like adaptors. Mol. Cell. Biol. 32:4510–4522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O'Donnell AF, Apffel A, Gardner RG, Cyert MS. 2010. Alpha-arrestins Aly1 and Aly2 regulate intracellular trafficking in response to nutrient signaling. Mol. Biol. Cell 21:3552–3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Risinger AL, Kaiser CA. 2008. Different ubiquitin signals act at the Golgi and plasma membrane to direct GAP1 trafficking. Mol. Biol. Cell 19:2962–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rotin D, Kumar S. 2009. Physiological functions of the HECT family of ubiquitin ligases. Nat. Rev. Mol. Cell Biol. 10:398–409 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt A, Beck T, Koller A, Kunz J, Hall MN. 1998. The TOR nutrient signalling pathway phosphorylates NPR1 and inhibits turnover of the tryptophan permease. EMBO J. 17:6924–6931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Soetens O, De Craene JO, Andre B. 2001. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J. Biol. Chem. 276:43949–43957 [DOI] [PubMed] [Google Scholar]
- 17. Springael JY, Andre B. 1998. Nitrogen-regulated ubiquitination of the Gap1 permease of Saccharomyces cerevisiae. Mol. Biol. Cell 9:1253–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Xiao K, et al. 2007. Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc. Natl. Acad. Sci. U. S. A. 104:12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]