Abstract
Perenniporia species are basidiomycetes, resupinate shelf fungi responsible for white rot decay of wood. Here, we report for the first time an intracavitary pulmonary fungal ball due to a species of Perenniporia that has not been recognized so far as a human pathogen. The fungus was identified by sequencing of the partial ribosomal operon of a culture from a clinical specimen.
CASE REPORT
A 55-year-old nonsmoking male from Assam, India, presented with complaints of intermittent right-sided nonanginal chest pain and 5 to 6 episodes of minor hemoptysis over a period of 1 month. The patient gave a history of having received antitubercular therapy 3 years before with a 6-month-long regimen containing rifampin, isoniazid, ethambutol, and pyrazinamide for sputum-smear-positive pulmonary tuberculosis, with which he had been compliant until declared cured by his physician. The patient was found to have uncontrolled diabetes mellitus as revealed by an HbA1c level of 13%. He was previously evaluated in Assam, where a chest radiograph showed a cavity in the right upper and middle pulmonary zone. A contrast-enhanced computerized tomography (CECT) scan of his thorax revealed the presence of fibroparenchymal lesions with mild traction bronchiectasis in the right lung (sequelae of healed pulmonary tuberculosis) associated with a fungal ball in the right lower lobe (Fig. 1). The patient was treated with CT-guided percutaneous intracavitary injection of amphotericin B, though records of the dosing schedule could not be obtained. The patient was then referred to Delhi for mycological diagnosis and further management of the case. The patient underwent a fiber optic bronchoscopy (FOB) to rule out the possibility of reactivation of tuberculosis and for an investigative work-up for hemoptysis. The FOB showed no endobronchial lesion, active bleeding, or blood clots in the bronchi. A diagnostic bronchoalveolar lavage (BAL) fluid sample was taken from the apical segment of the right lower and postero-anterior basal segment of the right upper lobe.
Fig 1.
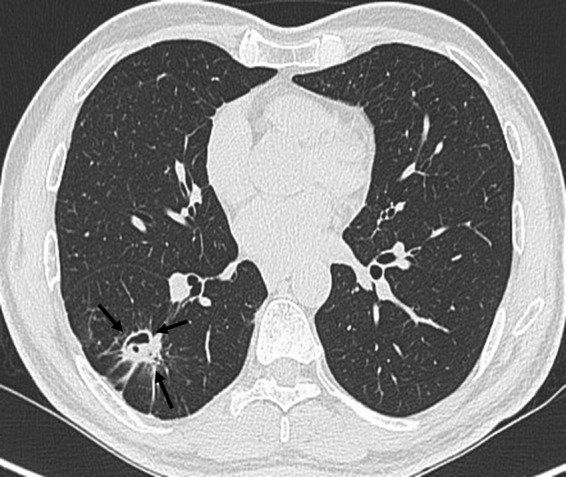
High-resolution CECT scan of the patient showing a fungal ball within a cavity located in the lower lobe of the right lung (arrows).
Mycological investigations.
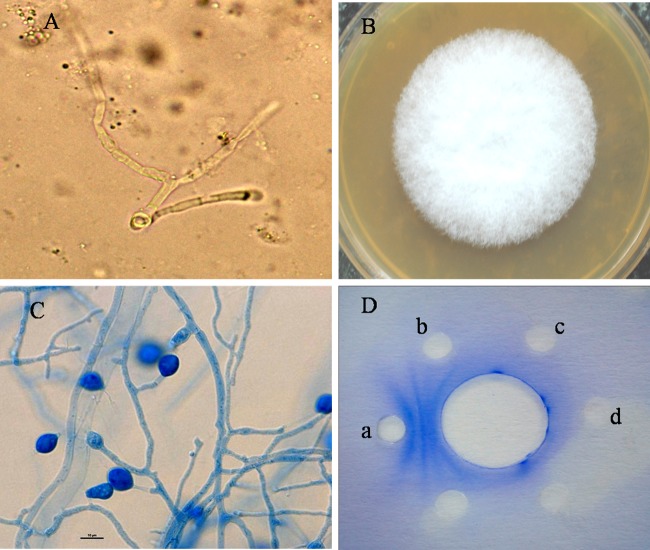
Direct microscopy of KOH wet mounts of the BAL fluid specimen revealed hyaline septate hyphae (Fig. 2A). Cultures yielded multiple white, cottony colonies of identical molds on Sabouraud's glucose agar (SGA) plates incubated for 7 days at 28°C and 37°C. Subcultures on potato dextrose agar (PDA) incubated at 28°C and 37°C showed dense white cottony growth after 7 days (Fig. 2B), and slide cultures of the mold isolates on PDA at 28°C revealed hyaline septate hyphae with chlamydospore-like cells (Fig. 2C). No clamp connections or hyphal pegs were seen during up to 3 weeks of incubation. The isolate was assigned accession no. VPCI 85/P/10 (CBS 130020) for molecular identification and antifungal susceptibility testing. BAL fluid was also investigated microscopically after Gram and Ziehl-Neelsen (ZN) staining and cultured for aerobic pathogens and Mycobacterium spp. The Gram and ZN stains were negative, and cytology was negative for any malignant cells. No Mycobacterium spp. were isolated after 6 weeks of incubation.
Fig 2.
(A) KOH wet mount of BAL fluid specimen of the patient showing hyaline septate hyphae (magnification, ×400). (B) White cottony growth on PDA culture plate seen after 7 days of incubation at 28°C. (C) Slide culture of the isolate on PDA showing hyaline septate hyphae with chlamydospores (magnification, ×400). (D) Ouchterlony's agar gel double diffusion test of the patient's serum showing two precipitin bands against a Perenniporia species isolate (a) and negative results with antigens of A. fumigatus (b), A. flavus (c), and A. terreus (d).
Immunodiffusion of the patient's serum demonstrated precipitins against culture filtrate antigen prepared from the patient's isolate as described previously (Fig. 2D) (3, 4). In contrast, negative results were found in tests using antigens of Aspergillus fumigatus, Aspergillus flavus, Aspergillus terreus, and Aspergillus niger. It was evident from these findings that the patient was suffering from posttuberculosis fibrocavitary disease of the right lobe with a fungal ball. Since no episodes of hemoptysis in the recent past were reported by the patient, no active intervention was done and he was managed conservatively on an outpatient basis. He was briefed about the possibility of recurrent episodes of hemoptysis, for which he was prescribed ethamsylate and cough suppressants, and was advised to consult a nearby medical facility for the same in his hometown in Assam.
The identification of 85/P/10 (CBS 130020) was done by sequencing of the internal transcribed spacer (ITS) ribosomal DNA (rDNA) region and D1/D2 large subunit (LSU) regions as described previously (3, 4). GenBank BLAST searches were performed for species identification. The LSU sequence of CBS 130020 showed 99% identity with Megasporoporia setulosa GU566007 (strain MG38) and 100% identity with an unidentified Perenniporia species, strain 1V2/2 (GQ982883). The ITS region sequence of the isolate exhibited 100% identity with the Perenniporia strain 1V2/2 (GQ982890). The nearest neighbor, Megasporoporia setulosa JF894111, showed 90% similarity. The ITS and LSU nucleotide sequences for the isolate CBS 130020 were deposited in GenBank under the accession numbers JX271779 and JX292098, respectively.
ITS sequences from reference isolates belonging to the genera Abundisporus (n = 5), Agaricomycetes (n = 2), Donkioporia (n = 1), Megasporoporia (n = 6), Microporellus (n = 1), Perenniporia (n = 53), Perenniporiella (n = 7), Polyporus (n = 1), Pyrofomes (n = 1), and Trametes (n = 1), described by Guglielmo et al. (14), Robledo et al. (23), Pinruan et al. (20), Yuan et al. (32), and Zhao and Cui (33), were included in the analyses (Table 1 ). Evolutionary analyses were conducted in MEGA5 (30) with the maximum likelihood method. Evolutionary distances were computed using the Kimura 2-parameter method (16) with 1,000 bootstrap replicates (10). A discrete gamma distribution was used to model evolutionary rate differences among sites.
Table 1.
Details of species and internal transcribed spacer (ITS) sequence database accession numbers of reference isolates used in the present study for phylogenetic analysis of Perenniporia species (CBS 130020)
| Species | Isolate code | Origin | ITS accession no. | Reference |
|---|---|---|---|---|
| Perenniporiella chaquenia | MUCL 47648 | Argentina | FJ411084 | 23 |
| Perenniporiella chaquenia | MUCL 49758 | Argentina | FJ411085 | 23 |
| Perenniporiella chaquenia | MUCL 47647 | Argentina | FJ411083 | 23 |
| Perenniporiella pendula | MUCL 47129 | Cuba | FJ411082 | 23 |
| Perenniporiella pendula | MUCL 46034 | Cuba | FJ411081 | 23 |
| Perenniporiella micropora | MUCL 43581 | Cuba | FJ411086 | 23 |
| Perenniporiella neofulva | MUCL 45091 | Cuba | FJ411080 | 23 |
| Perenniporia tephropora | Cui 9029 | China | HQ876601 | 33 |
| Perenniporia tephropora | Cui 6331 | China | HQ848473 | 33 |
| Perenniporia maackiae | Cui 8929 | HQ654102 | 33 | |
| Perenniporia corticola | Dai 7330 | HQ654094 | 33 | |
| Perenniporia corticola | Cui 2655 | HQ654093 | 33 | |
| Perenniporia corticola | Cui 1248 | China | HQ848472 | 33 |
| Perenniporia minor | Cui 5738 | China | HQ848475 | 33 |
| Perenniporia minor | Cui 5782 | China | HQ883475 | 33 |
| Perenniporia straminea | Cui 8858 | HQ654104 | 33 | |
| Perenniporia straminea | Cui 8718 | China | HQ876600 | 33 |
| Perenniporia ohiensis | Cui 5714 | HQ654103 | 33 | |
| Perenniporia ohiensis | MUCL 41036 | USA | FJ411096 | 23 |
| Perenniporia detrita | MUCL 42649 | French Guyana | FJ411099 | 23 |
| Perenniporia ochroleuca | MUCL 39563 | Australia | FJ411097 | 23 |
| Perenniporia ochroleuca | MUCL 39726 | Taiwan | FJ411098 | 23 |
| Perenniporia ochroleuca | Cui 8817 | China | HQ848476 | 33 |
| Perenniporia ochroleuca | Dai 11486 | HQ654105 | 33 | |
| Perenniporia nanlingensis | Cui 7620 | China | HQ848477 | 33 |
| Perenniporia nanlingensis | Cui 7541 | China | HQ848479 | 33 |
| Perenniporia minutissima | Dai 11643 | China | HQ876602 | 33 |
| Perenniporia tenuis | Cui 5523 | China | HQ848474 | 33 |
| Perenniporia truncatospora | Dai 5125 | HQ654098 | 33 | |
| Perenniporia japonica | Cui 7047 | HQ654097 | 33 | |
| Perenniporia rhizomorpha | Cui 7507 | HQ654107 | 33 | |
| Perenniporia medulla-panis | Dai 10780 | HQ654099 | 33 | |
| Perenniporia medulla-panis | Dai 8736 | HQ654100 | 33 | |
| Perenniporia medulla-panis | MUCL 45934 | Thailand | FJ411091 | 23 |
| Perenniporia medulla-panis | MUCL 51629 | USA | FJ411090 | 23 |
| Perenniporia medulla-panis | MUCL 47876 | China | FJ411089 | 23 |
| Perenniporia medulla-panis | MUCL 49581 | Poland | FJ411088 | 23 |
| Perenniporia medulla-panis | MUCL 43250 | Norway | FJ411087 | 23 |
| Megasporoporia setulosa | JV1008 102J | USA | JF894111 | Vlasak et al.,a unpublished data |
| Megasporoporia setulosa | JV1008 51J | USA | JF894109 | Vlasak et al.,a unpublished data |
| Megasporoporia setulosa | JV1008_102J | USA | JF894110 | Vlasak et al.,a unpublished data |
| Perenniporia subadusta | Cui 8459 | China | HQ876606 | 33 |
| Microporellus violaceo-cinerascens | MUCL 45229 | Ethiopia | FJ411106 | 23 |
| Trametes versicolor | M126 | HM595570 | 32 | |
| Megasporoporia sp. | Dai 12306 | JQ314362 | Li et al.,b unpublished data | |
| Megasporoporia sp. | Dai 12278 | JQ314361 | Li et al.,b unpublished data | |
| Perenniporia sp. | E7373 | Indonesia | AJ537408 | Bougher et al.,c unpublished data |
| Perenniporia subacida | Cui 3643 | FJ613655 | 33 | |
| Perenniporia subacida | Dai 8224 | China | HQ876605 | 33 |
| Perenniporia subacida | MUCL 31402 | Japan | FJ411103 | 23 |
| Perenniporia narymica | Dai 10510 | HQ654101 | 33 | |
| Agaricomycetes sp. | CK | JN630804 | Sheikhi et al.,d unpublished data | |
| Agaricomycetes sp. | India01 | HM167516 | R. Sasidhara and T. Thirunalasundari, unpublished data | |
| Perenniporia sp. | 1V2/2 | GQ982890 | Pinruan et al.,e unpublished data | |
| Perenniporia sp. | CBS 130020 | India | JX271779 | Present study |
| Megasporoporia sp. | Dai 12170 | JQ314363 | Li et al.,b unpublished data | |
| Perenniporia fergusii | Gilbertson 16116 | China | HQ876607 | 33 |
| Polyporus arcularius | CulTENN7883 | Costa Rica | AF516524 | 17 |
| Perenniporia robiniophila | Cui 5644 | China | HQ876609 | 33 |
| Perenniporia robiniophila | Cui 7144 | China | HQ876608 | 33 |
| Perenniporia robiniophila | Dai 10416 | HQ654096 | 33 | |
| Perenniporia robiniophila | Cui 9174 | China | HQ876610 | 33 |
| Perenniporia vicina | MUCL 44779 | Ethiopia | FJ411095 | 23 |
| Perenniporia fraxinea | Cui 8885 | China | HQ876611 | 33 |
| Perenniporia formosana | Dai 5245 | China | HQ876612 | 33 |
| Perenniporia fraxinea | Cui 7154 | HQ654095 | 33 | |
| Perenniporia fraxinea | MUCL 39326 | France | FJ411094 | 23 |
| Perenniporia fraxinea | DP83 | Italy | AM269789 | 14 |
| Pyrofomes demidoffii | MUCL 41034 | Russia | FJ411105 | 23 |
| Donkioporia expansa | MUCL 35116 | Belgium | FJ411104 | 23 |
| Perenniporia martius | MUCL 41677 | Argentina | FJ411092 | 23 |
| Perenniporia martius | MUCL 41678 | Argentina | FJ411093 | 23 |
| Perenniporia martius | Cui 7992 | China | HQ876603 | 33 |
| Perenniporia latissima | Cui 6652 | China | HQ876604 | 33 |
| Abundisporus sp. | MUCL 49566 | China | FJ411108 | 23 |
| Abundisporus violaceus | MUCL 38617 | Zimbabwe | FJ411100 | 23 |
| Abundisporus sclerosetosus | MUCL 41438 | Singapore | FJ411101 | 23 |
| Abundisporus roseoalbus | MUCL 49583 | China | FJ411102 | 23 |
| Abundisporus roseoalbus | MUCL 49622 | China | FJ411107 | 23 |
J. Vlasak, J. Kout, Jr., J. Vlasak, and L. Ryvarden.
H. J. Li, B. K. Cui, and Y. C. Dai.
N. L. Bougher, I. C. Tommerup, S. R. H. Langrell, S. Q. Bolsenbroek, and J. M. Catchpole.
F. Sheikhi, M. Roayaei Ardakani, and N. Enayatizamir.
U. Pinruan, N. Rungjindamai, R. Choeyklin, S. Lumyong, and G. Jones.
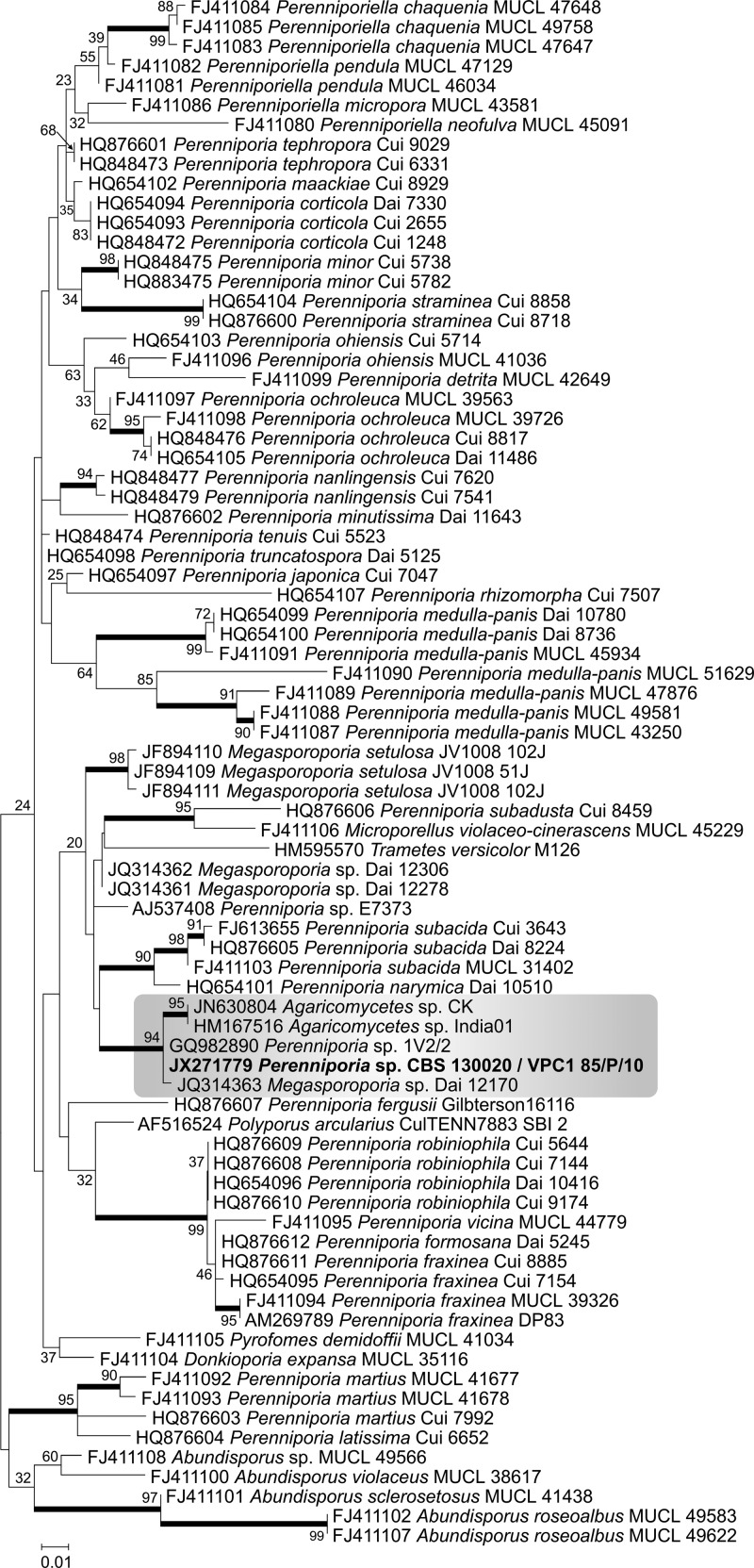
The complete alignment included 79 sequences. Aligned sequences of the ITS were 866 bp long, including 340 invariable characters, 324 variable parsimony-informative (37.4%) characters, and 108 singletons. Positions containing gaps and missing data were eliminated. The tree comprised 31 described Perenniporia species, including the generic type species P. medulla-panis. All of the unresolved deeper branches exhibited bootstrap values below 80%, raising concern about the cluster of related species. Furthermore, the tree (outside the ancestral Abundisporus branches) included species of Donkioporia, Megasporoporia, Polyporus, Pyrofomes, and Trametes, which may indicate possible misidentification. The clinical isolate CBS 130020 shared a supported clade (bootstrap, 94%) with unidentified Agaricomycetes and a Megasporoporia sp. and was found to be identical to the unnamed basidiomycetous endophyte 1V2/2, described by Pinruan et al. (20) (Fig. 3). The nearest taxa are Perenniporia subacida and Perenniporia narymica, located in a sister clade at a 90% bootstrap level.
Fig 3.
Molecular phylogenetic tree based on ITS sequences generated in this study by the maximum likelihood method based on the Kimura 2-parameter model. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1,000 replicates) is shown next to the branches (bootstrap support values of >90% are indicated in bold). GenBank accession numbers are indicated before the strain code.
Antifungal susceptibility testing (AFST) of the isolate was performed by the CLSI broth microdilution method (5). The antifungals tested were amphotericin B (Sigma, St. Louis, MO), fluconazole (Pfizer, Groton, CT), itraconazole (Lee Pharma, Hyderabad, India), voriconazole (Pfizer), posaconazole (Schering-Plough, Kenilworth, NJ [now Astellas]), isavuconazole (Basilea Pharmaceutica, Basel, Switzerland), flucytosine (Sigma), caspofungin (Merck, Whitehouse Station, NJ), micafungin (Astellas, Toyama, Japan), and anidulafungin (Pfizer). For the broth microdilution test, RPMI 1640 medium with glutamine without bicarbonate (Sigma) buffered to pH 7 with 0.165 mol/liter 3-N-morpholinepropanesulfonic acid (Sigma) was used. Isolates were grown on PDA for 8 days at 37°C, and the inoculum was adjusted to a final density of 1.0 × 104 to 5.0 × 104 hyphal fragments/ml measured by a spectrophotometer. Drug-free and mold-free controls were included, and microtiter plates were incubated at 35°C for 72 h. CLSI-recommended quality control strains Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 and reference strains Aspergillus fumigatus ATCC 204305 and Aspergillus flavus ATCC 204304 were included. The MIC endpoints were read visually; for azoles and amphotericin B, MICs were defined as the lowest concentration at which there was 100% inhibition of growth compared with the drug-free control wells. For echinocandins, minimal effective concentrations (MECs) were defined as the lowest concentration of drug that led to the growth of small, rounded, and compact hyphal forms. For the isolate, the lowest MIC was that of posaconazole (0.06 μg/ml), followed by itraconazole (0.5 μg/ml), voriconazole (2 μg/ml), and isavuconazole (2 μg/ml). Amphotericin B had a MIC of 0.25 μg/ml. All three echinocandins showed good activity (MECs, 0.125 to 0.5 μg/ml). Fluconazole and flucytosine did not show any activity (64 μg/ml).
The clinical significance of white, cottony, rapidly growing filamentous molds from pulmonary samples is poorly understood. In the past, cultures have often been discarded as purported contaminants, because morphological identification of these nonsporulating fungi was impossible, and hence, they could not be attributed to any of the known pathogens. Today, we know that many of these cultures are of basidiomycete affinity (9). The most common of these is Schizophyllum commune, which is recognizable morphologically by the presence of hyphal pegs (26) and sometimes by clamp connections and by the formation of abortive fruiting bodies (3). Occasionally, another basidiomycete repeatedly isolated from pulmonary infections in humans is Hormographiella aspergillata, the anamorph of the Agaricales mushroom Coprinopsis cinereus (11, 13, 27, 28, 31). The Coprinopsis species are recognizable in culture by the formation of arthroconidial anamorphs (9). Recently, some lesser-known basidiomycete species, such as Cyclomyces tabacinus (18), Irpex lacteus (2), Inonotus (Phellinus) tropicalis (7, 29), Oxyporus corticola (1), and Volvariella volvacea (25), have been added as causative agents of pulmonary and fatal deep-seated mycoses in humans and animals. Other basidiomycetes such as Phanerochaete chrysosporium (anamorph, Sporotrichum pruinosum) and Bjerkandera adusta have repeatedly been isolated from pulmonary sites and may also be pathogenic in some settings (12, 15).
The majority of infections caused by filamentous basidiomycetes are associated with chronic colonization of cavities in lungs or sinuses (3, 21, 26). Occasionally, however, this may lead to fatal dissemination and cerebral involvement, implying that this fungal group may have a neurotropic potential (22). As long as there is a paucity of information on fungus and host responses for filamentous basidiomycetes, infections by these fungi should be treated with caution. The present case demonstrates a novel agent of fungal ball due to a Perenniporia species. The case was diagnosed by CECT showing intracavitary mass, by bronchoscopy, by direct demonstration (KOH wet mount), and by isolation of the species in culture from BAL fluid. Serological analysis demonstrated precipitins against the etiologic agent. Many patients with fungal ball are asymptomatic, but the most frequent symptom is hemoptysis. Less commonly, patients develop chest pain, dyspnea, malaise, and wheezing. In the present case, the patient was afebrile with a history of mild hemoptysis. In asymptomatic patients, no treatment is required, regular observation being sufficient in most cases. There is no consistent evidence that fungal ball responds to antifungal agents, and such drugs rarely achieve the effective concentrations within the lung cavities (19).
The isolate did not show the characteristics that facilitated recognition of a filamentous basidiomycete such as the presence of clamp connections and/or crystals, the formation of spicules along the hyphae, and mushroom- or basidiocarp-type fruiting bodies. Since most clinical isolates are monokaryons, neither clamps nor fruiting structures are produced. The isolate was proven to be affiliated with the filamentous basidiomycetes through sequencing. Definite identification was impossible, because it showed 100% identity with an as-yet-unidentified species attributed to Perenniporia and it was close to several unnamed basidiomycete species (Fig. 3). The nearest named species was Megasporoporia setulosa, at a 10% ITS distance. Taxonomically, Perenniporia and Megasporoporia belong to the order Poriales in the Agaricomycetes, Basidiomycota. Fungi of this group are characterized morphologically by formation of porate, often resupinate fruiting bodies which are flat on the substrate with the hymenium on the outer side on rotten branches. The genus Perenniporia contains numerous species growing as saprobes on dead wood (6, 33). They are ubiquitously present under almost all types of climatic conditions, degrading wood by decomposition of lignin and, to a limited extent, cellulose, leading to white rot. Most species are presently known from herbarium materials only and have not been sequenced. The fact that the sequence of our strain did not have a match in GenBank is therefore not surprising, as there are not enough data in GenBank to identify unknown sterile basidiomycetes with a high degree of confidence by ITS and/or D1/D2 sequencing (24). Through GenBank, similarities also were found with other genera of shelf fungi, such as Trametes, Donkioporia, and Pyrofomes (Fig. 3). The taxonomy of these fungi has insufficiently been resolved using modern, nonmorphological techniques, and the sequences available thus far do not clearly resolve the genera (Fig. 3). Therefore, description of our strain as a novel species in any of the genera mentioned does not seem to be appropriate. In our tree (Fig. 3), the name Perenniporia is widely distributed (31 described species). The generic type species, P. medulla-panis, included in the tree is represented by strains studied by Decock and Stalpers, who rectified the generic typification (8). This and several other Perenniporia species show variability in the sequenced gene. Perenniporia medulla-panis occupies a central location compared to the remaining species, and the main branches lack statistical support, indicating concerns due to related taxa. Therefore, attribution of our strain to the genus Perenniporia is justified.
Perenniporia species have never been reported as etiologic agents of human disease before. Similarly to Schizophyllum, the fungus produces large amounts of airborne basidiospores which are easily inhaled. The frequency with which this leads to colonization of pulmonary cavities or systemic dissemination is presently unknown. This report extends the genera of basidiomycetous fungi implicated in pulmonary infections and underscores the utility of molecular methods in the identification or verification of these often unidentifiable molds when conventional mycological techniques fail to identify the incriminated fungi (21).
Nucleotide sequence accession numbers.
The ITS and LSU nucleotide sequences for the isolate CBS 130020 were deposited in GenBank under the accession numbers JX271779 and JX292098, respectively.
ACKNOWLEDGMENTS
This work was carried out, in part, with financial assistance from the Department of Biotechnology (reference no. BT/39/NE/TBP/2010), New Delhi, India.
J.F.M. received grants from Astellas, Merck, Pfizer, Schering-Plough, Gilead, and Janssen Pharmaceuticals. He has been a consultant to Basilea and Merck and received speaker's fees from Merck, Pfizer, Schering-Plough, Gilead, and Janssen Pharmaceuticals. For all other authors, there are no potential conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Footnotes
Published ahead of print 15 August 2012
REFERENCES
- 1. Brockus CW, et al. 2009. Disseminated Oxyporus corticola infection in a German shepherd dog. Med. Mycol. 47:862– 868 [DOI] [PubMed] [Google Scholar]
- 2. Buzina W, Lass-Flörl C, Kropshofer G, Freund MC, Marth E. 2005. The polypore mushroom Irpex lacteus, a new causative agent of fungal infections. J. Clin. Microbiol. 43:2009– 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chowdhary A, et al. 2012. Schizophyllum commune as an emerging fungal pathogen: a review and report of two cases. Mycoses [Epub ahead of print.] doi:10.1111/j.1439-0507.2012.02190.x [DOI] [PubMed] [Google Scholar]
- 4. Chowdhary A, et al. 2011. Bipolaris hawaiiensis as etiologic agent of allergic bronchopulmonary mycosis: first case in a paediatric patient. Med. Mycol. 49:765– 769 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antimicrobial susceptibility testing of filamentous fungi, 2nd ed, M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6.Cui BK, Zhao CL. 2012. Morphological and molecular evidence for a new species of Perenniporia (Basidiomycota) from Tibet, southwestern China. Mycoscience doi:10.1007/s10267-011-0180-x [Google Scholar]
- 7. Davis CM, et al. 2007. Basidiomycetous fungal Inonotus tropicalis sacral osteomyelitis in X-linked chronic granulomatous disease. Pediatr. Infect. Dis. J. 26:655– 656 [DOI] [PubMed] [Google Scholar]
- 8. Decock C, Stalpers JA. 2006. Proposal to conserve the name Perenniporia against Physisporus with a conserved type (Basidiomycota). Taxon 55:227– 238 [Google Scholar]
- 9. de Hoog GS, Guarro J, Gené J, Figueras MJ. 2009. Atlas of clinical fungi, 3rd ed. CBS, Utrecht, The Netherlands: [Google Scholar]
- 10. Felsenstein J. 1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783– 791 [DOI] [PubMed] [Google Scholar]
- 11. Gené J, Guillamón JM, Guarro J, Pujol I, Ulfig K. 1996. Molecular characterization, relatedness and fungal susceptibility of the basidiomycetous Hormographiella species and Coprinus cinereus from clinical and environmental sources. Antonie Van Leeuwenhoek 70:49– 57 [DOI] [PubMed] [Google Scholar]
- 12. González GM, Sutton DA, Thompson E, Tijerina R, Rinaldi MG. 2001. In vitro activities of approved and investigational antifungal agents against 44 clinical isolates of basidiomycetous fungi. Antimicrob. Agents Chemother. 45:633– 635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guarro J, Gené J, De Vroey C, Guého E. 1992. Hormographiella, a new genus of hyphomycetes from clinical sources. Mycotaxon 45:179– 190 [Google Scholar]
- 14. Guglielmo F, Bergemann SE, Gonthier P, Nicolotti G, Garbelotto M. 2007. A multiplex PCR-based method for the detection and early identification of wood rotting fungi in standing trees. J. Appl. Microbiol. 103:1490– 1507 [DOI] [PubMed] [Google Scholar]
- 15. Khan ZU, Randhawa HS, Kowshik T, Gaur SN, de Vries GA. 1988. The pathogenic potential of Sporotrichum pruinosum isolated from the human respiratory tract. J. Med. Vet. Mycol. 26:145– 151 [PubMed] [Google Scholar]
- 16. Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111– 120 [DOI] [PubMed] [Google Scholar]
- 17. Krüger D, Gargas A. 2004. The basidiomycete genus Polyporus—an emendation based on phylogeny and putative secondary structure of ribosomal RNA molecules. Feddes Repertorium 115:530– 546 [Google Scholar]
- 18. Marriott D, Kwong T, Harkness J, Ellis D. 2006. Cyclomyces tabacinus as a cause of deep tissue infection: the first case report. Mycoses 49:147– 149 [DOI] [PubMed] [Google Scholar]
- 19. Pennington JE. 1980. Aspergillus lung disease. Med. Clin. North Am. 64:475– 490 [DOI] [PubMed] [Google Scholar]
- 20. Pinruan U, et al. 2010. Occurrence and diversity of basidiomycetous endophytes from the oil palm, Elaeis guineensis in Thailand. Fungal Divers. 41:71– 88 [Google Scholar]
- 21. Pounder JI, et al. 2007. Discovering potential pathogens among fungi identified as nonsporulating molds. J. Clin. Microbiol. 45:568– 571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rihs JD, Padhye AA, Good CB. 1996. Brain abscess caused by Schizophyllum commune: an emerging basidiomycete pathogen. J. Clin. Microbiol. 34:1628– 1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robledo GL, Amalfi M, Castillo G, Rajchenberg M, Decock C. 2009. Perenniporiella chaquenia sp. nov. and further notes on Perenniporiella and its relationships with Perenniporia (poriales, basidiomycota). Mycologia 101:657– 673 [DOI] [PubMed] [Google Scholar]
- 24. Romanelli AM, Sutton DA, Thompson EH, Rinaldi MG, Wickes BL. 2010. Sequence-based identification of filamentous basidiomycetous fungi from clinical specimens: a cautionary note. J. Clin. Microbiol. 48:741– 752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salit RB, et al. 2010. Death by edible mushroom: first report of Volvariella volvacea as an etiologic agent of invasive disease in a patient following double umbilical cord blood transplantation. J. Clin. Microbiol. 48:4329– 4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sigler L, de la Maza LM, Tan G, Egger KN, Sherburne RK. 1995. Diagnostic difficulties caused by a nonclamped Schizophyllum commune isolate in a case of fungus ball of the lung. J. Clin. Microbiol. 33:1979– 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Speller DCE, MacIver AG. 1971. Endocarditis caused by a Coprinus species: a fungus of the toadstool group. J. Med. Vet. Mycol. 4:370– 374 [DOI] [PubMed] [Google Scholar]
- 28. Surmont I, Van Aelst F, Verbanck J, de Hoog GS. 2002. A pulmonary infection caused by Coprinus cinereus (Hormographiella aspergillata) diagnosed after a neutropenic episode. Med. Mycol. 40:217– 219 [DOI] [PubMed] [Google Scholar]
- 29. Sutton DA, et al. 2005. Identification and first report of Inonotus (Phellinus) tropicalis as an etiologic agent in a patient with chronic granulomatous disease. J. Clin. Microbiol. 43:982– 987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731– 2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Verweij PE, et al. 1997. Fatal pulmonary infection caused by the basidiomycete Hormographiella aspergillata. J. Clin. Microbiol. 35:2675– 2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuan ZL, Rao LB, Chen YC, Zhang CL, Wu YG. 2011. From pattern to process: species and functional diversity in fungal endophytes of Abies beshanzuensis. Fungal Biol. 115:197– 213 [DOI] [PubMed] [Google Scholar]
- 33. Zhao CL, Cui BK. 2012. A new species of Perenniporia (Polyporales, Basidiomycota) described from southern China based on morphological and molecular characters. Mycol. Prog. 11:555– 560 [Google Scholar]