Abstract
The contents of 2'-O-methylguanosine and 1-methyladenosine in unfractionated tRNA obtained from Thermus thermophilus HB27 were found to increase significantly when the bacterium was grown at a higher temperature (80 degrees C). S-Adenosyl-L-methionine-dependent tRNA (guanosine-2')-methyltransferase (EC 2.1.1.34) and tRNA (adenine-1)-methyltransferase (EC 2.1.1.36) were detected in a cell-free extract of the thermophile, and both of them were partially purified. tRNA (guanosine-2')-methyltransferase specifically catalyzed the methylation of the guanylate residue at position 19 from the 5' end of Escherichia coli tRNAMetf. The amounts of these methyltransferases in the cells and their thermal characteristics seemed to be independent of the growth temperature of the bacterial cells from which the enzymes were extracted. It was inferred that the temperature dependence of the methylation process in vivo is accounted for, not by temperature dependence of enzyme formation, but by that of the enzyme activity.
Full text
PDF
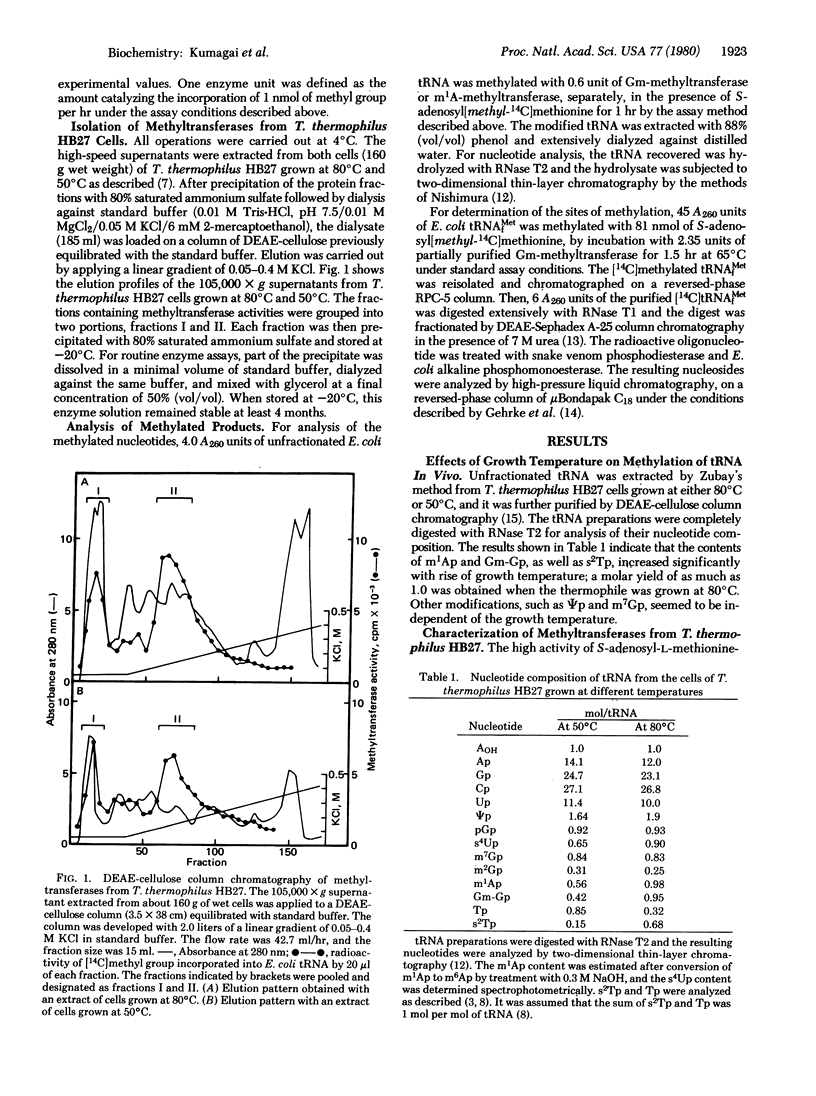



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Koh H., Söll D. The effect of growth temperatures on the in vivo ribose methylation of Bacillus stearothermophilus transfer RNA. Arch Biochem Biophys. 1973 Jan;154(1):277–282. doi: 10.1016/0003-9861(73)90058-1. [DOI] [PubMed] [Google Scholar]
- Cory S., Marcker K. A. The nucleotide sequence of methionine transfer RNA-M. Eur J Biochem. 1970 Jan;12(1):177–194. doi: 10.1111/j.1432-1033.1970.tb00836.x. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Grüter F., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1979 Jan;6(1):r1–r19. [PMC free article] [PubMed] [Google Scholar]
- Gefter M. L. The in vitro synthesis of 2'-omethylguanosine and 2-methylthio 6N (gamma,gamma, dimethylallyl) adenosine in transfer RNA of Escherichia coli. Biochem Biophys Res Commun. 1969 Aug 7;36(3):435–441. doi: 10.1016/0006-291x(69)90583-x. [DOI] [PubMed] [Google Scholar]
- Gehrke C. W., Kuo K. C., Davis G. E., Suits R. D., Waalkes T. P., Borek E. Quantitative high-performance liquid chromatography of nucleosides in biological materials. J Chromatogr. 1978 Mar 21;150(2):455–476. doi: 10.1016/s0021-9673(00)88205-9. [DOI] [PubMed] [Google Scholar]
- HOLLEY R. W. Large-scale preparation of yeast "soluble" ribonucleic acid. Biochem Biophys Res Commun. 1963 Jan 31;10:186–188. doi: 10.1016/0006-291x(63)90048-2. [DOI] [PubMed] [Google Scholar]
- HOSKINSON R. M., KHORANA H. G. STUDIES ON POLYNUCLEOTIDES. XLI. PURIFICATION OF PHENYLALANINE-SPECIFIC TRANSFER RIBONUCLEIC ACID FROM YEAST BY COUNTERCURRENT DISTRIBUTION. J Biol Chem. 1965 May;240:2129–2134. [PubMed] [Google Scholar]
- Harada F., Kimura F., Nishimura S. Primary sequence of tRNA val from Escherichia coli B. I. Oligonucleotide sequences of digests of Escherichia coli tRNA val with RNase T and pancreatic RNase. Biochemistry. 1971 Aug 17;10(17):3269–3277. doi: 10.1021/bi00793a017. [DOI] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Structure of yeast phenylalanine transfer RNA at 2.5 A resolution. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4414–4418. doi: 10.1073/pnas.72.11.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelicke A., von der Haar F., Sprinzl M., Cramer F. The structure of the anticodon loop of tRNAPhe from yeast as deduced from spectroscopic studies on oligonucleotides. Biopolymers. 1975 Jan;14(1):155–171. doi: 10.1002/bip.1975.360140112. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Oshima T., Sakaki Y., Wakayama N., Watanabe K., Ohashi Z. Biochemical studies on an extreme thermophile Thermus thermophilus: thermal stabilities of cell constituents and a bacteriophage. Experientia Suppl. 1976;26:317–331. doi: 10.1007/978-3-0348-7675-9_26. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Seeman N. C., Suddath F. L., Rich A., Sussman J. L., Kim S. H. Hydrogen bonding in yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4866–4870. doi: 10.1073/pnas.72.12.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seno T., Kobayashi M., Nishimura S. Recovery of transfer RNA functions by combining fragmented Escherichia coli formylmethionine transfer RNA. Biochim Biophys Acta. 1969 Oct 22;190(2):285–303. doi: 10.1016/0005-2787(69)90080-x. [DOI] [PubMed] [Google Scholar]
- Taya Y., Nishimura S. Biosynthesis of 5-methylaminomethyl-2-thiouridylate. I. Isolation of a new tRNA-methylase specific for 5-methylaminomethyl-2-thiouridylate. Biochem Biophys Res Commun. 1973 Apr 16;51(4):1062–1068. doi: 10.1016/0006-291x(73)90035-1. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Kuchino Y., Yamaizumi Z., Kato M., Oshima T., Nishimura S. Nucleotide sequence of formylmethionine tRNA from an extreme thermophile, Thermus thermophilus HB8. J Biochem. 1979 Oct;86(4):893–905. doi: 10.1093/oxfordjournals.jbchem.a132621. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Iijima K., Yamaizumi Z., Nishimura S. Purification and thermal stability of several amino acid-specific tRNAs from an extreme thermophile, Thermus thermophilus HB8. J Biochem. 1980 Jan;87(1):1–13. doi: 10.1093/oxfordjournals.jbchem.a132713. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Nishimura S. CD spectra of 5-methyl-2-thiouridine in tRNA-Met-f from an extreme thermophile. Nucleic Acids Res. 1976 Jul;3(7):1703–1713. doi: 10.1093/nar/3.7.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Oshima T., Saneyoshi M., Nishimura S. Replacement of ribothymidine by 5-methyl-2-thiouridine in sequence GT psi C in tRNA of an extreme thermophile. FEBS Lett. 1974 Jul 1;43(1):59–63. doi: 10.1016/0014-5793(74)81105-1. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Shinma M., Oshima T., Nishimura S. Heat-induced stability of tRNA from an extreme thermophile, Thermus thermophilus. Biochem Biophys Res Commun. 1976 Oct 4;72(3):1137–1144. doi: 10.1016/s0006-291x(76)80250-1. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Taylor M. W., Brock T. D. Thermal stability of ribosomes and RNA from Thermus aquaticus. Biochim Biophys Acta. 1970 Apr 15;204(2):512–520. doi: 10.1016/0005-2787(70)90171-1. [DOI] [PubMed] [Google Scholar]


