Abstract
Munc13s are presynaptic proteins that mediate synaptic vesicle priming and thereby control the size of the readily releasable pool of vesicles. During high synaptic activity, Munc13-1 and its closely related homolog, ubMunc13-2, bind Ca2+/calmodulin, resulting in enhanced priming activity and in changes of short-term synaptic plasticity characteristics. Here, we studied whether bMunc13-2 and Munc13-3, two remote isoforms of Munc13-1 with a neuronal subtype-specific expression pattern, mediate synaptic vesicle priming and regulate short-term synaptic plasticity in a Ca2+/calmodulin-dependent manner. We identified a single functional Ca2+/calmodulin binding site in these isoforms and provide structural evidence that all Munc13s employ a common mode of interaction with calmodulin despite the lack of sequence homology between their Ca2+/calmodulin binding sites. Electrophysiological analysis showed that, during high-frequency activity, Ca2+/calmodulin binding positively regulates the priming activity of bMunc13-2 and Munc13-3, resulting in an increase in the size of the readily releasable pool of vesicles and subsequently in strong short-term synaptic enhancement of neurotransmission. We conclude that Ca2+/calmodulin-dependent regulation of priming activity is structurally and functionally conserved in all Munc13 proteins, and that the composition of Munc13 isoforms in a neuron differentially controls its short-term synaptic plasticity characteristics.
INTRODUCTION
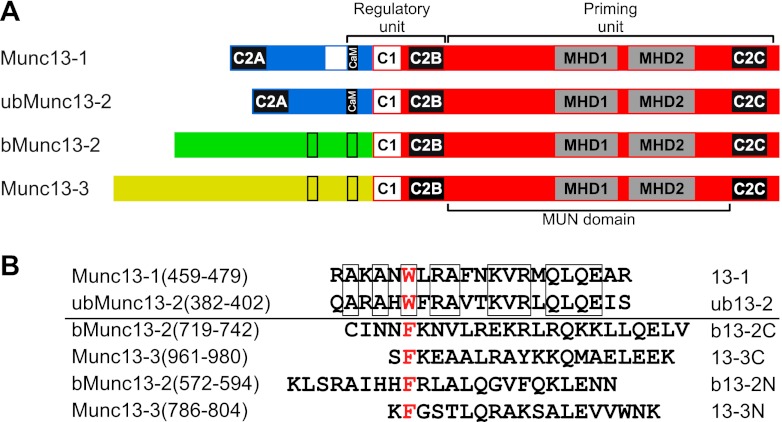
Munc13 proteins are key mediators of synaptic vesicle (SV) priming, an essential process in Ca2+-regulated neurotransmitter release that renders SVs fusion competent prior to exocytosis (38, 50, 54). Genetic ablation of Munc13 expression eliminates evoked and spontaneous release due to a complete loss of the readily releasable pool (RRP) of fusion-competent SVs, underscoring the indispensable role of Munc13s for neuronal function (4, 51). The mammalian genome encodes four major Munc13 proteins, Munc13-1, ubMunc13-2 (the ubiquitously expressed splice variant of Munc13-2), bMunc13-2 (the brain-specific splice variant of Munc13-2), and Munc13-3 (2, 7), which share the C-terminal MUN domain that is sufficient for priming function (5, 49). Modulation of priming activity occurs through an N-terminal regulatory unit comprising the Ca2+/phospholipid-binding C2B domain (47), a diacylglycerol-binding C1 domain (6, 39), and a Ca2+/calmodulin (CaM)-binding domain (22, 57) (Fig. 1A). Thus, Munc13 activity can be regulated by Ca2+ either directly via the C2B domain or indirectly via the C1 and the CaM-binding domain to control release efficacy and synaptic short-term plasticity (STP).
Fig 1.
CaM-binding sites of Munc13 proteins. (A) Domain structure of the Munc13 protein family. All major Munc13 proteins share the MUN domain within their highly homologous C-terminal part and the C2B and C1 domains within their N-terminal regulatory unit. Functional CaM-binding sites (filled bars) were so far only established in the closely related isoforms Munc13-1 and ubMunc13-2. Bioinformatic prediction revealed potential CaM recognition motifs in the heterologous N termini of bMunc13-2 and Munc13-3 (open bars). (B) Munc13-derived CaM-binding peptides. The localization within the respective Munc13 isoform, amino acid sequence, and acronym used is given. Note that bMunc13-2 and Munc13-3 contain two potential CaM recognition motifs, referred to as N and C terminal in our nomenclature. Only the peptides 13-1 and ub13-2 are highly homologous (57% identity; identical amino acids are boxed). The hydrophobic anchor residue of the diverse CaM-binding sequences (Trp-464 in Munc13-1, Trp-387 in ubMunc13-2, Phe-580 and Phe-723 in bMunc13-2, and Phe-787 and Phe-962 in Munc13-3; red) typically defines position 1 of the recognition motifs and is replaced by the photoreactive amino acid p-benzoyl-Phe (Bpa) to obtain the corresponding photoprobes.
STP plays a pivotal role in higher brain functions (13) and is thought to correlate with changes in the residual presynaptic Ca2+ concentration ([Ca2+]i). During and after repetitive activity, two forms of STP can be differentiated, namely, short-term depression (STD), where the neuron exhibits a gradual decrease in synaptic transmission, and short-term enhancement (STE), where an increase in synaptic transmission during activity is observed (58). Depending on the Munc13 isoform expressed, cultured hippocampal neurons respond to high-frequency stimulation with moderate STD (in the presence of Munc13-1) or STE (in the presence of ubMunc13-2) (22, 42, 47). As one important molecular link between [Ca2+]i signaling and STP phenomena, we have previously found that Munc13-1 and ubMunc13-2 bind Ca2+/CaM through an evolutionarily conserved CaM-binding site (22). Neurons expressing a CaM-insensitive Munc13-1 exhibit stronger STD during high-frequency action potential (AP) trains than neurons expressing wild-type Munc13-1. Remarkably, neurons expressing a CaM-insensitive ubMunc13-2 exhibit STD, whereas expression of wild-type ubMunc13-2 leads to STE during and after a high-frequency AP train (22).
While Munc13-1 is expressed throughout the rodent brain, Munc13-2 and Munc13-3 exhibit distinct and neuronal subtype-specific expression patterns and likely modulate neurotransmitter release in concert with Munc13-1 (2, 3, 10, 42). However, the priming function of bMunc13-2 and Munc13-3 has not yet been proven experimentally. Moreover, it is unknown whether the heterologous N termini of bMunc13-2 and Munc13-3 feature functional CaM-binding sites to enable Ca2+-dependent regulation of priming. The minimal CaM-binding sites of Munc13-1 and ubMunc13-2 comprise a highly homologous 21-amino-acid stretch, but due to the lack of sequence homology, we did not recognize potential CaM-binding sites in bMunc13-2 and Munc13-3 in our initial study (22). Later, we used bioinformatic tools based on biophysical and structural criteria to predict two nonconserved CaM recognition motifs in each of these isoforms and validated CaM binding of the four corresponding synthetic peptides (15). However, this finding posed further important questions as to (i) whether both potential CaM-binding sites in bMunc13-2 and in Munc13-3 bind CaM at the protein level, (ii) whether the nonconserved CaM recognition motifs found within the Munc13 family converge to a common binding mode, and (iii) whether Ca2+/CaM binding of bMunc13-2 and Munc13-3 plays a role in STP.
We now present biochemical data indicating that of the two potential Ca2+/CaM binding sites in bMunc13-2 and Munc13-3, only one is functional at the protein level. We used molecular modeling with distance constraints derived from cross-linking experiments to gain insights into the structure of Munc13 peptide-CaM complexes. Finally, we conducted a series of electrophysiological experiments to investigate the role of Ca2+/CaM binding of bMunc13-2 and Munc13-3 in neurotransmitter release and STP. We provide structural and functional evidence that all Munc13s differentially control STP through nonconserved Ca2+/CaM binding sites.
MATERIALS AND METHODS
Peptide synthesis and PAL.
Munc13-derived peptides and photoprobes were synthesized by using standard solid-phase fluorenylmethoxycarbonyl (Fmoc) chemistry and the photoreactive amino acid Bpa (Novabiochem) (20). Peptides with an N-terminal Cys (either originating from the native amino acid sequence or from an artificial elongation) were S-carboxamidomethylated with iodoacetamide prior to their use in cross-linking experiments when subsequent mapping of cross-linked sites was intended. PAL-based competition and Ca2+ titration experiments with recombinant CaM were performed as described previously, and photoadduct formation was monitored by SDS-PAGE and linear matrix-assisted laser desorption ionization–time-of-flight–mass spectrometry (MALDI-TOF-MS) (15).
Cloning and mutagenesis of Munc13 constructs.
bMunc13-2(366-780) and Munc13-3(711-1063) were amplified by PCR from the full-length bMunc13-2 and Munc13-3 clones (GenBank accession numbers U24071 and U75361 [7]) and cloned via EcoRI/XhoI into pGEX-4T-1 (GE Healthcare Life Sciences). Site-directed mutagenesis of these clones was performed with a QuikChange kit (Stratagene) under conditions suggested by the manufacturer, generating the following plasmids: pGEX-bMunc13-2(366-780)F580R, pGEX-bMunc13-2(366-780)F723R, pGEX-bMunc13-2(366-780)F580R/F723R, pGEX-bMunc13-2(366-780)F723R/K724E/R728E/R731E, pGEX-Munc13-3(711-1063)F787R, pGEX-Munc13-3(711-1063)F962R, and pGEX-Munc13-3(711-1063)F787R/F962R. To generate full-length C-terminally VENUS-tagged bMunc13-2 and Munc13-3, the VENUS cassette of pVENUS-VGLUT1 (described in reference 34; the authors also provided the plasmid) was excised using AgeI/NotI and ligated into the same sites in bMunc13-2-pEGFP-N1 or Munc13-3-pEGFP-N1 (6), replacing the enhanced green fluorescent protein (EGFP) cassette. Site-directed mutagenesis was applied to these constructs to generate bMunc13-2F723R/K724E/K728E/R731E-VENUS and Munc13-3F962R-VENUS. The resulting constructs and wild-type variants were subcloned via XhoI/NotI into the pCAGIG vector (Addgene plasmid 11159) (28) that carries an EGFP cassette downstream of an internal ribosomal entry site. All vectors were sequenced prior to use.
Cosedimentation assay.
Glutathione S-transferase (GST)-fused bMunc13-2 and Munc13-3 fragments were expressed in Escherichia coli BL21(DE3) by using the respective pGEX-4T1 plasmids in the GST Gene Fusion system (GE Healthcare Life Sciences). Cells were lysed by sonication, Triton X-100 (final concentration 0.1%) was added to the suspension, and proteins were solubilized by shaking for 20 min on ice. Cell debris was pelleted by ultracentrifugation (100,000 × g, 30 min, 4°C), and the supernatant was filtrated through a sterile 0.22-μm filter. Glutathione-Sepharose 4B beads were prepared according to the GST Gene Fusion system handbook and incubated overnight at 4°C with the filtrated supernatant to allow for binding of the GST fusion proteins. The beads were washed 4× with buffer A (20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM EDTA) containing protease inhibitors and dithiothreitol (DTT), equilibrated with either buffer E (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM EDTA) or buffer C (50 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM EDTA; 4 mM CaCl2), and incubated with 30 ng/μl of bovine brain CaM (Roche) in the respective buffers for 4 h at 4°C. The beads were washed three times with either buffer E or C, boiled for 3 min in SDS sample buffer, and analyzed by SDS-PAGE on precast NuPAGE 12% Bis-Tris gels (Invitrogen) using a morpholinepropanesulfonic acid (MOPS) buffer system. Proteins were visualized by colloidal Coomassie staining, and gels were documented with an Odyssey near-infrared fluorescence imager (LI-COR), allowing for quantitation of proteins on the basis of the Coomassie fluorescence in the 700-nm channel (26). The fluorescence signals from the bands corresponding to the GST-Munc13 fusion proteins and to the cosedimented CaM were quantified in two independent experiments and normalized to protein size. The resulting so-called intensity-per-kDa ratios were used for estimating the stoichiometry of the interactions.
CD spectroscopy.
Peptides were dissolved in phosphate buffer (100 mM K2HPO4-KH2PO4, pH 7.5) at a concentration range of 100 to 150 μM, and final concentrations were determined spectrophotometrically in the near-UV range. To estimate the absorption coefficient of peptide b13-2C-mut (no aromatic residue), absorption spectra of samples with known concentrations of b13-2C were recorded in the far-UV range and the absorption coefficient at 220 nm (per peptide bond) was calculated. Far-UV-circular dichroism (CD) measurements in the presence of increasing trifuoroethanol (TFE) concentrations were performed at 4°C in a 0.5-mm fused silica cuvette on a Jasco J-810 spectropolarimeter. Up to 30 single far-UV-CD spectra were accumulated from 200 to 260 nm with a scan rate of 20 nm/min, 4-s response, and bandwidth of 1 nm. The dynode voltage did not exceed ≈600 mV. No concentration dependence was observed in the presence of TFE, indicating that the Munc13 peptides did not form oligomers.
Mapping of cross-linked sites in Munc13 peptide-CaM complexes. (i) Photoaffinity labeling.
Labeling of CaM with Munc13 photoprobes and mapping of the photoadducts by MS was performed according to our recently introduced workflow based on isotopically labeled [15N]CaM (14). To identify and sequence cross-linked peptides, PAL reaction mixtures were subjected to in-solution digestion with trypsin and subsequently analyzed by offline liquid chromatography (LC)-MALDI-MS on a syringe pump-based capillary (300 μm inner diameter) LC system coupled to a Bruker Ultraflex MALDI-TOF/TOF mass spectrometer (14). To complement mass spectrometric sequencing, a ligand release assay by on-target CNBr cleavage was used for confirmation of cross-linked sites as described previously (14, 23). In this assay, photoincorporation into the Met side chain is indicated by a newly appearing signal corresponding to the methylthiocyanate derivative (mass shift of +73.00 mass units) of the respective benzophenone-containing tryptic peptide derived from the photoprobe.
(ii) Chemical cross-linking.
The cross-linking reactions with N-succinimidyl-p-benzoyldihydrocinnamate (SBC) were conducted in a two-step fashion (24). First, the amine-reactive site of SBC was allowed to react with CaM. For this, bovine brain CaM (Calbiochem) was diluted to 8.5 μM in 10 mM HEPES buffer (pH 7.2) containing a Ca2+/chelator (EGTA) system to adjust the free Ca2+ concentration to 30 nM. After incubation for 15 min at room temperature, a 20- or 50-fold molar excess of a freshly prepared solution of SBC in dimethylsulfoxide (DMSO) was added and the mixture was incubated for 30 min. Nonreacted SBC was quenched with ammonium bicarbonate (20 mM final concentration) and removed by microfiltration with YM-3 centrifugation units (Millipore). For the photoreaction, the respective Munc13 peptide (10 μM) was mixed with SBC-labeled CaM before irradiating the mixture with UV light (365 nm). Analysis of the cross-linking reactions by SDS-PAGE and linear MALDI-TOF-MS was performed as described previously (14). Cross-linked products were separated from nonreacted CaM by SDS-PAGE, and gel bands of interest were subjected to in-gel digestion with trypsin (45) for 2 h at 37°C in the presence of the trypsin enhancer ProteaseMAX surfactant (Promega) according to the manufacturer's protocol. As described recently, tryptic peptide mixtures were analyzed by nano-high-performance liquid chromatography (HPLC) (Ultimate; Dionex) coupled to MALDI-TOF/TOF-MS (Ultraflex; Bruker) (14, 24) or by nano-HPLC (Ultimate; Dionex) coupled to a nano-electrospray ionization-linear ion trap (ESI-LTQ)-Orbitrap-MS (LTQ-Orbitrap XL; ThermoFisher Scientific) (24) to detect candidate cross-linked peptides with high mass accuracy, confirm their identity, and assign the cross-linking sites. Briefly, data-dependent MS/MS was conducted in the linear ion trap on the five most abundant precursor ion signals from the Orbitrap full MS scan (resolution, 60,000). Fragment ions were generated by collision-induced dissociation (CID) in the LTQ and analyzed either in the LTQ or the Orbitrap (resolution, 7,500). To increase the chance of fragmentation of less abundant peaks, a dynamic exclusion was enabled in such a way that after 3 repeats of fragmentation of one precursor, it was set to the exclusion list for 120 s. Cross-linked products were identified by analyzing the MS data with the CoolToolBox software program, which is a major upgrade of the VirtualMassSpectrometryLab (VMSL) software (12). MS/MS data of cross-linked products were identified using General Protein Mass Analysis for Windows (GPMAW 8.2; Lighthouse Data) (36). For the structural analysis of SBC-cross-linked peptides, Lys residues of CaM and Ala, Ile, Leu, Lys, Arg, Met, and Phe residues of Munc13 peptides were considered potential reaction sites of the N-hydroxysuccinimide (NHS) ester and benzophenone moiety, respectively. The restrictions applied for the photophore were based on prior knowledge (17) and our own experiences with its preferred reaction sites.
Computational modeling.
Structure predictions of Munc13 peptide-CaM complexes were performed as described previously (14). Briefly, the structures of the Munc13 peptides were modeled using the PepFold program (29) on the basis of secondary-structure predictions from JuFo (31) and PSIPRED (30). Structures of different peptide-CaM complexes (Protein Data Bank [PDB] entries 1CDL, 1CKK, 1QS7, 1QTX, 1WRZ, 2BBM, 2F3Y, and 2O60) were used as a starting point for fast global protein docking searches with the PatchDock server (18), thereby accounting for the broad variety of CaM-binding modes (from parallel to antiparallel) and conformations (from open to highly compact). Phe-Cβ/Trp-Cβ–Met-Cε distances of ≤8 Å and Lys-Cα–X-Cα distances of ≤20 Å were implemented as experimental distance constraints derived from PAL and chemical cross-linking, respectively. PatchDock solutions were refined by local searches using the RosettaDock protein-protein docking server (27). Two thousand structures were first calculated independently and ranked by energy. From the 10 energetically favored structures, the final model was selected on the basis of its root mean square deviations (RMSD) from the input structure and optimal conformance with all experimental distance constraints. Model quality was evaluated with RosettaDock using the selected structures as the starting point for the calculation of an additional 1,000 models. The energy and solvent-accessible surface area (SASA) of those structures were plotted as a function of deviation from the starting structure (RMSD) to provide for a local minimum. MolProbity (11) was used for model evaluation and refinement, PyMol 0.99rc6 (www.pymol.org) was used for visualization of structures, and MultiProt (46) was used for structure-structure alignments.
Transfection of neurons and electrophysiological rescue experiments.
Munc13-1 and Munc13-2 knockout (KO) mice were published previously (4, 51). Microisland cultures of mouse hippocampal neurons were prepared and cultured as described previously (37). Prior to transfection, large amounts of DNA were prepared using the EndoFree Plasmid Maxi kit (Qiagen). Transfections were performed using the CalPhos mammalian transfection kit (Clontech). In brief, 4 μg of DNA was used to transfect one coverslip in a 6-well plate. The transfection reagents were mixed according to the manufacturer's protocol and were applied on day in vitro 3 to 4 neurons in NBA medium (Gibco) for 15 min at 5% CO2. The medium then was changed to Hanks balanced salt solution (HBSS) (Gibco) that was kept at 10% CO2 for 1 h prior to the transfection. After 30 min, the medium was changed back to the standard culture medium. Transfection efficiencies were typically 0 to 0.5% for all products. Similar fluorescence intensities were observed in neurons expressing bMunc13-2WT, bMunc13-2CaM*, Munc13-3WT, and Munc13-3CaM*, which was most likely due to the presence of free EGFP originating from the internal ribosomal entry site.
Transfected neurons (i.e., neurons presenting green fluorescence) were recorded at day in vitro 12 to 15. Neurons were whole-cell voltage clamped at −70 mV, and excitatory postsynaptic currents (EPSCs) were evoked by depolarization of the cell membrane potential from −70 to 0 mV for 2 ms. During our experiments, we observed that neurons with strong fluorescence exhibit lower EPSC amplitudes than neurons with low fluorescence. Thus, the EGFP and/or the Munc13-VENUS variant may cause cytotoxicity or otherwise negatively affect neurotransmission when expressed at high levels, thereby preventing any correlation between fluorescence intensities and EPSC amplitudes. Sucrose pulse experiments, phorbol ester stimulation, and high-frequency stimulation experiments were performed as described previously (21, 39, 42). The vesicular release probability (Pvr) was calculated by dividing the charge transfer during an EPSC by the charge transfer during the response to hypertonic sucrose. Whole-cell voltage clamp recordings were acquired using the Axon Multiclamp 700B amplifier, Digidata 1440A data acquisition system, and the pCLAMP 10 software (Molecular Devices). Analysis was performed using Axograph X software (Axograph). The n values indicate the number of cells tested. Data are expressed as means ± standard errors of the means (SEM). The averages describing the degree of depression and facilitation represent the last 10 data points (for bMunc13-2) or 5 data points (for Munc13-3). Statistical significance was tested using the nonparametric unpaired Mann-Whitney test. P values of <0.05 were considered significant.
RESULTS
Characterization of CaM-binding peptides derived from bMunc13-2 and Munc13-3.
In a previous study (15), we identified the sequence stretches bMunc13-2(572-594), bMunc13-2(719-742), Munc13-3(786-804), and Munc13-3(961-980) as potential CaM binding sites (Fig. 1B) and showed in photoaffinity labeling (PAL) experiments that the corresponding photoprobes Bpa580-b13-2N, Bpa723-b13-2C, Bpa787-13-3N, and Bpa962-13-3C are all capable of binding to CaM. Here, we extend our findings by characterizing the specificity and Ca2+ sensitivity of CaM binding to these peptides. As already established for Bpa464-13-1 and Bpa387-ub13-2 (15), photoadduct formation of CaM with Bpa580-b13-2N, Bpa723-b13-2C, Bpa787-13-3N, and Bpa962-13-3C was suppressed by increasing concentrations of the respective unmodified wild-type peptides, whereas peptide analogs containing a hydrophilic, charged Arg residue instead of the hydrophobic anchor residue were less efficient (see Fig. S1 in the supplemental material). This finding indicates that photoprobe binding is displaceable and thus specific, and that the anchor position is crucial for CaM binding of all Munc13 peptides.
To fulfill their role as Ca2+ sensors, Munc13-CaM complexes must be able to form at submicromolar Ca2+ concentrations ([Ca2+]). We used our PAL-based Ca2+ titration assay (15) to determine the effective [Ca2+] necessary to trigger interaction between CaM and the Munc13 photoprobes. We found that Ca2+-dependent photoadduct formation of Bpa580-b13-2N, Bpa723-b13-2C, Bpa787-13-3N, and Bpa962-13-3C was initiated at free [Ca2+] of 20 to 30 nM (see Fig. S2 in the supplemental material), confirming previous findings with Bpa464-13-1 and Bpa387-ub13-2 (15). However, a half-maximal effective [Ca2+] of approximately 100 nM has been determined for the binding of full-length ubMunc13-2 to immobilized CaM (57). It is therefore likely that the extremely high Ca2+ sensitivity seen here is due to the use of truncated CaM-binding peptides, and we assume it to be about one order of magnitude lower for full-length Munc13 proteins.
bMunc13-2 and Munc13-3 bind CaM stoichiometrically.
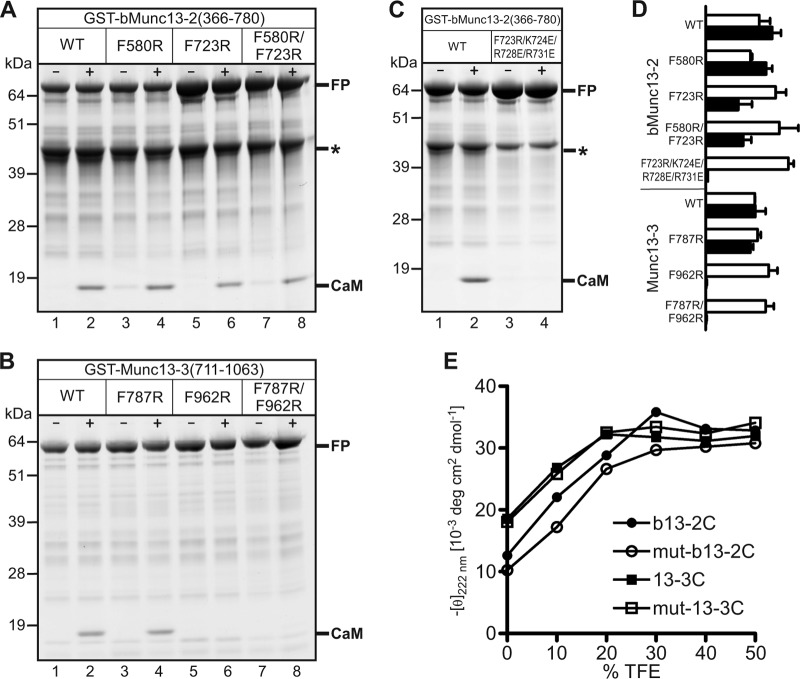
The presence of two potential CaM-binding sites in bMunc13-2 and Munc13-3 led us to consider different possibilities for complex formation with CaM (see Fig. S3 in the supplemental material). To investigate which of these occurs at the protein level, we used GST–bMunc13-2(366-780) and GST–Munc13-3(711-1063) in cosedimentation experiments and found that they interact with CaM in a strictly Ca2+-dependent manner (Fig. 2A and B, lanes 1 and 2). We analyzed the stoichiometry of the complexes by quantification of the fluorescence signals of protein-bound Coomassie dye and normalization of band intensities to protein size. Similar intensity-per-kDa ratios for the GST–bMunc13-2(366-780) and CaM bands (Fig. 2A, lane 2, and D) as well as for the GST–Munc13-3(711-1063) and CaM bands (Fig. 2B, lane 2, and D) indicated the formation of 1:1 complexes, suggesting that only one of the two CaM-binding sites in bMunc13-2 and Munc13-3 is occupied. To determine the relevant site, we replaced the respective hydrophobic anchor residues with an Arg residue and tested CaM binding of the mutated constructs. CaM binding remained unchanged for GST–bMunc13-2F580R (Fig. 2A, lane 4, and D) and GST–Munc13-3F787R (Fig. 2B, lane 4, and D), whereas it was considerably impaired for GST–bMunc13-2F723R and GST–bMunc13-2F580R/F723R (Fig. 2A, lanes 6 and 8, and D) or even abolished for GST–Munc13-3F962R and GST–Munc13-3F787R/F962R (Fig. 2B, lanes 6 and 8, and D). Although the F723R mutation in GST–bMunc13-2(366-780) reduced its CaM binding by a factor of two to three already, we sought to generate a variant completely lacking CaM binding. We generated the construct GST–bMunc13-2F723R/K724E/R728E/R731E where, in addition to the F723R mutation, the three amino acids defining the basic patch of the amphipathic α-helix (Lys-724, Arg-728, and Arg-731) were replaced by acidic Glu. These mutations completely abolished the bMunc13-2–CaM interaction (Fig. 2C and D). To test whether the loss of binding was due to the removal of the hydrophobic anchor residue and the basic patch or due to a perturbation of the α-helical structure, we subjected the mutated CaM-binding peptides Arg723-Glu724,728,731-b13-2C (mut-b13-2C) and Arg962-13-3C (mut-13-3C) to CD spectroscopy. We observed that the propensity to form an α-helix was not substantially altered for mut-b13-2C and mut-13-3C compared to their wild-type counterparts (Fig. 2E), and we concluded that the mutations do not perturb the overall structure of bMunc13-2 and Munc13-3, a hypothesis that was later confirmed by our functional characterization of the CaM-insensitive Munc13 variants.
Fig 2.
CaM binding of GST-Munc13 fusion proteins and their mutant variants. (A to C) Representative Coomassie-stained SDS-PAGE gels showing cosedimentation of CaM with GST–bMunc13-2(366-780) and GST–Munc13-3(711-1063) in the absence (−) or presence (+) of Ca2+. The use of various mutant variants revealed that CaM binding was unchanged for GST–bMunc13-2F580R (A) and GST–Munc13-3F787R (B), diminished for GST–bMunc13-2F723R and GST–bMunc13-2F580R/F723R (A), and abolished for GST–Munc13-3F962R, GST–Munc13-3F787R/F962R (B), and GST–bMunc13-2F723R/K724E/R728E/R731E (C). Where applicable, binding stoichiometry was determined by comparison of fluorescence intensities from fusion protein (FP) and CaM bands, each divided by the molecular mass of the respective protein to correct for its size (and thus its binding capacity for Coomassie dye molecules). Calculation of the intensity-per-kDa ratios also accounted for differences in the load of fusion protein as seen in panel A, lanes 1 to 4 versus lanes 5 to 8. The protein species marked with an asterisk in panels A and C was not considered for quantification, as mass spectrometric peptide mapping (not shown) indicated that it corresponds to a truncated GST–bMunc13-2 fragment not containing any of the two CaM-binding sites. (D) Histogram depicting the quantification of gel bands. For all constructs tested, the intensity-per-kDa ratios were plotted for FP (open bars) and CaM bands (closed bars) in arbitrary units. Error bars indicate the standard deviations from two independent experiments. (E) Secondary-structure analysis of CaM-binding peptides and their mutant variants by CD spectroscopy. Plotted is the negative molar ellipticity at 222 nm (an indicator of α-helicity) as a function of TFE concentration (a helix-inducing agent).
Taken together, we provide strong evidence that bMunc13-2 and Munc13-3 bind CaM predominantly through a single CaM-binding site, though we cannot completely rule out binding to the secondary putative CaM-binding site because of limitations related to GST-based cosedimentation experiments (e.g., failure to detect weak interactions, inaccessibility of binding sites due to protein aggregation, or autoinhibition by another protein sequence). However, the established CaM-binding sites spatially align with those of Munc13-1/ubMunc13-2 in terms of distance to the C1 domain (Fig. 1A), and mutation of these sites completely abolished CaM binding.
All four Munc13 isoforms employ a common mode of interaction with CaM.
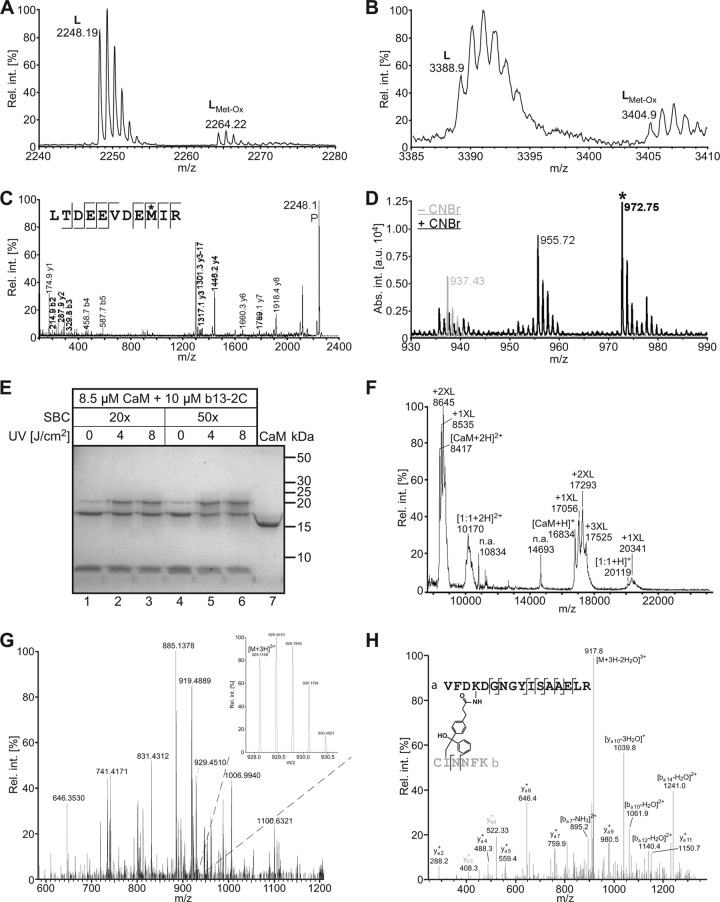
The CaM-binding sites of Munc13s are diverse in their sequences and do not belong to any of the common recognition motif families (40, 56), raising the possibility that they employ different modes of interaction. To gain information on the structure of bMunc13-2 and Munc13-3 complexes with CaM, we combined two cross-linking techniques and mapped the cross-linked sites within peptide-CaM complexes by mass spectrometry. First, we took a PAL approach and subjected the photoadduct of CaM and Bpa723-b13-2C to our established analytical workflow (14), which led to the detection of the cross-linked peptides Bpa723-b13-2C(1-6)–CaM(116-126) and Bpa723-b13-2C(1-6)–CaM(127-148) (Fig. 3A and B). Sequencing of Bpa723-b13-2C(1-6)–CaM(116-126) by MS clearly identified Met-124 as the site of photoincorporation into CaM (Fig. 3C). However, the fragment ion mass spectrum of the larger cross-linked peptide, Bpa723-b13-2C(1-6)–CaM(127-148), did not lead to an unequivocal ion series (not shown) and therefore was subjected to on-target CNBr cleavage. As originally introduced by Kage et al. (23) and recently confirmed by us (14), this treatment leads to the release of the photoprobe fragment, but only when the cross-link was formed through a Met side chain. We detected such an indicative signal (mass shift of +73 mass units) upon CNBr cleavage of Bpa723-b13-2C(1-6)–CaM(127-148) and concluded that Met-144 is the second site of photoincorporation into CaM (Fig. 3D). Similarly, Met-124/Met-144 were confirmed as cross-linked sites when CaM was labeled with Bpa962-13-3C and analyzed in the same fashion (see Fig. S4 in the supplemental material). Together, our PAL data show that, in the bound state, the hydrophobic anchor residues of bMunc13-2 and Munc13-3 are coordinated by Met-124 and Met-144 of CaM, which is in complete agreement with our previous findings for Munc13-1/ubMunc13-2 (14). We thus considered it likely that the CaM-binding peptides of all four Munc13 isoforms are similarly positioned in complex with CaM.
Fig 3.
Mapping of contact sites in bMunc13-2–CaM complexes. Covalent complexes of the Munc13 peptide b13-2C and CaM were generated either by PAL (A to D) or by chemical cross-linking with SBC (E to H), and the cross-linked sites were mapped by MS. (A and B) Detection of the cross-linked peptides Bpa723-b13-2C(1-6)–CaM(116-126) ([M + H]+calculated = 2,248.03 Da) (A) and Bpa723-b13-2C(1-6)–CaM(127-148) ([M + H]+calculated = 3,388.48 Da) (B) by LC-MALDI-MS. The signals with a mass increment of +16 mass units represent the same peptide with an oxidized Met. (C) Sequencing of Bpa723-b13-2C(1-6)–CaM(116-126) by MS. In the fragment ion mass spectrum, P denotes the precursor signal, and only b and y ions are labeled for the sake of clarity. On the basis of the conclusive N- and C-terminal ion series, Met-124 (asterisk) was identified as the site of photoincorporation into CaM. (D) CNBr cleavage of Bpa723-b13-2C(1-6)–CaM(127-148). The indicative signal at m/z 972.75 (asterisk) was absent before (−CNBr) but present after (+CNBr) cleavage and represents the methyl thiocyanate derivative of Bpa723-b13-2C(1-6) ([M + H]+calculated = 899.41 Da + 73.00 Da = 972.42 Da). Appearance of this signal upon CNBr cleavage clearly indicated photoincorporation into a Met side chain, most likely Met-144 rather than Met-145, as linkage through the former was somewhat more compatible with the fragment ion mass spectrum of Bpa723-b13-2C(1-6)–CaM(127-148) (not shown). The signal at m/z 955.72 (mass increment of −17 mass units) most likely represents the loss of ammonia by cyclization of the N-terminal S-carboxamidomethyl-Cys (19). (E) Coomassie-stained gel showing the influence of different conditions (excess of SBC, irradiation energy) on cross-linking yield. The faint bands corresponding to b13-2C–CaM complexes, which also appeared in the absence of UV light (lanes 1 and 4), are probably caused by a slight degree of photoreaction in ambient light. Control CaM in lane 7 was dissolved in water. (F) Detection of cross-linked b13-2C–CaM complexes by MALDI-TOF-MS. The mass spectrum shows the sample from lane 6 of the gel in panel E. Although the spectrum is dominated by signals for non-cross-linked CaM carrying up to three cross-linker molecules, the signals for 1:1 b13-2C–CaM adducts are clearly visible (m/z range of 20,000 to 21,000 for singly and 10,000 to 11,000 for doubly charged ions). (G) LC-MS/MS analysis of the cross-linked b13-2C–CaM complex after tryptic in-gel digestion. Although only of low abundance in the mass spectrum shown, the triply charged signal at m/z 929.1158 (see inset) identified the candidate cross-linked peptide b13-2C(1-6)–CaM(91-106) on the basis of its high mass accuracy ([M + H]+observed = 2,785.332 Da; [M + H]+calculated = 2,785.329 Da; mass deviation of 0.003 Da). (H) Sequencing of b13-2C(1-6)–CaM(91-106) by MS. The fragment ion mass spectrum confirmed the identity of the cross-linked peptide and revealed Lys-94 of CaM(91-106) to be linked to Ile-2 of b13-2C(1-6). Cross-linking at Cys-1 of b13-2C could not be excluded on the basis of the mass spectrometric data but was considered unlikely, as reaction to this site is unusual for benzophenones and would most likely lead to steric hindrance of tryptic cleavage. Rel. int., relative intensity in percent; abs. int., absolute intensity in arbitrary units.
To confirm this hypothesis, we complemented the PAL data by chemical cross-linking with the aim of generating sets of distance constraints for molecular modeling. Using the novel heterobifunctional reagent N-succinimidyl-p-benzoyldihydrocinnamate (SBC) (24), CaM was first labeled by the amine-reactive function and then photo-cross-linked with the respective Munc13 peptide. As exemplified here for the cross-linking reaction of b13-2C and CaM, analysis by SDS-PAGE revealed the formation of a covalent b13-2C–CaM complex at an apparent molecular mass of ∼20 kDa (Fig. 3E), and mass spectrometric analysis clearly confirmed the presence of a b13-2C–CaM complex with 1:1 stoichiometry (Fig. 3F). To map the cross-linked sites, the respective bands were subjected to tryptic in-gel digestion, and the resulting peptide mixtures were analyzed by LC-MS/MS. As shown in the example survey spectrum in Fig. 3G, the minor signal at m/z 929.1158 agreed well with the triply charged cross-linked peptide b13-2C(1-6)–CaM(91-106). Subsequent sequencing by MS led to a fragment ion mass spectrum that confirmed the composition of the cross-linked peptides and revealed Ile-2 of b13-2C(1-6) as the reaction site of the benzophenone moiety of SBC (Fig. 3H). We were able to map four different intermolecular cross-links within the b13-2C–CaM complex and 13 within the 13-3C–CaM complex, in addition to the two contact sites derived from PAL (Table 1). Comparable sets of cross-linked sites were previously obtained for 13-1 and ub13-2 with homobifunctional amine-reactive reagents (14) and confirmed here with SBC (Table 1; also see Fig. S5 in the supplemental material).
Table 1.
Summary of cross-linked sites within Munc13 peptide-CaM complexesc
| Method | [M + H]+observed | [M + H]+calculated | CaM | Munc13 peptide |
|---|---|---|---|---|
| PAL | 2,248.19 | 2,248.03 | 116LTDEEVDEMIR126 | b13-2C; 1CINNFK6a |
| PAL | 3,388.92 | 3,388.48 | 127EADIDGDGQVNYEEFVQMMTAK148 | b13-2C; 1CINNFK6a |
| SBC | 2,781.419 | 2,781.409 | 14EAFSLFDKDGDGTITTK30 | b13-2C; 11EKRLR15 |
| SBC | 2,785.332 | 2,785.329 | 91VFDKDGNGYISAAELR106 | b13-2C; 1CINNFK6a |
| SBC | 2,691.393 | 2,691.388 | 91VFDKDGNGYISAAELR106 | b13-2C; 11EKRLR15 |
| SBC | 2,748.410 | 2,748.399 | 91VFDKDGNGYISAAELR106 | b13-2C; 7NVLREK12 |
| PAL | 2,534.32 | 2,534.13 | 116LTDEEVDEMIR126 | 13-3C; 1CSFKEAALR9a |
| PAL | 3,675.03 | 3,674.58 | 127EADIDGDGQVNYEEFVQMMTAK148 | 13-3C; 1CSFKEAALR9a |
| SBC | 2,709.235b | 2,709.227 | 75KMKDTDSEEEIR86 | 13-3C; 14QMAELEEK21 |
| SBC | 2,967.430 | 2,967.408 | 91VFDKDGNGYISAAELR106 | 13-3C; 14QMAELEEK21 |
| SBC | 2,911.463 | 2,911.462 | 91VFDKDGNGYISAAELR106 | 13-3C; 5EAALRAYK12 |
| SBC | 3,039.563 | 3,039.557 | 91VFDKDGNGYISAAELR106 | 13-3C; 5EAALRAYKK13 |
| SBC | 3,057.433 | 3,057.429 | 14EAFSLFDKDGDGTITTK30 | 13-3C; 14QMAELEEK21 |
| SBC | 3,068.393 | 3,068.386 | 76MKDTDSEEEIREAFR90 | 13-3C; 14QMAELEEK21 |
| SBC | 3,071.415 | 3,071.422 | 76MKDTDSEEEIR86 | 13-3C; 10AYKKQMAELEEK21 |
| SBC | 3,095.507 | 3,095.503 | 91VFDKDGNGYISAAELR106 | 13-3C; 13KQMAELEEK21 |
| SBC | 3,129.584 | 3,129.578 | 14EAFSLFDKDGDGTITTK30 | 13-3C; 5EAALRAYKK13 |
| SBC | 3,185.526 | 3,185.523 | 14EAFSLFDKDGDGTITTK30 | 13-3C; 13KQMAELEEK21 |
| SBC | 3,457.705 | 3,457.698 | 91VFDKDGNGYISAAELR106 | 13-3C; 10AYKKQMAELEEK21 |
| SBC | 4,014.002b | 4,013.955 | 91VFDKDGNGYISAAELR106 | 13-3C; 5EAALRAYKKQMAELEEK21 |
| SBC | 4,674.323 | 4,675.314 | 1ADQLTEEQIAEFKEAFSLFDK21 | 13-3C; 5EAALRAYKK13 |
| SBC | 1,375.730 | 1,375.755 | 75KMK77 | 13-1; 10AFNKVR15 |
| SBC | 2,163.062 | 2,163.069b | 22DGDGTITTKELGTVMR37 | 13-1; 3AK4 |
| SBC | 2,454.225 | 2,454.219 | 14EAFSLFDKDGDGTITTK30 | 13-1; 1RAK3 |
| SBC | 1,059.540 | 1,059.591 | 75KMK77 | ub13-2; 10AVTK13 |
| SBC | 1,357.732 | 1,357.687 | 75KMK77 | ub13-2; 5AHWFR9 |
| SBC | 2,753.404 | 2,753.403 | 14EAFSLFDKDGDGTITTK30 | ub13-2; 10AVTKVR15 |
N-terminal Cys was S-carboxamidomethylated.
Observed and calculated molecular mass refer to a cross-linked peptide species with a single oxidation of Met.
Connected sequences were identified in photoaffinity labeling (PAL) or chemical cross-linking (SBC) experiments. Cross-linked residues are underlined. Italic letters in the photoprobe fragments indicate the Phe-Bpa exchange. Note that in some cases it was not possible to identify a single cross-linked residue in the Munc13 peptides on the basis of the MS/MS data. Out of the two or three candidate residues, the most likely reaction site was selected according to prior knowledge (17) and our own experiences with reaction preferences of benzophenone moieties and were used to define the respective constraints for molecular modeling. See reference 14 for additional sites derived from PAL and chemical cross-linking with the homobifunctional amine-reactive reagents BS3 and BS2G. [M + H]+ units are Da.
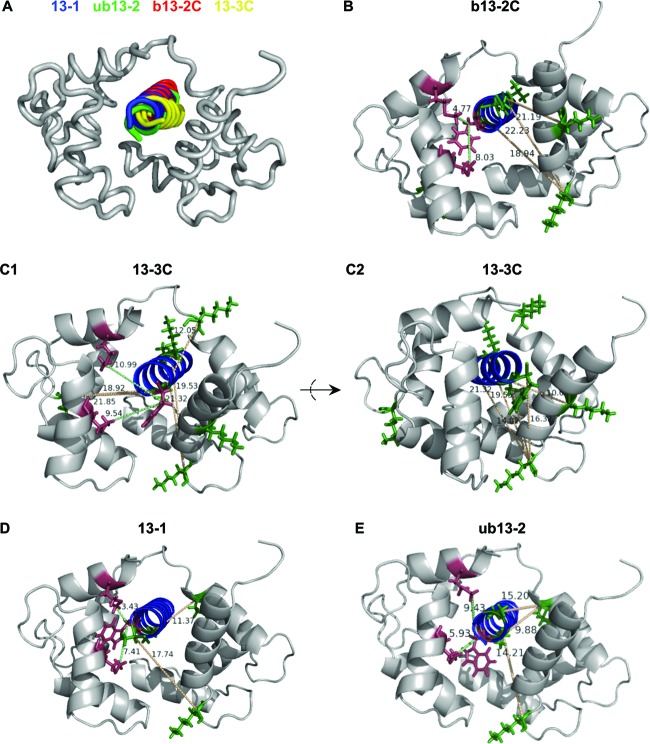
We next used the cross-linked sites (Table 1) as experimental distance constraints for the creation of low-resolution structural models of Munc13 peptide-CaM complexes. In agreement with our CD spectroscopic data (Fig. 2E), b13-2C and 13-3C were predicted to form an α-helix and were docked as such to the structures of different peptide-CaM complexes by using the PatchDock server. The distance constraints were best compatible with compact structures in which CaM enwraps the peptide ligand in an antiparallel manner. The highest score (i.e., the energetically most favored structure) was obtained when the CaM complex of a peptide derived from neuronal NO synthase (nNOS; PDB entry 2O60) was used as the template. Such a compact conformation was further supported by intramolecular cross-links found between the N- and C-terminal CaM domains (see Table S1 in the supplemental material), confirming their close proximity during binding of b13-2C and 13-3C. Most importantly, the models obtained for the CaM complexes of b13-2C and 13-3C resembled those of 13-1 and ub13-2 (14), and superimposition of the CaM structures revealed that the CaM-binding peptides of all four Munc13 isoforms align well (Fig. 4A). For model refinement, the final PatchDock solutions were used as starting structures for local searches using the RosettaDock server. Ten energetically favored structures were filtered for optimal conformance with the experimental distance constraints (i.e., for the lowest root mean-squared deviation [RMSD] from the PatchDock model [see Table S2 in the supplemental material]) to create the final structural models for the Munc13 peptide-CaM complexes. The resulting structures are highly related, all featuring antiparallel binding of the α-helical peptides through similar contact points and a compact overall shape (Fig. 4B to E). We thus conclude that all four Munc13 isoforms employ a common mode of interaction with CaM.
Fig 4.
Computational modeling of Munc13-CaM complexes. Docking of 13-1, ub13-2, b13-2C, and 13-3C was performed with PatchDock by implementation of the experimental distance constraints derived from PAL (≤8 Å for Phe-Cβ–Met-Cε and Trp-Cβ–Met-Cε) and chemical cross-linking (≤20 Å for Lys-Cα–X-Cα). (A) Structure alignment showing that the CaM conformation derived from the nNOS peptide-CaM complex is the best-fitting structure for all four Munc13 peptides. CaM structures were superimposed to visualize the virtually identical mode of binding for 13-1 (blue), ub13-2 (green), b13-2C (red), and 13-3C (yellow). (B to E) Energetically best models after refinement with RosettaDock and filtering according to the experimental distance constraints. The CaM complexes of b13-2C (B), 13-3C (C1 and C2), 13-1 (D), and ub13-2 (E) are shown. The distances between amino acids, which were experimentally found to be connected, are given in Å and are indicated by green lines for PAL (Phe-Cβ–Met-Cε in panels B and C and Trp-Cβ–Met-Cε in panels D and E) and by yellow lines for chemical cross-linking (Lys-Cα–X-Cα). The peptides' hydrophobic anchor residues (Phe in panels B and C, Trp in panels D and E) which were replaced by Bpa are shown in red, as are the corresponding sites of photoincorporation into CaM (Met-124 and Met-144). Chemically cross-linked Lys residues of CaM and their corresponding cross-linked sites in the Munc13 peptides are shown in green. The CaM complex of 13-3C is presented in two different views (C1 and C2) to facilitate visualization of the numerous experimental constraints derived from chemical cross-linking.
The role of Ca2+/CaM binding of bMunc13-2 and Munc13-3 in STP.
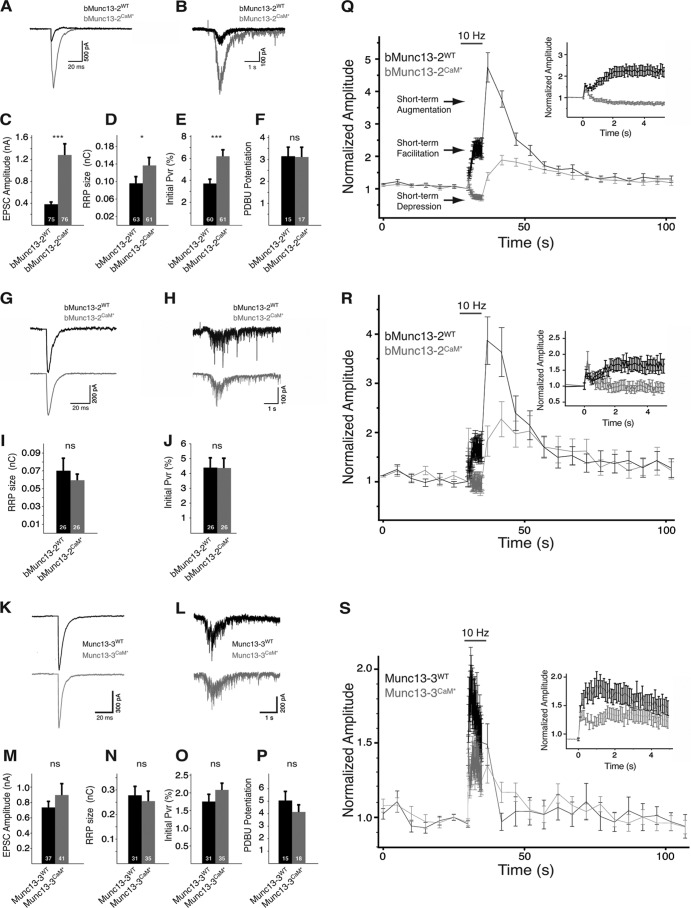
To investigate how Ca2+/CaM binding regulates the activity of bMunc13-2 and Munc13-3 in vivo, we performed a series of electrophysiological experiments. C-terminally VENUS-tagged bMunc13-2WT, Munc13-3WT, and their CaM-insensitive variants, bMunc13-2F723R/K724E/K728E/R731E (bMunc13-2CaM*) and Munc13-3F962E (Munc13-3CaM*), were studied in autaptic hippocampal Munc13-1/2 double knockout (DKO) neurons, which exhibit no evoked or spontaneous release. Expression of bMunc13-2WT clearly rescued the block of synaptic transmission, and a small average EPSC amplitude of 0.383 ± 0.04 nA was measured (n = 75) (Fig. 5A and C), comparable to the amplitudes measured in autaptic hippocampal neurons from Munc13-1 KO mice still expressing ubMunc13-2 and bMunc13-2 (∼0.1 nA) (2, 42). We therefore consider it unlikely that the small amplitudes seen in our rescue experiments represent an artifact of low expression levels. Rather, they might indicate that bMunc13-2 is only capable of rescuing a subset of synapses in a given hippocampal neuron, a notion that is supported by the FM1-34 uptake experiments by Rosenmund et al. (42), who showed that in neurons from Munc13-1 KO mice, only a small fraction of synapses remain active. Surprisingly, bMunc13-2CaM* rescued synaptic transmission more efficiently, with average EPSC amplitudes of 1.28 ± 0.2 nA (n = 76) (Fig. 5A and C). To estimate the size of the RRP of fusion-competent SVs, we used the postsynaptic response elicited upon application of hypertonic sucrose solution (43) and obtained average RRP sizes of 0.095 ± 0.015 nC (n = 63) and 0.136 ± 0.018 nC (n = 61) for bMunc13-2WT and bMunc13-2CaM*, respectively (Fig. 5B and D). The vesicular release probability (Pvr, the probability of a SV to be released upon an AP) was 3.8% ± 0.4% (n = 60) for bMunc13-2WT-expressing neurons and 6.3% ± 0.6% (n = 61) for bMunc13-2CaM*-expressing neurons (Fig. 5E). No differences were seen in the potentiation of the synaptic response by phorbol ester (39), indicating that the C1 domain activation of bMunc13-2 regulates neurotransmitter release independently of CaM binding in a fashion similar to that of Munc13-1/ubMunc13-2 (Fig. 5F).
Fig 5.
Basal synaptic transmission and STP characteristics of Munc13-1/2 DKO neurons rescued by bMunc13-2, Munc13-3, or their CaM-insensitive variants. Wild-type bMunc13-2 and Munc13-3 (bMunc13-2WT and Munc13-3WT, respectively) and their CaM-insensitive variants (bMunc13-2CaM* and Munc13-3CaM*, respectively) were expressed in glutamatergic, autaptic hippocampal neurons from Munc13-1/2 DKO mice, and synaptic transmission was monitored using the whole-cell voltage clamp technique. Example traces (A) and averaged amplitudes (C) of evoked excitatory postsynaptic currents (EPSCs) in neurons expressing either bMunc13-2WT or bMunc13-2CaM* are shown. Example traces (B) and averaged RRP size (D) released upon application of hypertonic sucrose solution (0.5 M) for 6 s and plot of the average Pvr (E) are also shown. (F) Phorbol dibutyrate (PDBu; 1 μM) was applied for a period of 60 s, and the potentiation of evoked EPSCs, normalized to the average EPSC amplitude before application, was plotted. Shown are example traces of evoked EPSC (G) and sucrose application (H) from neurons that were matched according to their initial EPSC amplitudes and averaged RRP size (I) and Pvr (J) resulting from this data set. (K and L) Example traces of EPSCs and responses to application of sucrose recorded in neurons expressing Munc13-3WT or Munc13-3CaM*. Average EPSC amplitude (M), RRP size (N), Pvr (O), and potentiation in response to a 2-min application of 1 μM PDBu (P) in Munc13-3WT- and Munc13-3CaM*-expressing neurons, as described in panels A to F, are shown. (Q) STP in neurons expressing bMunc13-2WT (n = 74) or bMunc13-2CaM* (n = 73). A 5-s stimulation at 10-Hz frequency (gray horizontal bar) was applied during a period of stimulation at 0.2 Hz. All data points are normalized to the first EPSC of the 10-Hz train. The different STP phases observed are indicated by arrows. Inset: magnification of the 10-Hz stimulation period. (R) STP characteristics in amplitude-matched neurons (bMunc13-2WT, n = 25; bMunc13-2CaM*, n = 24) as described for panel Q. (S) STP in neurons expressing Munc13-3WT (n = 37) or Munc13-3CaM* (n = 31) as described for panel Q. In all bar graphs, n values are indicated at the bottom of the bar. ***, P < 0.001; *, P < 0.05; ns, not significant.
The finding that bMunc13-2WT and bMunc13-2CaM* differ in all basic parameters of synaptic transmission (i.e., EPSC amplitude, RRP size, and Pvr) was unexpected, as it was not observed for the CaM-insensitive variants of Munc13-1, ubMunc13-2 (22), and Munc13-3 (see below). However, we noticed that bMunc13-2WT- and bMunc13-2CaM*-expressing neurons that exhibit similar initial EPSC amplitudes also exhibit similar RRP and Pvr. We therefore analyzed a subset of the data by matching bMunc13-2WT- and bMunc13-2CaM*-expressing neurons from the same culture according to the criterion that their initial EPSC amplitude was within a window of ±100 pA. After matching, average EPSC amplitudes of 0.42 ± 0.05 nA (bMunc13-2WT; n = 26) and 0.415 ± 0.05 nA (bMunc13-2CaM*; n = 26) were calculated (Fig. 5G). In this data set, neither the average RRP sizes (bMunc13-2WT, 0.07 ± 0.014 nC [n = 26]; bMunc13-2CaM*, 0.06 ± 0.006 nC [n = 26]) (Fig. 5H and I) nor the Pvr (bMunc13-2WT, 4.41% ± 0.7% [n = 26]; bMunc13-2CaM*, 4.38% ± 0.55% [n = 26]; Fig. 5J) were significantly different. These results imply that the differences in RRP size and Pvr in the full data set are not related to an effect of the point mutations on intrinsic protein function, as such changes should not depend on the initial EPSC size. Rather, we speculate that these changes result from differences in the expression levels of bMunc13-2WT and bMunc13-2CaM*. However, we could not test this hypothesis, because Ca2+-phosphate transfection of primary neurons results in very low transfection efficiencies that are incompatible with Western blot detection. Moreover, immunocytochemical comparison using a bMunc13-2 antibody (52) was inconclusive due to somatic background staining in wild-type and Munc13-1/2 DKO neurons (not shown) similar to that observed in the retina (10), and immunostaining of the C-terminal VENUS tag would be masked by the free EGFP derived from an EGFP cassette in the pCAGIG plasmid. In any case, the analysis of amplitude-matched neurons provided a sub-data set in which the characteristics of basal neurotransmission mediated by bMunc13-2WT and bMunc13-2CaM* were comparable and enabled us to dissect the specific effect of CaM binding on STP.
We next studied basal synaptic transmission in the presence of Munc13-3 by expressing Munc13-3WT and Munc13-3CaM* in hippocampal Munc13-1/2 DKO neurons, where Munc13-3 expression is not detectable by morphological or functional analysis (2, 51). Munc13-3WT rescued the loss of synaptic transmission and an average EPSC amplitude of 0.73 ± 0.07 nA (n = 37) (Fig. 5K and M) was recorded, indicating that Munc13-3 is capable of mediating SV priming. Munc13-3CaM*-expressing neurons exhibited a slightly, but not significantly, higher average EPSC amplitude (0.89 ± 0.14 nA; n = 41) (Fig. 5K and M). RRP sizes were comparable (Munc13-3WT, 0.28 ± 0.034 nC [n = 31]; Munc13-3CaM*, 0.25 ± 0.04 nC [n = 35]) (Fig. 5L and N), resulting in a slightly higher Pvr of Munc13-3CaM*-expressing neurons (Munc13-3WT, 1.75% ± 0.21% [n = 31]; Munc13-3CaM*, 2.08% ± 0.19% [n = 35]) (Fig. 5O). The phorbol ester-mediated potentiation of synaptic transmission was slightly higher for Munc13-3WT (5.04 ± 0.7; n = 15) than for Munc13-3CaM* (4.1 ± 0.53; n = 18) (Fig. 5P). Taking these results together, we observed small but not significant differences in basal synaptic transmission between neurons expressing Munc13-3WT and Munc13-3CaM*.
To investigate STP characteristics in bMunc13-2WT-, bMunc13-2CaM*-, Munc13-3WT-, and Munc13-3CaM*-expressing neurons, we measured their response to high-frequency AP trains at a frequency of 10 Hz. During such a train, STD is seen as a gradual decrease of the EPSC size relative to the baseline EPSC obtained with 0.2-Hz stimulation, while facilitation, an early phase of STE, is seen as an increase of the EPSC size. Following the train, a several-second increase in EPSC amplitude represents augmentation, a later phase of STE (58). Neurons expressing bMunc13-2WT showed facilitation during the train, reaching an average of 2.1- ± 0.21-fold (n = 74) of the baseline transmission level, followed by a 4.74- ± 0.45-fold augmentation 2 s after the high-frequency train (Fig. 5Q). These values agree well with data obtained from Munc13-1 KO neurons, which express both isoforms of Munc13-2 (42), indicating that bMunc13-2 is the major splice variant expressed in cultured hippocampal neurons. Remarkably, neurons expressing bMunc13-2CaM* initially showed facilitation that was not sustained and rapidly switched to STD, reaching steady-state depression levels of 0.65- ± 0.04-fold (n = 73) of the basal transmission levels (Fig. 5Q). Following the train, reduced augmentation was seen, reaching 1.38- ± 0.11-fold of the basal synaptic transmission levels (Fig. 5Q). We next analyzed the STP behavior of neurons in the amplitude-matched group that exhibit similar Pvr to exclude the possibility that the STP differences between neurons expressing bMunc13-2WT and bMunc13-2CaM* arise solely due to their different Pvr. Strikingly, this analysis yielded comparable results, indicating that intrinsic functional differences between bMunc13-2WT and bMunc13-2CaM* account for the different STP characteristics they induce (for bMunc13-2WT, facilitation of 1.81- ± 0.28-fold and augmentation 3.86- ± 0.5-fold, n = 25; for bMunc13-2CaM*, depression of 0.90- ± 0.13-fold and augmentation of 1.82- ± 0.3-fold, n = 24) (Fig. 5R). We next analyzed STP in hippocampal neurons expressing Munc13-3WT and observed moderate frequency facilitation during the high-frequency stimulation train, which was peaking after ∼1 s to 1.94- ± 0.19-fold of the baseline levels and then decaying to 1.61- ± 0.17-fold at the end of the train (n = 37) (Fig. 5S). Following the train, a short-lasting, minor augmentation was seen (1.49- ± 0.14-fold) (Fig. 5S). In contrast, Munc13-3CaM*-expressing neurons exhibited significantly reduced facilitation during the early phase of the train (1.34- ± 0.09-fold; n = 31), with comparable augmentation at the end of and following the train (1.32- ± 0.11-fold and 1.34- ± 0.11-fold) (Fig. 5S). Thus, it appears that Ca2+/CaM regulation of Munc13-3 activity plays a role during the early phase of the high-frequency train, but its overall effect on STP is lower than that of Ca2+/CaM regulation of the other Munc13s.
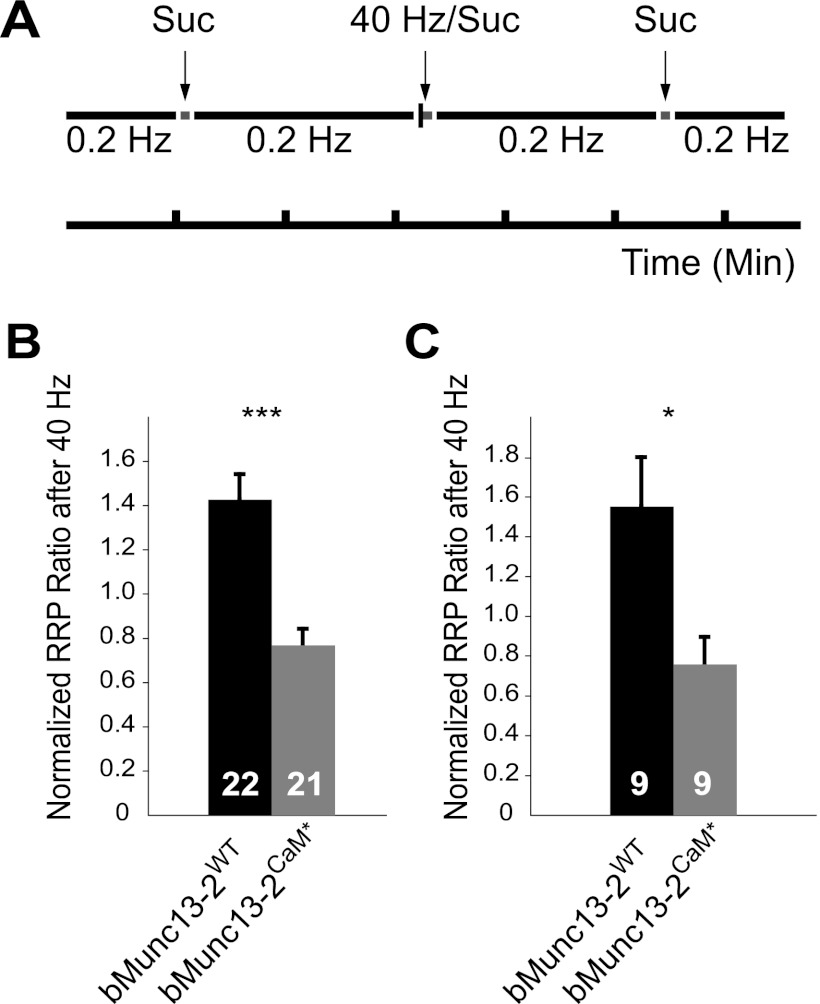
To gain insight into the mechanism of STP modulation by Ca2+/CaM-Munc13 binding, we studied the modulation of RRP size after high-frequency activity at 40 Hz (Fig. 6A). We observed a strong increase in RRP size to 1.43- ± 0.11-fold (n = 22) of its value under resting conditions in neurons expressing bMunc13-2WT. In contrast, the RRP size was reduced to 0.77- ± 0.07-fold (n = 21) of its value under resting conditions in neurons expressing bMunc13-2CaM* (Fig. 6B). Importantly, almost the same is seen in the amplitude-matched data set (bMunc13-2WT, 1.55- ± 0.25-fold, n = 9; bMunc13-2CaM*, 0.76- ± 0.13-fold, n = 9) (Fig. 6C). The release probabilities after high-frequency activity, as estimated by dividing the averaged augmentation ratio of the EPSC amplitude by the ratio of RRP change after the 40-Hz train, were identical. We did not carry out the corresponding experiment for Munc13-3, as neurons expressing Munc13-3WT and Munc13-3CaM* exhibit similar augmentation ratios and thus were not expected to show significant differences under our experimental conditions. However, we consider it likely that Ca2+/CaM binding to Munc13-3 regulates STP in the early phase of the AP train by RRP size modulation, as this mechanism also was previously proposed for Munc13-1 and ubMunc13-2 based on an experimental paradigm similar to that used here (22). In that study, the CaM-insensitive variants of Munc13-1 and ubMunc13-2 showed a reduction in RRP size after an AP train at 10 Hz without corresponding changes in Pvr compared to their wild-type variants.
Fig 6.
Activity-dependent modulation of RRP size by Ca2+/CaM–bMunc13-2 binding. (A) Experimental design used to determine the changes in RRP size following high-frequency stimulation in neurons expressing bMunc13-2WT or bMunc13-2CaM*. The RRP was measured using three sucrose applications (Suc). The first application was used to quantify the RRP size under resting conditions, the second was given 0.5 s after a 40-Hz train for 2.5 s and served to evaluate activity-dependent RRP modulation, and the third was used to correct for RRP rundown during the experiment. The ratio between the RRP size after a 40-Hz stimulation and the predicted RRP size under resting conditions corrected for rundown is plotted for the entire data set (B) and for the amplitude-matched group (C). In all bar graphs, n values are indicated at the bottom of the bar. ***, P < 0.001; *, P < 0.05.
Taken together, our electrophysiological data indicate that Ca2+/CaM interaction with bMunc13-2 or Munc13-3 enhances priming during periods of high neuronal activity. Consequently, these neurons are able to sustain high rates of neurotransmitter release during high-frequency AP trains, leading to a transition from STD to STE in the case of bMunc13-2 or to enhanced STE in the case of Munc13-3.
DISCUSSION
Activity-dependent STP phenomena have been observed in virtually every neuron type studied so far and are considered a fundamental feature of neurotransmission (58). They contribute to the reliability, fidelity, and endurance of synaptic transmission and are pivotal in many higher brain functions, such as cortical gain control (1), working memory (32), motor control (33), sensory adaptation (8), or sound localization (9). However, little is known about the molecular mechanisms underlying STP. One common presynaptic mechanism proposed for both STD and STE is Ca2+-dependent RRP size regulation. Accordingly, the buildup of [Ca2+]i during strong synaptic activity leads to SV fusion and thus to RRP exhaustion but also to an increased rate of RRP replenishment, probably through the activation of a molecular machinery that replenishes fused SVs. When the replenishment rate is higher than the fusion rate, STE prevails, while STD is observed when the fusion rate is faster than the replenishment rate (35). As essential priming factors, CAPS (21) and Munc13 proteins control the RRP size, but only Munc13s have been shown to be regulated by Ca2+ (22, 39, 47).
Based on our in vitro finding that bMunc13-2 and Munc13-3 contain only one functional Ca2+/CaM binding site, which can be inactivated by targeted mutagenesis (Fig. 2), we generated CaM-insensitive full-length variants and studied their STP characteristics along with those of their wild-type counterparts. We found that Ca2+/CaM binding of bMunc13-2 leads to a transition from STD to STE, while Ca2+/CaM binding of Munc13-3 leads to stronger STE (Fig. 5). Moreover, we show that Ca2+/CaM binding is necessary for the refilling of the RRP after high-frequency stimulation (Fig. 6). Our physiological analysis presented here and previously (22), together with the structural insights indicating a common mode of Ca2+/CaM interaction for all Munc13 isoforms (Fig. 4), lead to the conclusion that the regulation of priming activity through Ca2+/CaM complexes is conserved within the Munc13 protein family despite the lack of consistent sequence homology. We propose that Ca2+/CaM signaling to all Munc13s transduces elevations in residual [Ca2+]i into an increased size and refilling rate of the RRP, two parameters that essentially determine the extent of and the recovery from STD (16, 44, 48, 53). Thus, our data contribute to the STP mechanism proposed above and highlight Munc13s as important components of the molecular machinery that controls STP.
Within the Munc13 protein family, a role in SV priming and STP has been established only for the closely related isoforms Munc13-1 and ubMunc13-2. Here, we show directly by electrophysiological rescue experiments that bMunc13-2 and Munc13-3 exhibit priming activity, as they rescue synaptic transmission in neurons deficient of all Munc13s (Fig. 5). During our electrophysiological analysis, we observed that distinct STP characteristics are conferred to a neuron upon expression of bMunc13-2 and Munc13-3, adding to earlier findings reporting different forms of STP upon expression of Munc13-1 and ubMunc13-2 (42). It thus appears that each Munc13 isoform is capable of inducing unique STP characteristics. Neurons expressing ubMunc13-2 (22, 42, 47), bMunc13-2, and Munc13-3 (this paper) display facilitation during an AP train at a frequency of 10 Hz, whereas expression of Munc13-1 leads to depression (42). Interestingly, the two Munc13-2 splice variants seem to differ in efficacy, as bMunc13-2-expressing neurons exhibit stronger facilitation and augmentation (1.8- and 3.9-fold) compared to ubMunc13-2 (1.3- and 2.1-fold) (22, 47), leading to increased activity-dependent outputs in neurons equipped with bMunc13-2. Munc13-3 expression in hippocampal neurons leads to a mild facilitation during an AP train that decays during the train (Fig. 5S) and is followed by a small, transient augmentation. Of note, Munc13-3 is the only Munc13 isoform that is not present in the hippocampus of the rodent brain and may therefore induce different STP characteristics in the subset of neurons that normally express it, e.g., cerebellar neurons (2). In view of these findings, it is very likely that different STP outputs are shaped in distinct synapses in vivo by particular Munc13 isoforms or particular combinations thereof.
Ca2+/CaM-dependent regulation of Munc13s appears to be a highly important feature of SV priming in vertebrates and invertebrates. It likely operates in all UNC-13 proteins, as CaM recognition motifs similar to that of Munc13-1 exist in the UNC-13 homologs of Caenorhabditis elegans and Drosophila melanogaster, and CaM binding of the latter was confirmed experimentally (55). Moreover, during the evolution of different Munc13s, at least three distinct types of CaM-binding sites in Munc13-1/ubMunc13-2, bMunc13-2, and Munc13-3 appeared that converged to a common structure and function, thereby preserving the Ca2+/CaM-dependent regulation of priming activity. Interestingly, a recent study reported a similar phenomenon regarding the conserved C-terminal MUN domain of Munc13-1 (25). Part of this domain is structurally similar to vesicle tethering factors but has a distinct primary structure (sequence homology of <10%), indicating that MUN domain-containing proteins such as Munc13s, CAPS, and others have a common role in membrane traffic.
Our structural models of the Munc13 peptide-CaM complexes indicate that the N-terminal part of the Munc13 peptides contact the C-terminal CaM domain through hydrophobic residues in positions 1, 5, and 8 (13-1, ub13-2, and 13-3C) or 1, 5, and 10 (b13-2C) of the motifs. This interaction domain is in agreement with the high-resolution nuclear magnetic resonance (NMR) structure derived from the CaM complex of the C-terminally elongated 34-amino-acid peptide Munc13-1459-492 and is referred to as the C module (41). However, this published structure indicated that Trp-489 of Munc13-1 (position 26 of the motif) is embedded in the hydrophobic cleft of the N-terminal CaM domain to form a second interaction domain, the N module. Within this modular architecture, the complex adopts an extended conformation, as the C and N modules are connected by central flexible linkers in both CaM and Munc13-1459-492. Interestingly, NMR titration experiments indicated that the formation of the C module can occur at resting [Ca2+], while a higher [Ca2+] is required for the formation of the N module (41). This sequential binding mode likely enables CaM-Munc13 complexes to sense [Ca2+]i over a broad concentration range, an essential feature to fulfill their role in Ca2+-dependent RRP size regulation. Intriguingly, bMunc13-2 and Munc13-3 contain neither the 1-5-8-26 CaM recognition motif of Munc13-1/ubMunc13-2 (41) nor any other hydrophobic cluster with a spacing of 20 to 30 amino acids from the first anchor residue. Nevertheless, in view of the conserved function, it is likely that some type of sequential interaction occurs during CaM binding of all Munc13 isoforms. We consider our electrophysiological data evidence in support of this notion, but detailed structural information on the CaM complexes of larger bMunc13-2 and Munc13-3 protein fragments is needed to prove or disprove this hypothesis.
In conclusion, we show here that the Ca2+/CaM-dependent regulation of SV priming is structurally and functionally conserved within the Munc13 family, and that this regulation is mediated through nonconserved Ca2+/CaM-binding sites, which are likely the result of convergent evolution. Our data establish the Ca2+/CaM-Munc13 complexes as components of the synaptic molecular machinery that plays a pivotal role in determining the STP characteristics of a neuron. The evolution of four Munc13 proteins regulated by Ca2+/CaM enables the mammalian brain to differentially shape STP outputs in distinct synapses, constituting a mechanism for the fine-tuning of neuronal plasticity, which is fundamental for many higher brain functions.
Supplementary Material
ACKNOWLEDGMENTS
N.L. was a recipient of a Feodor Lynen Fellowship of the Minerva Foundation.
We thank H. Kawabe for helpful discussion and A. Poulopoulos for critically reading the manuscript. We are grateful to F. Benseler, I. Thanhäuser, D. Schwerdtfeger, A. Galinski, L. van Werven, M. Uecker, and T. Liepold for excellent technical support and to the staff of the MPIEM animal facility for the management of mouse colonies.
We declare no conflicts of interest.
Footnotes
Published ahead of print 10 September 2012
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1. Abbott LF, Varela JA, Sen K, Nelson SB. 1997. Synaptic depression and cortical gain control. Science 275:220–224 [DOI] [PubMed] [Google Scholar]
- 2. Augustin I, Betz A, Herrmann C, Jo T, Brose N. 1999. Differential expression of two novel Munc13 proteins in rat brain. Biochem. J. 337:363–371 [PMC free article] [PubMed] [Google Scholar]
- 3. Augustin I, et al. 2001. The cerebellum-specific Munc13 isoform Munc13-3 regulates cerebellar synaptic transmission and motor learning in mice. J. Neurosci. 21:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Augustin I, Rosenmund C, Sudhof TC, Brose N. 1999. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 400:457–461 [DOI] [PubMed] [Google Scholar]
- 5. Basu J, et al. 2005. A minimal domain responsible for Munc13 activity. Nat. Struct. Mol. Biol. 12:1017–1018 [DOI] [PubMed] [Google Scholar]
- 6. Betz A, et al. 1998. Munc13-1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron 21:123–136 [DOI] [PubMed] [Google Scholar]
- 7. Brose N, Hofmann K, Hata Y, Südhof TC. 1995. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C(2)-domain proteins. J. Biol. Chem. 270:25273–25280 [DOI] [PubMed] [Google Scholar]
- 8. Chung S, Li X, Nelson SB. 2002. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron 34:437–446 [DOI] [PubMed] [Google Scholar]
- 9. Cook DL, Schwindt PC, Grande LA, Spain WJ. 2003. Synaptic depression in the localization of sound. Nature 421:66–70 [DOI] [PubMed] [Google Scholar]
- 10. Cooper B, et al. 2012. Munc13-independent vesicle priming at mouse photoreceptor ribbon synapses. J. Neurosci. 32:8040–8052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davis IW, et al. 2007. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35:W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Koning LJ, et al. 2006. Computer-assisted mass spectrometric analysis of naturally occurring and artificially introduced cross-links in proteins and protein complexes. FEBS J. 273:281–291 [DOI] [PubMed] [Google Scholar]
- 13. Deng PY, Klyachko VA. 2011. The diverse functions of short-term plasticity components in synaptic computations. Commun. Integr. Biol. 4:543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dimova K, et al. 2009. Structural insights into the calmodulin-Munc13 interaction obtained by cross-linking and mass spectrometry. Biochemistry 48:5908–5921 [DOI] [PubMed] [Google Scholar]
- 15. Dimova K, Kawabe H, Betz A, Brose N, Jahn O. 2006. Characterization of the Munc13-calmodulin interaction by photoaffinity labeling. Biochim. Biophys. Acta 1763:1256–1265 [DOI] [PubMed] [Google Scholar]
- 16. Dittman JS, Regehr WG. 1998. Calcium dependence and recovery kinetics of presynaptic depression at the climbing fiber to Purkinje cell synapse. J. Neurosci. 18:6147–6162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dorman G, Prestwich GD. 1994. Benzophenone photophores in biochemistry. Biochemistry 33:5661–5673 [DOI] [PubMed] [Google Scholar]
- 18. Duhovny D, Nussinov R, Wolfson H. 2002. Efficient unbound docking of rigid molecules, p 185–200 In Algorithms in bioinformatics, vol 2452 Springer, Berlin, Germany [Google Scholar]
- 19. Geoghegan KF, et al. 2002. Cyclization of N-terminal S-carbamoylmethylcysteine causing loss of 17 Da from peptides and extra peaks in peptide maps. J. Proteome Res. 1:181–187 [DOI] [PubMed] [Google Scholar]
- 20. Jahn O, Eckart K, Brauns O, Tezval H, Spiess J. 2002. The binding protein of corticotropin-releasing factor: ligand-binding site and subunit structure. Proc. Natl. Acad. Sci. U. S. A. 99:12055–12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jockusch WJ, et al. 2007. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell 131:796–808 [DOI] [PubMed] [Google Scholar]
- 22. Junge HJ, et al. 2004. Calmodulin and Munc13 form a Ca2+ sensor/effector complex that controls short-term synaptic plasticity. Cell 118:389–401 [DOI] [PubMed] [Google Scholar]
- 23. Kage R, Leeman SE, Krause JE, Costello CE, Boyd ND. 1996. Identification of methionine as the site of covalent attachment of a p-benzoyl-phenylalanine-containing analogue of substance P on the substance P (NK-1) receptor. J. Biol. Chem. 271:25797–25800 [DOI] [PubMed] [Google Scholar]
- 24. Krauth F, Ihling CH, Ruttinger HH, Sinz A. 2009. Heterobifunctional isotope-labeled amine-reactive photo-cross-linker for structural investigation of proteins by matrix-assisted laser desorption/ionization tandem time-of-flight and electrospray ionization LTQ-Orbitrap mass spectrometry. Rapid Commun. Mass Spectrom. 23:2811–2818 [DOI] [PubMed] [Google Scholar]
- 25. Li W, et al. 2011. The crystal structure of a Munc13 C-terminal module exhibits a remarkable similarity to vesicle tethering factors. Structure 19:1443–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luo S, Wehr NB, Levine RL. 2006. Quantitation of protein on gels and blots by infrared fluorescence of Coomassie blue and Fast Green. Anal. Biochem. 350:233–238 [DOI] [PubMed] [Google Scholar]
- 27. Lyskov S, Gray JJ. 2008. The RosettaDock server for local protein-protein docking. Nucleic Acids Res. 36:W233–W238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matsuda T, Cepko CL. 2004. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. U. S. A. 101:16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maupetit J, Derreumaux P, Tuffery P. 2010. A fast method for large-scale de novo peptide and miniprotein structure prediction. J. Comput. Chem. 31:726–738 [DOI] [PubMed] [Google Scholar]
- 30. McGuffin LJ, Bryson K, Jones DT. 2000. The PSIPRED protein structure prediction server. Bioinformatics 16:404–405 [DOI] [PubMed] [Google Scholar]
- 31. Meiler J, Muller M, Zeidler A, Schmaschke F. 2001. Generation and evaluation of dimension-reduced amino acid parameter representations by artificial neural networks. J. Mol. Model. 7:360–369 [Google Scholar]
- 32. Mongillo G, Barak O, Tsodyks M. 2008. Synaptic theory of working memory. Science 319:1543–1546 [DOI] [PubMed] [Google Scholar]
- 33. Nadim F, Manor Y. 2000. The role of short-term synaptic dynamics in motor control. Curr. Opin. Neurobiol. 10:683–690 [DOI] [PubMed] [Google Scholar]
- 34. Nagai T, et al. 2002. A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat. Biotechnol. 20:87–90 [DOI] [PubMed] [Google Scholar]
- 35. Neher E, Sakaba T. 2008. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59:861–872 [DOI] [PubMed] [Google Scholar]
- 36. Peri S, Steen H, Pandey A. 2001. GPMAW–a software tool for analyzing proteins and peptides. Trends Biochem. Sci. 26:687–689 [DOI] [PubMed] [Google Scholar]
- 37. Pyott SJ, Rosenmund C. 2002. The effects of temperature on vesicular supply and release in autaptic cultures of rat and mouse hippocampal neurons. J. Physiol. 539:523–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rettig J, Neher E. 2002. Emerging roles of presynaptic proteins in Ca++-triggered exocytosis. Science 298:781–785 [DOI] [PubMed] [Google Scholar]
- 39. Rhee J-S, et al. 2002. Beta phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell 108:121–133 [DOI] [PubMed] [Google Scholar]
- 40. Rhoads A, Friedberg F. 1997. Sequence motifs for calmodulin recognition. FASEB J. 11:331–340 [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez-Castaneda F, et al. 2010. Modular architecture of Munc13/calmodulin complexes: dual regulation by Ca2+ and possible function in short-term synaptic plasticity. EMBO J. 29:680–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rosenmund C, et al. 2002. Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron 33:411–424 [DOI] [PubMed] [Google Scholar]
- 43. Rosenmund C, Stevens CF. 1996. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16:1197–1207 [DOI] [PubMed] [Google Scholar]
- 44. Sakaba T, Neher E. 2001. Calmodulin mediates rapid recruitment of fast-releasing synaptic vesicles at a calyx-type synapse. Neuron 32:1119–1131 [DOI] [PubMed] [Google Scholar]
- 45. Schulz DM, Ihling C, Clore GM, Sinz A. 2004. Mapping the topology and determination of a low-resolution three-dimensional structure of the calmodulin-melittin complex by chemical cross-linking and high-resolution FTICRMS: direct demonstration of multiple binding modes. Biochemistry 43:4703–4715 [DOI] [PubMed] [Google Scholar]
- 46. Shatsky M, Nussinov R, Wolfson HJ. 2004. A method for simultaneous alignment of multiple protein structures. Proteins 56:143–156 [DOI] [PubMed] [Google Scholar]
- 47. Shin OH, et al. 2010. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat. Struct. Mol. Biol. 17:280–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stevens CF, Sullivan J. 1998. Synaptic plasticity. Curr. Biol. 8:R151–R153 [DOI] [PubMed] [Google Scholar]
- 49. Stevens DR, et al. 2005. Identification of the minimal protein domain required for priming activity of Munc13-1. Curr. Biol. 15:2243–2248 [DOI] [PubMed] [Google Scholar]
- 50. Sudhof TC. 2004. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27:509–547 [DOI] [PubMed] [Google Scholar]
- 51. Varoqueaux F, et al. 2002. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc. Natl. Acad. Sci. U. S. A. 99:9037–9042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Varoqueaux F, Sons MS, Plomp JJ, Brose N. 2005. Aberrant morphology and residual transmitter release at the Munc13-deficient mouse neuromuscular synapse. Mol. Cell. Biol. 25:5973–5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang LY, Kaczmarek LK. 1998. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 394:384–388 [DOI] [PubMed] [Google Scholar]
- 54. Wojcik SM, Brose N. 2007. Regulation of membrane fusion in synaptic excitation-secretion coupling: speed and accuracy matter. Neuron 55:11–24 [DOI] [PubMed] [Google Scholar]
- 55. Xu XZ, et al. 1998. Retinal targets for calmodulin include proteins implicated in synaptic transmission. J. Biol. Chem. 273:31297–31307 [DOI] [PubMed] [Google Scholar]
- 56. Yap KL, et al. 2000. Calmodulin target database. J. Struct. Funct. Genomics 1:8–14 [DOI] [PubMed] [Google Scholar]
- 57. Zikich D, et al. 2008. Vesicle priming and recruitment by ubMunc13-2 are differentially regulated by calcium and calmodulin. J. Neurosci. 28:1949–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zucker RS, Regehr WG. 2002. Short-term synaptic plasticity. Annu. Rev. Physiol. 64:355–405 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.