Abstract
Between 2008 and 2011, population-based candidemia surveillance was conducted in Atlanta, GA, and Baltimore, MD. Surveillance had been previously performed in Atlanta in 1992 to 1993 and in Baltimore in 1998 to 2000, making this the first population-based candidemia surveillance conducted over multiple time points in the United States. From 2,675 identified cases of candidemia in the current surveillance, 2,329 Candida isolates were collected. Candida albicans no longer comprised the majority of isolates but remained the most frequently isolated species (38%), followed by Candida glabrata (29%), Candida parapsilosis (17%), and Candida tropicalis (10%). The species distribution has changed over time; in both Atlanta and Baltimore the proportion of C. albicans isolates decreased, and the proportion of C. glabrata isolates increased, while the proportion of C. parapsilosis isolates increased in Baltimore only. There were 98 multispecies episodes, with C. albicans and C. glabrata the most frequently encountered combination. The new species-specific CLSI Candida MIC breakpoints were applied to these data. With the exception of C. glabrata (11.9% resistant), resistance to fluconazole was very low (2.3% of isolates for C. albicans, 6.2% for C. tropicalis, and 4.1% for C. parapsilosis). There was no change in the proportion of fluconazole resistance between surveillance periods. Overall echinocandin resistance was low (1% of isolates) but was higher for C. glabrata isolates, ranging from 2.1% isolates resistant to caspofungin in Baltimore to 3.1% isolates resistant to anidulafungin in Atlanta. Given the increase at both sites and the higher echinocandin resistance, C. glabrata should be closely monitored in future surveillance.
INTRODUCTION
Candida species are a leading cause of health care-associated bloodstream infections (BSIs) in the U.S., accounting for approximately 10% of health care-associated BSIs and one of the highest crude mortality rates (37). Candidemia infections increase the risk of patient mortality and increase both the length of stay and cost associated with hospitalization (11, 17). To implement appropriate control measures, it is important to understand the epidemiology of BSIs caused by Candida species. Although the species distribution has changed over the last 3 decades, more than 95% of candidemia infections have been caused by the five most common species: Candida albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei (4, 26, 36). The most dramatic temporal change has been the shift from C. albicans to non-C. albicans species as causative agents of the majority of BSIs (1, 4, 10, 12, 16, 26, 36). The significance of this trend is that non-C. albicans species, especially C. glabrata, can be less susceptible to fluconazole, the most inexpensive and readily available antifungal agent used to treat candidemia (10, 25, 28, 31). The shift to non-C. albicans species is also significant because the newest class of antifungal agents, the echinocandins, is not currently recommended as primary therapy against C. parapsilosis, the third most common species in the United States (10, 16, 20, 25).
Sentinel and population-based surveillance play complementary roles in our understanding of candidemia. Sentinel surveillance can encompass many facilities over broad geographic areas, but the makeup of individual institutions may not be representative of whole populations. The advantage of population-based surveillance is that it allows the calculation of population-based incidence rates (9, 10, 12, 16, 25, 28, 29). Antifungal susceptibility testing of Candida isolates collected as part of surveillance has played an increasingly important role in surveillance strategies for epidemiological evaluation of antifungal resistance (2, 6, 9, 10, 12, 28, 33, 34). With the advent of standardized susceptibility testing methodology (7), past and present surveillance can be directly compared to monitor temporal trends in antifungal resistance. Global sentinel surveillance revealed an increasing trend in resistance to fluconazole among C. parapsilosis, C. guilliermondii, and C. lusitaniae, while the resistance rates among C. albicans, C. glabrata, and C. tropicalis fluctuated slightly or remained stable (28). Because of the recent appearance of echinocandins in clinical practice, temporal surveillance data for susceptibility to these drugs are limited, but an early report indicated that susceptibility rates were stable (23). Temporal changes in antifungal susceptibility for Candida species to any antifungal agent have not yet been described using population-based surveillance in a single U.S. city.
The Centers for Disease Control and Prevention (CDC) and state partners in the Emerging Infections Program (EIP) have undertaken two active, population-based surveillance studies to determine the incidence of candidemia, the distribution of Candida species causing BSI, and the prevalence of antifungal drug resistance (10, 12). In both studies, surveillance was conducted in two geographic areas: Atlanta, GA (1992 to 1993), and Baltimore City and Baltimore County, MD (1998 to 2000). CDC and EIP partners again conducted population-based surveillance for candidemia in 2008 to 2011, returning to the previous areas of Atlanta, GA, and Baltimore, MD. By returning to previous areas of surveillance, changes in species distribution and antifungal susceptibility can be described. Here, we report the laboratory results of that surveillance including molecular determination of Candida species and antifungal susceptibility testing against nine antifungal agents. This is the first time that susceptibility to echinocandins has been determined within a U.S. population-based collection of isolates.
MATERIALS AND METHODS
Case and isolate definitions.
Isolates were obtained from persons with an incident episode of candidemia (defined below) identified between 1 March 2008 and 28 February 2011 for residents of Georgia Health District 3 (the eight counties comprising metropolitan Atlanta, GA) (population, 3.8 million) and between 1 June 2008 and 31 May 2011 for residents of Baltimore City or Baltimore County, Maryland (population, 1.4 million). The isolate retrieval rate from identified incident cases in Atlanta was 71%, and in Baltimore it was 92%. An incident episode of candidemia was defined as the 30 days following the first positive blood culture for Candida species in a resident of the surveillance area. Positive cultures of the same species within the 30-day period were considered part of the incident case episode and were not captured, but positive cultures of a different species during a single episode were captured. Cultures drawn more than 30 days after the incident case were considered a new incident episode and assigned a new case number. Isolates were received from 17 hospitals in Baltimore, with an average of 87 isolates per hospital (range, 1 to 562), and from 24 hospitals in Atlanta, with an average of 63 isolates per hospital (range, 2 to 310).
Isolate storage, DNA extraction, PCR amplification, and sequencing.
Prior to use, all isolates were stored in glycerol at −70°C. Isolates were identified by both conventional biochemical means at the referring institutions and molecular means using a Luminex assay or DNA sequencing of the D1/D2 subunit of the 28S ribosomal DNA (rDNA) at the CDC as previously described (8).
Antifungal susceptibility testing.
Antifungal susceptibility testing was performed by broth microdilution with fluconazole, itraconazole, voriconazole, posaconazole, flucytosine, anidulafungin, caspofungin, and micafungin as described by the Clinical and Laboratory Standards Institute (CLSI) M27-A3 document guidelines (7) using frozen RPMI medium microbroth trays custom manufactured by Trek Diagnostics (Cleveland, OH). Susceptibility testing for amphotericin B was performed by Etest as per the manufacturer's instructions (bioMérieux, Durham, NC). Results were read visually after 24 h for fluconazole, voriconazole, posaconazole (24), caspofungin, micafungin, anidulafungin, amphotericin B, and flucytosine (14) and after 48 h for itraconazole. For all but amphotericin B, the MIC was read as the lowest concentration of drug that caused a significant decrease in growth compared to the control well. Recently approved CLSI 24-h resistance breakpoints for fluconazole, voriconazole, and the echinocandins were used (21, 22, 27): isolates of C. albicans, C. tropicalis, and C. parapsilosis with MICs of ≥8 μg/ml and isolates of C. glabrata with MICs of ≥64 μg/ml were considered resistant to fluconazole; C. krusei was considered intrinsically resistant to fluconazole; isolates of C. albicans, C. tropicalis, and C. parapsilosis with MICs of ≥1 μg/ml and C. krusei isolates with MICs of ≥2 μg/ml were considered resistant to voriconazole; isolates of C. albicans, C. tropicalis, and C. krusei with MICs of ≥1 μg/ml were considered resistant to caspofungin, micafungin, and anidulafungin; isolates of C. parapsilosis with MICs of ≥8 μg/ml were considered resistant to caspofungin, micafungin, and anidulafungin; isolates of C. glabrata with MICs of ≥0.5 μg/ml were considered resistant to caspofungin and anidulafungin, while those with MICs of ≥0.25 μg/ml were considered resistant to micafungin. For C. dubliniensis, the C. albicans breakpoints were used. All other species were considered to have no current breakpoints. Amphotericin B susceptibility was determined using Etest as per the manufacturer's instructions, and MIC values were read at 24 h. Quality-control isolates C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were included on each day of testing. As prior population-based surveillance was conducted in both Atlanta (1992 to 1993) (12) and Baltimore (1998 to 2000) (10) and as susceptibility to fluconazole was previously determined for all isolates, we applied the new breakpoints to the previous surveillance results and compared them to the current results.
Multispecies episodes.
A multispecies episode was defined as occurring when a Candida species other than the incident isolate species was recovered from a patient within the 30 days encompassing the incident episodic period or when two species were recovered simultaneously as part of the incident episode.
Defining multidrug resistance.
To screen for multidrug-resistant (MDR) isolates, the most frequently used antifungal agents in our surveillance areas were selected: fluconazole and voriconazole representing the azoles; caspofungin, micafungin, and anidulafungin representing the echinocandins; and amphotericin B as the polyene. Isolates were considered multidrug resistant if they were resistant to one or more representative antifungal(s) of two of the above classes. Since there are no resistance breakpoints for amphotericin B, the arbitrary but accepted breakpoint of 2.0 μg/ml for resistance was used.
RESULTS
Cases.
A total of 2,227 incident episodes had one or more isolates available, from which 2,329 eligible isolates were collected (Table 1). Incident isolates were available for 2,136 (80%) patients. There were 94 episodes with two species (42 in Atlanta and 52 in Baltimore) and 1 episode with three species, 1 episode with four species, and 1 episode with five species, all in Baltimore. Candida albicans was the most frequently isolated species, comprising 34% of the isolates from Baltimore and 41% of the isolates from Atlanta (Fig. 1). Candida glabrata was the second most prevalent species from both sites (27% in Atlanta and 31% in Baltimore), followed by C. parapsilosis and C. tropicalis. In Baltimore, C. dubliniensis was the fifth most prevalent species, but in Atlanta, C. lusitaniae was the fifth most prevalent species. Overall, C. albicans, C, glabrata, C. parapsilosis, C. tropicalis, and C. krusei comprised 95% of the isolates; with the addition of C. dubliniensis and C. lusitaniae, they comprised 98% of the isolates. A total of 217 (8%) case patients had more than one 30-day episode of candidemia. Of those, 69 (32%) were C. glabrata, 45 (21%) were C. albicans, 43 (20%) were C. parapsilosis, and 29 (13%) were C. tropicalis.
Table 1.
Species distribution from surveillance of 2008 to 2011
| Species | No. of isolates (% of total) |
||
|---|---|---|---|
| Combined total | Atlanta | Baltimore | |
| C. albicans | 877 (38) | 489 (41) | 388 (34) |
| C. glabrata | 670 (29) | 318 (27) | 352 (31) |
| C. parapsilosis | 389 (17) | 212 (18) | 177 (16) |
| C. tropicalis | 241 (10) | 103 (9) | 138 (12) |
| C. dubliniensis | 36 (2) | 5 | 31 (3) |
| C. lusitaniae | 34 (1) | 22 (2) | 12 (1) |
| C. krusei | 32 (1) | 19 (2) | 13 (1) |
| C. orthopsilosis | 13 | 6 | 7 |
| C. metapsilosis | 10 | 8 | 2 |
| C. guilliermondii | 8 | 5 | 3 |
| C. nivariensis | 4 | 3 | 1 |
| C. fermentati | 4 | 2 | 2 |
| C. bracarensis | 2 | 1 | 1 |
| C. catenulata | 2 | 0 | 2 |
| C. pararugosa | 2 | 1 | 1 |
| C. famata | 1 | 1 | 0 |
| C. kefyr | 1 | 1 | 0 |
| C. pelliculosa | 1 | 0 | 1 |
| C. norvegensis | 1 | 1 | 0 |
| C. rugosa | 1 | 1 | 0 |
| Total | 2,329 | 1,198 | 1,131 |
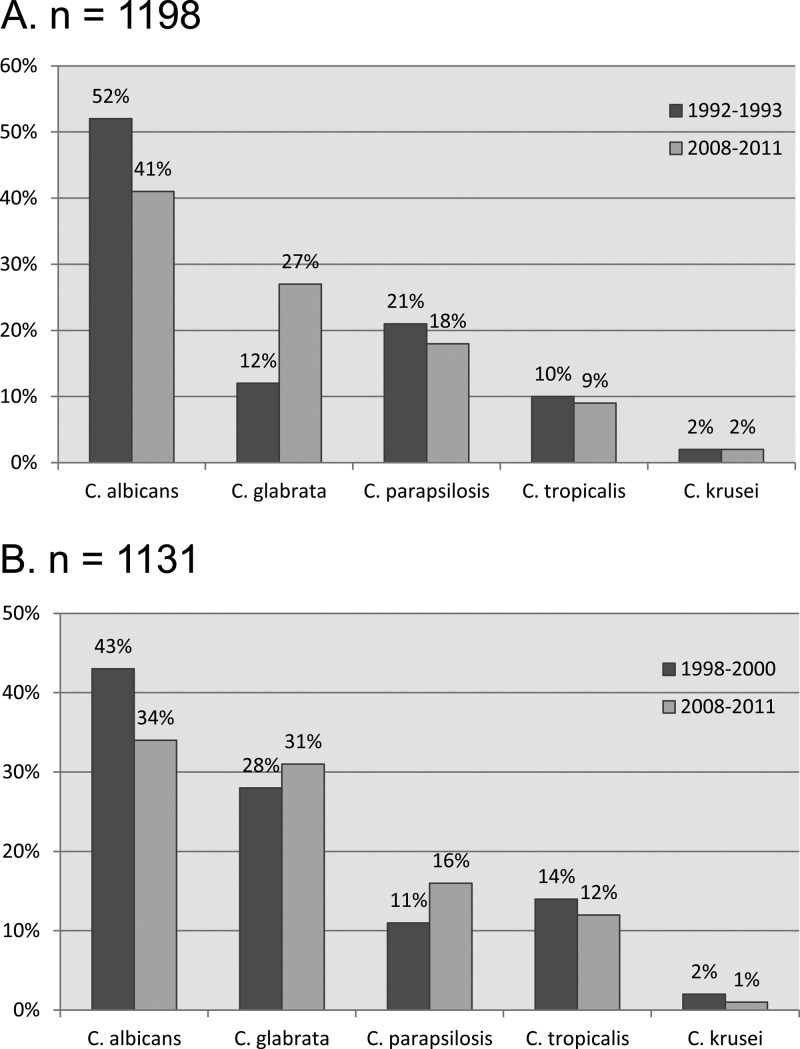
Fig 1.
Differences in species distribution between past (1992 to 1993) and present (2008 to 2011) surveillance in Atlanta (A) and Baltimore (B).
Susceptibility.
Among the 2,329 isolates tested (Tables 2, 3), overall resistance to fluconazole was 7.3%, primarily due to C. glabrata and C. krusei, which together comprised 31% of the total isolates for which breakpoints were available but 68% of the fluconazole-resistant isolates. Resistance to fluconazole varied by species, with C. glabrata having the highest rate (11.9% of isolates), followed by C. tropicalis (6.2%), C. dubliniensis (5.6%), C. parapsilosis (4.1%), and C. albicans (2.3%). Resistance to itraconazole was high (21.1% of isolates), again primarily due to C. glabrata (59.6% of isolates were resistant) and C. krusei (21.9% resistant). Overall, voriconazole resistance was low (1.0% of isolates), ranging from 0% among C. krusei to 2.5% among C. tropicalis isolates. There are no currently approved breakpoints for posaconazole, but the MIC90 value for most species was ≤0.5 μg/ml (Table 2).
Table 2.
In vitro susceptibilities of all 2008–2011 surveillance Candida isolates
| Species (no. of isolates) | Antifungal agent | MIC (μg/ml) |
% Resistant isolates (no.) | ||
|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | |||
| All isolates (2,329) | Fluconazole | 0.125–256 | 1 | 16 | 7.3 (165) |
| Voriconazole | 0.008–16 | 0.06 | 0.25 | 1.0 (16) | |
| Itraconazole | 0.015–16 | 0.25 | 1 | 21.1 (493) | |
| Posaconazole | 0.008–16 | 0.125 | 1 | ||
| Caspofungin | 0.008–16 | 0.06 | 0.25 | 1.0 (22) | |
| Anidulafungin | 0.008–4 | 0.03 | 1 | 1.0 (23) | |
| Micafungin | 0.008–4 | 0.03 | 1 | 1.1 (25) | |
| Flucytosine | 0.125–256 | 0.125 | 1 | 0.8 (19) | |
| Amphotericin B | 0.002–2 | 0.125 | 0.38 | ||
| C. albicans (877) | Fluconazole | 0.125–256 | 0.5 | 1 | 2.3 |
| Voriconazole | 0.008–16 | 0.03 | 0.125 | 0.9 | |
| Itraconazole | 0.03–16 | 0.125 | 0.25 | 1.6 | |
| Posaconazole | 0.008–16 | 0.125 | 0.25 | ||
| Caspofungin | 0.008–2 | 0.03 | 0.06 | 0.5 | |
| Anidulafungin | 0.008–2 | 0.015 | 0.06 | 0.3 | |
| Micafungin | 0.008–2 | 0.03 | 0.03 | 0.3 | |
| Flucytosine | 0.125–256 | 0.25 | 2 | 0.9 | |
| Amphotericin B | 0.002–0.5 | 0.094 | 0.19 | ||
| C. glabrata (670) | Fluconazole | 0.5–256 | 8 | 64 | 11.9 |
| Voriconazolea | 0.008–16 | 0.25 | 2 | ||
| Itraconazole | 0.06–16 | 1 | 4 | 59.6 | |
| Posaconazole | 0.008–16 | 0.5 | 2 | ||
| Caspofungin | 0.015–16 | 0.06 | 0.125 | 2.4 | |
| Anidulafungin | 0.008–4 | 0.06 | 0.125 | 2.7 | |
| Micafungin | 0.008–4 | 0.015 | 0.03 | 2.7 | |
| Flucytosine | 0.125–256 | 0.125 | 0.125 | 0.6 | |
| Amphotericin B | 0.002–2 | 0.25 | 0.5 | ||
| C. parapsilosis (389) | Fluconazole | 0.125–64 | 1 | 2 | 4.1 |
| Voriconazole | 0.008–2 | 0.03 | 0.06 | 0.8 | |
| Itraconazole | 0.015–1 | 0.25 | 0.5 | 1.5 | |
| Posaconazole | 0.008–1 | 0.125 | 0.25 | ||
| Caspofungin | 0.008–2 | 0.25 | 0.5 | 0 | |
| Anidulafungin | 0.008–8 | 1 | 2 | 0.3 | |
| Micafungin | 0.008–4 | 1 | 2 | 0 | |
| Flucytosine | 0.125–8 | 0.125 | 0.25 | 0 | |
| Amphotericin B | 0.002–2 | 0.064 | 0.19 | ||
| C. tropicalis (241) | Fluconazole | 0.125–128 | 0.5 | 2 | 6.2 |
| Voriconazole | 0.008–16 | 0.06 | 0.125 | 2.5 | |
| Itraconazole | 0.015–16 | 0.5 | 1 | 22.4 | |
| Posaconazole | 0.008–1 | 0.125 | 0.5 | ||
| Caspofungin | 0.008–2 | 0.03 | 0.125 | 0.8 | |
| Anidulafungin | 0.008–0.25 | 0.015 | 0.06 | 0 | |
| Micafungin | 0.008–1 | 0.03 | 0.125 | 1.2 | |
| Flucytosine | 0.125–128 | 0.125 | 0.25 | 1.7 | |
| Amphotericin B | 0.008–1 | 0.25 | 0.5 | ||
| C. dubliniensis (36)a | Fluconazole | 0.125–16 | 0.25 | 0.5 | 5.6 |
| Voriconazole | 0.008–0.125 | 0.015 | 0.03 | 0 | |
| Itraconazole | 0.03–0.5 | 0.125 | 0.25 | 0 | |
| Posaconazole | 0.03–0.25 | 0.06 | 0.125 | ||
| Caspofungin | 0.03–0.25 | 0.06 | 0.125 | 0 | |
| Anidulafungin | 0.015–1 | 0.03 | 0.06 | 2.8 | |
| Micafungin | 0.008–1 | 0.06 | 0.06 | 2.8 | |
| Flucytosine | 0.125–0.25 | 0.125 | 0.125 | 0 | |
| Amphotericin B | 0.006–0.125 | 0.023 | 0.064 | ||
| C. lusitaniae (34) | Fluconazole | 0.125–8 | 0.5 | 1 | |
| Voriconazole | 0.008–0.015 | 0.008 | 0.015 | ||
| Itraconazole | 0.03–1 | 0.25 | 0.5 | ||
| Posaconazole | 0.03–0.5 | 0.125 | 0.25 | ||
| Caspofungin | 0.03–1 | 0.25 | 0.5 | ||
| Anidulafungin | 0.008–1 | 0.25 | 0.5 | ||
| Micafungin | 0.015–2 | 0.25 | 0.5 | ||
| Flucytosine | 0.125–128 | 0.125 | 2 | ||
| Amphotericin B | 0.016–0.38 | 0.064 | 0.125 | ||
| C. krusei (32) | Fluconazoleb | 4–64 | 16 | 64 | 100 |
| Voriconazole | 0.015–0.5 | 0.25 | 0.25 | 0 | |
| Itraconazole | 0.125–2 | 0.5 | 1 | 21.9 | |
| Posaconazole | 0.125–1 | 0.25 | 0.5 | ||
| Caspofungin | 0.06–0.5 | 0.125 | 0.25 | 0 | |
| Anidulafungin | 0.015–0.25 | 0.03 | 0.06 | 0 | |
| Micafungin | 0.06–0.125 | 0.125 | 0.125 | 0 | |
| Flucytosine | 4–64 | 8 | 16 | 6.3 | |
| Amphotericin B | 0.023–1 | 0.5 | 0.75 | ||
| Other species (50) | Fluconazolea | 0.25–16 | 2 | 8 | |
| Voriconazolea | 0.008–0.25 | 0.06 | 0.25 | ||
| Itraconazolea | 0.06–2 | 0.25 | 1 | 18.0 | |
| Posaconazole | 0.03–2 | 0.25 | 0.5 | ||
| Caspofungin | 0.03–0.5 | 0.125 | 0.5 | ||
| Anidulafungin | 0.015–2 | 0.25 | 1 | ||
| Micafungin | 0.008–2 | 0.25 | 1 | ||
| Flucytosine | 0.125–8 | 0.125 | 0.25 | 0 | |
| Amphotericin B | 0.002–1 | 0.064 | 0.5 | ||
C. albicans breakpoints were applied.
Percent resistant was based on intrinsic resistance of C. krusei and did not follow actual MICs.
Table 3.
MICs for less common Candida species
| Species | No. of isolates | MIC range (μg/ml) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Fluconazole | Voriconazole | Itraconazole | Posaconazole | Caspofungin | Micafungin | Anidulafungin | Amphotericin B | ||
| C. orthopsilosis | 13 | 0.25–8 | 0.008–0.06 | 0.06–0.5 | 0.06–0.25 | 0.06–0.5 | 0.25–1 | 0.25–2 | 0.023–0.094 |
| C. metapsilosis | 10 | 1–4 | 0.015–0.06 | 0.125–0.5 | 0.03–0.25 | 0.06–0.5 | 0.125–0.5 | 0.125–0.5 | 0.023–0.19 |
| C. guilliermondii | 8 | 0.5–8 | 0.015–0.25 | 0.25–1 | 0.125–0.5 | 0.06–0.5 | 0.25–2 | 0.25–2 | 0.032–0.125 |
| C. nivariensis | 4 | 8–16 | 0.125–0.25 | 1–2 | 1–2 | 0.03–0.125 | 0.015–0.03 | 0.015–0.06 | 0.19–1 |
| C. fermentati | 4 | 1–16 | 0.03–0.125 | 0.5–1 | 0.25 | 0.25–0.5 | 0.25–1 | 0.25–1 | 0.032–0.5 |
Resistance to echinocandins was low overall, ranging from 1.0% of isolates to caspofungin or anidulafungin to 1.1% of isolates for micafungin. Again, resistance was primarily found in C. glabrata isolates, which accounted for 65% (20/31) of the isolates resistant to any echinocandin, followed by C. albicans (19% of the resistant isolates).
There are no established breakpoints for amphotericin B, but an MIC breakpoint of 2 μg/ml is widely considered to be elevated. Only four isolates had amphotericin B MIC values of 2 μg/ml: two C. glabrata isolates and one isolate each of C. tropicalis and C. parapsilosis.
Susceptibility by surveillance site.
The overall proportion of isolates that were resistant to fluconazole was slightly higher in Atlanta than in Baltimore. When analyzed by species, fluconazole resistance was nearly identical for C. albicans in Baltimore and Atlanta (Table 4). Fluconazole resistance was higher in Atlanta among C. glabrata, C. tropicalis, and C. parapsilosis isolates and nearly twice as high in Atlanta as in Baltimore for the last two species. Overall resistance to voriconazole was, again, almost identical in Atlanta and Baltimore, and only C. tropicalis isolates in Atlanta (3.8%) had resistance higher than 2%. Overall resistance to echinocandins was the same at the two sites (Tables 4).
Table 4.
In vitro susceptibilities of Candida surveillance isolates from 2008 to 2011 to selected antifungal agents in Atlanta and Baltimore
| Species and antifungal agent | Susceptibility profile of Atlanta isolates |
Susceptibility profile Baltimore isolates |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of isolatesa | MIC50 (μg/ml) | MIC90 (μg/ml) | % Resistant isolates | No. of isolatesa | MIC50 (μg/ml) | MIC90 (μg/ml) | % Resistant isolates | |
| All species | 1,141 | 1,068 | ||||||
| Fluconazole | 1 | 16 | 8.2 | 1 | 16 | 6.5 | ||
| Voriconazole | 0.06 | 0.5 | 1.1 | 0.06 | 0.5 | 0.9 | ||
| Caspofungin | 0.06 | 0.25 | 1.0 | 0.06 | 0.25 | 1.0 | ||
| Anidulafungin | 0.03 | 1 | 1.0 | 0.03 | 1 | 1.0 | ||
| Micafungin | 0.03 | 1 | 1.0 | 0.03 | 1 | 1.0 | ||
| C. albicans | 489 | 388 | ||||||
| Fluconazole | 0.5 | 2 | 2.2 | 0.5 | 1 | 2.3 | ||
| Voriconazole | 0.03 | 0.125 | 0.6 | 0.03 | 0.125 | 1.3 | ||
| Caspofungin | 0.03 | 0.06 | 0.6 | 0.03 | 0.06 | 0.3 | ||
| Anidulafungin | 0.015 | 0.06 | 0.4 | 0.03 | 0.06 | 0.3 | ||
| Micafungin | 0.03 | 0.03 | 0.4 | 0.015 | 0.03 | 0.3 | ||
| C. glabrata | 318 | 352 | ||||||
| Fluconazole | 8 | 64 | 13.2 | 8 | 64 | 10.8 | ||
| Voriconazole | 0.25 | 2 | 0.25 | 1 | ||||
| Caspofungin | 0.06 | 0.125 | 2.5 | 0.06 | 0.125 | 2.3 | ||
| Anidulafungin | 0.06 | 0.125 | 3.1 | 0.06 | 0.125 | 2.3 | ||
| Micafungin | 0.015 | 0.03 | 2.8 | 0.015 | 0.03 | 2.5 | ||
| C. parapsilosis | 212 | 177 | ||||||
| Fluconazole | 1 | 4 | 5.6 | 1 | 2 | 2.3 | ||
| Voriconazole | 0.03 | 0.06 | 0.9 | 0.03 | 0.06 | 0.6 | ||
| Caspofungin | 0.25 | 0.5 | 0 | 0.25 | 0.5 | 0 | ||
| Anidulafungin | 1 | 2 | 0 | 1 | 2 | 0.6 | ||
| Micafungin | 1 | 2 | 0 | 1 | 2 | 0 | ||
| C. tropicalis | 103 | 138 | ||||||
| Fluconazole | 0.5 | 2 | 8.6 | 0.5 | 2 | 4.3 | ||
| Voriconazole | 0.03 | 0.25 | 3.8 | 0.06 | 0.125 | 1.4 | ||
| Caspofungin | 0.03 | 0.125 | 0 | 0.03 | 0.06 | 1.4 | ||
| Anidulafungin | 0.015 | 0.06 | 0 | 0.015 | 0.06 | 0 | ||
| Micafungin | 0.03 | 0.06 | 0 | 0.03 | 0.125 | 1.4 | ||
| C. krusei | 19 | 13 | ||||||
| Fluconazole | 16 | 64 | 100 | 16 | 32 | 100 | ||
| Voriconazole | 0.25 | 0.5 | 0 | 0.125 | 0.25 | 0 | ||
| Caspofungin | 0.125 | 0.25 | 0 | 0.125 | 0.25 | 0 | ||
| Anidulafungin | 0.03 | 0.06 | 0 | 0.03 | 0.06 | 0 | ||
| Micafungin | 0.125 | 0.125 | 0 | 0.06 | 0.125 | 0 | ||
Number of isolates for which interpretive criteria are available.
Temporal comparison of susceptibility results.
We compared the previously published population-based surveillance results (10, 12) to the current results (Table 5). Resistance to fluconazole in C. albicans remained relatively unchanged in both Atlanta and Baltimore. Fluconazole resistance among C. glabrata isolates in Atlanta was 20% in 1993 and 13.2% in 2011; however, there were only 35 isolates available for testing in 1993, just over 10% of the number of isolates available for testing in 2011. Fluconazole resistance among C. glabrata isolates in Baltimore was 8.0% in 2008 and 10.8% in 2011. Resistance rates of C. parapsilosis to fluconazole were only slightly different over time in both Atlanta (from 1.9% to 5.6% of isolates) and Baltimore (from 0% to 2.3%), and the same was true for C. tropicalis in Atlanta (from 3.3% to 8.6% of isolates) and in Baltimore (from 8.8% to 4.3%).
Table 5.
Comparison of fluconazole susceptibilities from past and present surveillance in Atlanta and Baltimore
| Site and species | Year | No. of isolates tested | MIC50 (μg/ml) | MIC90 (μg/ml) | % Resistant isolates |
|---|---|---|---|---|---|
| Atlanta | |||||
| C. albicans | 1993 | 103 | 0.25 | 0.5 | 1.0 |
| 2011 | 489 | 0.5 | 2 | 2.2 | |
| C. glabrata | 1993 | 35 | 16 | 128 | 20 |
| 2011 | 318 | 8 | 64 | 13.2 | |
| C. parapsilosis | 1993 | 52 | 1 | 2 | 1.9 |
| 2011 | 212 | 1 | 4 | 5.6 | |
| C. tropicalis | 1993 | 30 | 1 | 2 | 3.3 |
| 2011 | 103 | 0.5 | 2 | 8.6 | |
| Baltimore | |||||
| C. albicans | 2000 | 232 | 0.125 | 1 | 3.4 |
| 2011 | 388 | 0.5 | 1 | 2.3 | |
| C. glabrata | 2000 | 150 | 4 | 32 | 8.0 |
| 2011 | 352 | 8 | 64 | 10.8 | |
| C. parapsilosis | 2000 | 58 | 0.5 | 1 | 0 |
| 2011 | 177 | 1 | 2 | 2.3 | |
| C. tropicalis | 2000 | 80 | 0.5 | 4 | 8.8 |
| 2011 | 138 | 0.5 | 2 | 4.3 |
Multidrug-resistant isolates.
Among isolates with acquired resistance, there were three MDR isolates from Atlanta, all C. glabrata isolates resistant to fluconazole and one or more echinocandins. There were six MDR isolates from Baltimore, five C. glabrata isolates and one C. albicans isolate all resistant to fluconazole and one or more echinocandins.
Multispecies incident episodes.
There were 42 multispecies episodes in Atlanta and 55 in Baltimore. In Atlanta, there were 23 episodes where two species were isolated simultaneously and 19 episodes where two species were isolated on different days during the 30-day episodic period (range, 1 to 27 days; average of 9 days apart). In Baltimore, there were 36 episodes with two species isolated simultaneously (including one patient with three species simultaneously), and 21 episodes where two species were isolated on different days (including one patient with four species isolated on four different days and one patient with two simultaneous species and then a different species 3 days later and then two different species again simultaneously) during the 30-day episodic period (range, 1 to 26 days; average of 7 days apart). Candida albicans was the most common species occurring in multispecies episodes, in Baltimore most often with C. glabrata (27 times) followed by C. parapsilosis (6 times) and in Atlanta most often with C. glabrata (12 times) or C. parapsilosis (7 times). Candida glabrata occurred with C. parapsilosis six times in Atlanta but only once in Baltimore.
DISCUSSION
There were a number of important results from this population-based surveillance. First, the distribution of species at both of the surveillance sites has changed, with the proportion of C. albicans infections decreasing and the proportion of C. glabrata and C. parapsilosis infections increasing. Second, multispecies episodes were frequently encountered. Third, when C. glabrata and C. krusei were not considered, overall resistance to fluconazole was low. Finally, although echinocandins are relatively new to the antifungal armamentarium, resistance was detected, especially among C. glabrata isolates.
Candida albicans has traditionally been the leading cause of candidemia worldwide, but temporal data show that the proportion of candidemia cases caused by non-C. albicans species, especially C. glabrata and C. parapsilosis, has been increasing (26, 30, 36). That trend has also been demonstrated in this surveillance: the proportion of C. albicans candidemia cases dropped from 52% to 41% in Atlanta and from 43% to 34% in Baltimore (Fig. 1). At the same time, the proportion of C. glabrata candidemia cases rose from 12% to 27% in Atlanta, and the proportion of C. parapsilosis candidemia cases rose from 11% to 16% in Baltimore. Among global population-based surveillance studies, these are both the lowest reported percentages of C. albicans and the highest reported percentages of C. glabrata (3, 5, 6, 9, 13, 19, 33, 34). Only recent surveillance studies in Scotland, Canada, Denmark, and the United States have reported proportions of C. glabrata candidemia at or above 20% (2, 9, 19, 28, 29, 33). This rise in the proportion of C. glabrata candidemia has been reported elsewhere in the United States. In surveillance from intensive care units, C. glabrata increased from the fourth most common Candida BSI in 1989 to second most common in 1999 (36). While the relative proportions of each species have changed over the last 20 years, C. albicans, C. glabrata, C. parapsilosis, and C. tropicalis isolates still comprise 93.5% of all isolates, a number that has not substantially changed in 20 years in the United States (12, 18).
In recent global surveillance, the percentage of multispecies candidemia has ranged from 1.2% to 3.4% of all episodes, with an average of 2.1% in any given surveillance (2, 5, 6, 15, 32–35). Our percentages were somewhat higher, with 3.6% of the episodes in Atlanta and 5.1% in Baltimore. However, these numbers are probably an underestimate as not all of the laboratories participating in the surveillance used chromogenic agar for initial plating of blood cultures; and even when chromogenic agar is used, only C. albicans/C. dubliniensis, C. tropicalis, and C. krusei can be reliably distinguished. Thirteen of the 23 simultaneous multispecies episodes in Atlanta and all but 1 of the 36 simultaneous multispecies episodes in Baltimore would have been readily distinguished on chromogenic agar, indicating the value of this clinical tool in species distinction. Timely and correct identification of isolates in multispecies episodes is essential for correct clinical management of the patient.
The overall proportion of resistance of Candida isolates to fluconazole did not change in Atlanta in the 16 years between the two surveillance reports or in Baltimore in the 10 years between surveillance reports. Applying the new breakpoints to the archived susceptibility data from 1992 to 1993 in Atlanta indicates that the overall fluconazole resistance rate among isolates was 7.0%. In 2008 to 2011 the overall fluconazole resistance rate was slightly higher at 8.2%, and an increase in the proportion of resistant isolates was noted for C. albicans, C. parapsilosis, and C. tropicalis. The proportion of fluconazole-resistant isolates that were identified as C. krusei and C. glabrata also increased only slightly from 64% to 65% of all resistant isolates. The largest increases in fluconazole resistance were found in Atlanta for C. parapsilosis, which increased from 1.9% to 5.6% of isolates, and for C. tropicalis, which increased from 3.3% to 8.6% of isolates. In Baltimore, in 1998 to 2000, the overall fluconazole resistance among tested isolates was 7.3%, which dropped to 6.5% in 2008 to 2010. The proportion of resistant isolates that were identified as C. krusei and C. glabrata again increased slightly from 60% to 72% of all resistant isolates. Resistance rates were slightly lower for C. albicans and C. tropicalis isolates and slightly higher for C. glabrata and C. parapsilosis isolates. Because this was the first nonglobal surveillance report employing the new species-specific Candida susceptibility breakpoints, it was not possible to directly compare our resistance rate results to older surveillance studies performed outside our institution.
Although the echinocandins are a relatively new class of antifungal agents, they were used during treatment in 61% of the patients in this surveillance (data not shown). Despite this high level of usage, there were very few resistant isolates (approximately 1% of total isolates). However, the one species with a notable level of resistance to echinocandins was C. glabrata (38). This is concerning, given that this low level of resistance has developed in a species already less susceptible to the triazole antifungals. The Infectious Disease Society of America guidelines recommend echinocandins as primary therapy for C. glabrata candidiasis in both neutropenic and nonneutropenic patients (20), so resistance should be monitored closely as usage increases.
In summary, candidemia remains an important cause of health care-associated BSI. Laboratories should be aware that mixed-species infections are not rare and that the two most prevalent species in those mixed infections (C. albicans and C. glabrata) may have very different antifungal susceptibility patterns. Although the overall rates of resistance to antifungal agents remain low, species-specific resistance raises some concerns, especially among isolates of MDR C. glabrata with resistance to both fluconazole and echinocandins. Currently, echinocandins are highly effective in vitro against all Candida species, and fluconazole remains highly effective in vitro against C. albicans. As the use of echinocandins becomes more commonplace in both empirical and primary therapy, it is imperative that we continue to monitor candidemia isolates for changing levels of resistance and use these data to update future treatment guidelines.
ACKNOWLEDGMENTS
We acknowledge the contributions of Shirley McClinton, Joyce Peterson, Randy Kuykendall, Eszter Deak, Kizee Etienne, Lalitha Gade, and Kaitlin Benedict at the CDC; Carolyn Kreiner, Helen Yoon, Debbie Lundy, Kim Holmes, Sandra Muhanuka, and Alethea Venida for assistance with data collection in Maryland; Janine Ladson, Lewis Perry, and the surveillance officers of the Georgia EIP for assistance with data collection; the hospitals in Atlanta Health District 3; and the Emerging Infections Programs in Georgia and Maryland.
This study received funding from the Office of Antimicrobial Resistance at the CDC.
The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Published ahead of print 8 August 2012
REFERENCES
- 1. Abi-Said D, et al. 1997. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin. Infect. Dis. 24: 1122– 1128 [DOI] [PubMed] [Google Scholar]
- 2. Arendrup MC, et al. 2011. National surveillance of fungemia in Denmark (2004 to 2009). J. Clin. Microbiol. 49: 325– 334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asmundsdóttir LR, Erlendsdóttir H, Gottfredsson M. 2002. Increasing incidence of candidemia: results from a 20-year nationwide study in Iceland. J. Clin. Microbiol. 40: 3489– 3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banerjee SN, et al. 1991. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am. J. Med. 91: 86S– 89S [DOI] [PubMed] [Google Scholar]
- 5. Chen S, et al. 2006. Active surveillance for candidemia, Australia. Emerg. Infect. Dis. 12: 1508– 1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cuenca-Estrella M, et al. 2005. In vitro susceptibilities of bloodstream isolates of Candida species to six antifungal agents: results from a population-based active surveillance programme, Barcelona, Spain, 2002–2003. J. Antimicrob. Chemother. 55: 194– 199 [DOI] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3, 3rd ed. Clinical Laboratory Standards Institute, Wayne, Pa. [Google Scholar]
- 8. Deak E, et al. 2010. Utility of a Luminex-based assay for multiplexed, rapid species identification of Candida isolates from an ongoing candidemia surveillance. Can. J. Microbiol. 56: 348– 351 [DOI] [PubMed] [Google Scholar]
- 9. Diekema DJ, et al. 2002. Epidemiology of candidemia: 3-year results from the emerging infections and the epidemiology of Iowa organisms study. J. Clin. Microbiol. 40: 1298– 1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hajjeh RA, et al. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42: 1519– 1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hassan I, Powell G, Sidhu M, Hart WM, Denning DW. 2009. Excess mortality, length of stay and cost attributable to candidaemia. J. Infect. 59: 360– 365 [DOI] [PubMed] [Google Scholar]
- 12. Kao AS, et al. 1999. The epidemiology of candidemia in two United States cities: results of a population-based active surveillance. Clin. Infect. Dis. 29: 1164– 1170 [DOI] [PubMed] [Google Scholar]
- 13. Laupland KB, Gregson DB, Church DL, Ross T, Elsayed S. 2005. Invasive Candida species infections: a 5 year population-based assessment. J. Antimicrob. Chemother. 56: 532– 537 [DOI] [PubMed] [Google Scholar]
- 14. Lockhart SR, Bolden CB, Iqbal N, Kuykendall RJ. 2011. Validation of 24-hour flucytosine MIC determination by comparison with 48-hour determination by the Clinical and Laboratory Standards Institute M27-A3 broth microdilution reference method. J. Clin. Microbiol. 49: 4322– 4325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lortholary O, et al. 2011. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob. Agents Chemother. 55: 532– 538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyon GM, Karatela S, Sunay S, Adiri Y, Candida Surveillance Study Investigators 2010. Antifungal susceptibility testing of Candida isolates from the Candida surveillance study. J. Clin. Microbiol. 48: 1270– 1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Morgan J, et al. 2005. Excess mortality, hospital stay, and cost due to candidemia: a case-control study using data from population-based candidemia surveillance. Infect. Control Hosp. Epidemiol. 26: 540– 547 [DOI] [PubMed] [Google Scholar]
- 18. Nguyen MH, et al. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100: 617– 623 [DOI] [PubMed] [Google Scholar]
- 19. Odds FC, et al. 2007. One year prospective survey of Candida bloodstream infections in Scotland. J. Med. Microbiol. 56: 1066– 1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pappas PG, et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48: 503– 535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfaller MA, et al. 2011. Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagnost. Microbiol. Infect. Dis. 70: 330– 343 [DOI] [PubMed] [Google Scholar]
- 22. Pfaller MA, et al. 2010. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist. Updat. 13: 180– 195 [DOI] [PubMed] [Google Scholar]
- 23. Pfaller MA, et al. 2008. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance. J. Clin. Microbiol. 46: 150– 156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pfaller MA, et al. 2011. Validation of 24-hour posaconazole and voriconazole MIC readings versus the CLSI 48-hour broth microdilution reference method: application of epidemiological cutoff values to results from a global Candida antifungal surveillance program. J. Clin. Microbiol. 49: 1274– 1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pfaller MA, Castanheira M, Messer SA, Moet GJ, Jones RN. 2011. Echinocandin and triazole antifungal susceptibility profiles for Candida spp., Cryptococcus neoformans, and Aspergillus fumigatus: application of new CLSI clinical breakpoints and epidemiologic cutoff values to characterize resistance in the SENTRY Antimicrobial Surveillance Program (2009). Diagn. Microbiol. Infect. Dis. 69: 45– 50 [DOI] [PubMed] [Google Scholar]
- 26. Pfaller MA, Diekema DJ. 2007. Epidemiology of invasive candidiasis: a persistent public health problem. Clin. Microbiol. Rev. 20: 133– 163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfaller MA, et al. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist. Updat. 14: 164– 176 [DOI] [PubMed] [Google Scholar]
- 28. Pfaller MA, et al. 2010. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J. Clin. Microbiol. 48: 1366– 1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfaller MA, et al. 1998. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: frequency of occurrence and antifungal susceptibility in the SCOPE Program. Diagn. Microbiol. Infect. Dis. 30: 121– 129 [DOI] [PubMed] [Google Scholar]
- 30. Pfaller MA, et al. 1999. Trends in species distribution and susceptibility to fluconazole among blood stream isolates of Candida species in the United States. Diagn. Microbiol. Infect. Dis. 33: 217– 222 [DOI] [PubMed] [Google Scholar]
- 31. Pfaller MA, Moet GJ, Messer SA, Jones RN, Castanheira M. 2011. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009). J. Clin. Microbiol. 49: 396– 399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Poikonen E, et al. 2010. Secular trend in candidemia and the use of fluconazole in Finland, 2004–2007. BMC Infect. Dis. 10: 312 doi:10.1186/1471-2334-10-312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sandven P, et al. 2006. Candidemia in Norway (1991 to 2003): results from a nationwide study. J. Clin. Microbiol. 44: 1977– 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. St-Germain G, et al. 2008. Epidemiology and antifungal susceptibility of bloodstream Candida isolates in Quebec: report on 453 cases between 2003 and 2005. Can. J. Infect. Dis. Med. Microbiol. 19: 55– 62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tortorano AM, et al. 2002. European Confederation of Medical Mycology (ECMM) prospective survey of candidaemia: report from one Italian region. J. Hosp. Infect. 51: 297– 304 [DOI] [PubMed] [Google Scholar]
- 36. Trick WE, et al. 2002. Secular trend of hospital-acquired candidemia among intensive care unit patients in the United States during 1989–1999. Clin. Infect. Dis. 35: 627– 630 [DOI] [PubMed] [Google Scholar]
- 37. Wisplinghoff H, et al. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39: 309– 317 [DOI] [PubMed] [Google Scholar]
- 38. Zimbeck AJ, et al. 2010. FKS mutations and elevated echinocandin MIC values among Candida glabrata isolates from U.S. population-based surveillance. Antimicrob. Agents Chemother. 54: 5042– 5047 [DOI] [PMC free article] [PubMed] [Google Scholar]