Abstract
Newcastle disease (ND) is a highly contagious, severe disease of poultry caused by pathogenic strains of Newcastle disease virus (NDV; or avian paramyxovirus-1). NDV is endemic in wild birds worldwide and one of the economically most important poultry pathogens. Most of the published strains are outbreak-associated strains, while the apathogenic NDV strains that occur in wild birds, posing a constant threat to poultry with their capability to convert into more virulent forms, have remained less studied. We screened for NDV RNA in cloacal and oropharyngeal samples from wild waterfowl in Finland during the years 2006 to 2010: 39 of 715 birds were positive (prevalence, 5.5%). The partial or full-length F genes of 37 strains were sequenced for phylogenetic purposes. We also characterized viruses derived from three NDV outbreaks in Finland and discuss the relationships between these outbreak-associated and the wild-bird-associated strains. We found that all waterfowl NDV isolates were lentogenic strains of class I or class II genotype I. We also isolated a genetically distinct class I strain (teal/Finland/13111/2008) grouping phylogenetically together with only strain HIECK87191, isolated in Northern Ireland in 1987. Together they seem to form a novel class I genotype genetically differing from other known NDVs by at least 12%.
INTRODUCTION
Type 1 avian paramyxovirus (APMV-1), commonly known as Newcastle disease virus (NDV), is a highly significant avian pathogen, with a strong impact on both commercial and backyard poultry. It has caused three major panzootics during the past 80 years. The genus Avulavirus, a member of the negative-sense RNA virus family Paramyxoviridae, is composed of the antigenically distinct avian paramyxovirus serotypes 1 to 11 (8, 19, 32). The name NDV is in some contexts reserved exclusively for velogenic poultry-derived strains (defined by the World Organization for Animal Health, OIE).
The fusion protein encoded by the F gene mediates the fusion between the viral envelope and the host cell membrane. The precursor F0 is cleaved by host cell proteases to F1 and F2 before gaining its functional activity (34, 39). The amino acid sequence of the cleavage site is considered to be a major pathogenicity factor, determining the protease specificity, and thereby the tissue tropism, of a given strain. NDVs are categorized as velogenic, mesogenic, and lentogenic depending on the severity of the disease they cause. Lentogens cause a mild or subclinical infection of the respiratory tract. Velogens, which are cleaved by proteases of the furin family allowing for systemic replication, affect the gastrointestinal tract and/or the nervous system, causing high mortality. The pathogenicity can be assessed by either an intracerebral pathogenicity index (ICPI) with a cutoff value of 0.7 for velogenic strains or molecularly by the cleavage site sequence of the fusion protein. Multiple basic amino acids at the C terminus of the F2 protein and phenylalanine at residue 117 of the F1 protein are indicative for high pathogenicity (OIE).
Within NDV, two clades can be distinguished (9): class I viruses, also designated group H or lineage 6 (3, 6, 14), comprise at least nine genotypes (23). They are almost exclusively lentogenic strains that have been found among waterfowl and at live-bird markets (35). Class II includes almost all of the velogenic strains and the majority of all published strains, as they are primarily responsible for the infections in poultry and pet birds (9). Class II viruses are further divided into genotypes I to XI (used in this study) (7, 17, 29–31, 40) or genetic lineages 1 to 6 (3). The genotypes of class II are separated as early (genotypes I to IV and IX) and recent or late (other genotypes, isolated after the 1960s). The division is characterized by different genome sizes, 15,186 nucleotides (nt) and 15,192 nt, respectively (10). A distinct sublineage (VIb) of class II viruses comprises the pigeon paramyxoviruses (PPMV-1). The group consists of a globally diverse range of strains and has further been divided into several sublineages (2). They were first seen in the Middle East in racing pigeons in the 1980s and have since then spread to all parts of the world (20). Endemic among feral and domestic pigeons, PPMV-1 is able to spread to and cause severe disease in wild and domestic bird populations, including commercial poultry (25).
Roughly, the NDV ecology can be divided into two host systems: wild waterfowl harboring the lentogenic strains and poultry where velogenic outbreaks are manifested. The ecology of NDVs in wild birds appears to be in many aspects similar to low-pathogenic avian influenza viruses (AIV), which are naturally hosted by wild waterfowl, although it is far less studied (21, 42). The highly pathogenic strains of both viruses seem to arise in a similar way during replication in high-density poultry populations, when mutations are accumulated at the fusion protein cleavage site (36, 37, 41).
The great intra- and intergenotype diversity of NDVs and their continuing evolution pose a diagnostic challenge, e.g., as demonstrated by class I viruses which are escaping detection by many conventional assays (24, 33). Constant monitoring of circulating viruses is required for evaluation of vaccine compatibility.
Here, we report our results from screening NDV from wild waterfowl during the years of 2006 to 2010 in Finland. The viruses were sequenced for phylogeny and to assess their pathogenicity by the deduced amino acid sequence of the fusion protein cleavage site. We also report three outbreaks that occurred in pheasants (Phasianus colchicus) in 2003, turkeys (Meleagris) in 2004, and hobby pigeons (Columba sp.) in 2008. Fourteen Finnish strains isolated before 2006 have been previously described in 2001 by Huovilainen et al. and in 2008 by Lindh et al. (18, 27). Our further aim was to elucidate the role of wild waterfowl in the ecology of NDV and the relationships between the wild-bird-derived and outbreak-derived NDVs described in this study.
MATERIALS AND METHODS
Sample overview.
Wild migratory waterfowl were sampled during the years of 2006 to 2010. The samples were collected by voluntary hunters and staff of the Finnish Game and Fisheries Research Institute, as part of an active surveillance program of AIV and NDV. Samples were collected from a total of 715 birds, from four main areas in Central and Southern Finland, where waterfowl nest in high numbers (16, 22). In addition, positive samples derived as part of passive surveillance (dead and clinically affected birds) and from NDV outbreaks were included in our study.
In 2003, NDV with an ICPI of 0.5 was isolated from a flock of pheasants that were partly kept outdoors. The pheasants were reared for release. Hatched chicks were kept in the holding for 5 days prior to release. During this time, a slightly higher mortality was seen (>10%), and the flock was destroyed when NDV was confirmed in two birds. The affected population consisted of 750 pheasants, 15 chickens, and 1,600 newly hatched chicks. This holding had traded birds with another backyard poultry holding.
During routine health controls in June 2004, antibodies to NDV were detected in western Finland in a flock of 12,000 fattening turkeys, although no clinical signs of disease were apparent. Consequently, NDV was isolated with an ICPI of 1.6. The birds were culled and the outbreak was controlled according to current regulations. Protection and surveillance zones were set around the infected premises, and all poultry within were tested serologically but remained seronegative. Wild waterfowl were suspected to be the source of this outbreak. At the same time, an outbreak by a highly similar virus was reported in Sweden. No connection was found, nor were the sources of the outbreaks confirmed at that time.
In 2008, NDV was confirmed in 6 dovecotes within 6 days. NDV was initially found in dovecote A, with 54 hobby pigeons, after the death of several birds. When the contacts were traced, NDV was found in dovecote B, with over 300 birds of several different species. Their contacts were again traced, and on days 3 and 4 two small dovecotes, C and D, which had been in contact with dovecote B, were found infected. The next day, cote E, which had been in contact with cote D, was found NDV positive. On the sixth day, dovecote F was found positive for NDV after contact with cotes A and B at a pigeon show. All 482 affected birds from the six affected cotes were culled. The dovecotes had been in contact by trade of birds or related products and during shows. All their contacts were investigated by molecular methods but no further NDV-positive cotes were found. All poultry holdings within close proximity with the infected cotes were also investigated, but no affected birds were found.
Sample handling.
Cloacal and oropharyngeal swabs from wild birds were collected using flocked nylon swabs and UTM (universal transport medium) (Copan). Samples were kept at room temperature or chilled but were not frozen until they reached the lab. At the lab, samples were centrifuged and supplied with additional antibiotics and stored at −80°C.
Organ suspensions from outbreak-associated birds and birds from passive surveillance were prepared for PCR studies and positives were confirmed by virus isolation in embryonated eggs (12).
RNA extraction and RT-PCR.
RNA was extracted from swab samples or allantoic fluids using a QIAmp viral RNA minikit (Qiagen). For the detection of NDV RNA, two different PCRs were used: an F gene-targeting reverse transcription (RT)-PCR for class II viruses, described by Seal et al. (35) and adjusted to comprise class I viruses by Huovilainen et al. (18), was used for studying samples collected in 2006 to 2009. A recently developed L gene-targeting real-time RT-PCR by Fuller et al. (13) was introduced in our lab in 2010 and used for studying samples from that year. All PCR-positive samples were subjected to virus isolation attempts and/or sequencing. Shortly, swab specimens were injected into 8- to 10-day-old embryonated chicken eggs, and allantoic fluids were harvested from eggs when embryos appeared dead or dying. The remaining eggs were harvested 6 days postinoculation and subjected to a second passage. The collected allantoic fluids were tested for hemagglutinating activity.
Serotyping and sequencing.
Distinction between class I and II APMV-1 was done using the previously described F gene RT-PCRs (18, 35). Primers for the sequencing of the whole F gene spanning the N- and C-terminal noncoding regions were designed for both classes (Table 1). An ∼600-nt region including the fusion protein cleavage site was sequenced for all the strains. The full-length F gene of the class I viruses and the causative agents of recent outbreaks were also sequenced.
Table 1.
Primers used for amplification of the full-length NDV F genes
| Class | Primer | Sequence | Position |
|---|---|---|---|
| I | s1Hf | TTG CCA AAT ACA AYC CGT TCA A | 5′ −188 |
| s1 h | GTG GTT ACY GAC TCT TGG ATT | 3′ 322 | |
| s2Hf | ACA ACC CTC CTT GCC CC | 5′ 264 | |
| s2 h | GGG AGA GTC ACC TGT ATA C | 3′ 868 | |
| s3Hf | GAC TCG CAA ACT CAG CTT CT | 5′ 828 | |
| s3 h | AGC TTG CTG TTA CTC TCT TCT A | 3′ 1462 | |
| s4Hf | ATC ACC TTG CGC CTT AGC | 5′ 1293 | |
| s4 h | AGT GCA ACT TGG CTG ACC | 3′ 1896 | |
| II | s1f | CTG TCG CAG TGA CYG CTA | 5′ −248 |
| s1r | CCCT GTC TGR GAT GAG GT | 3′ 176 | |
| s2f | ACA GGG TCA ATC ATA GTC AAG | 5′ 171 | |
| s2r | CCC AAG AGT TGA GTC TGT G | 3′ 850 | |
| s3f | TAG CTG GTG GNA ATA TGG ATT A | 5′ 721 | |
| s3r | ATC RAA TTC CCC ACT GAG C | 3′ 1322 | |
| s4f | ATA TCG CAA AAY TAT GGA GAA GC | 5′ 1221 | |
| s4r | GCY TCT CTT TCN TCA TTC TCT A | 3′ 1917 |
Amplifications of target sequences were done using the OneStep RT-PCR kit (Qiagen). Reaction mixes were prepared with 10 μl 5× PCR buffer, 2 μl deoxynucleoside triphosphates (dNTPs) (10 mM), 1 μl of each primer (50 pmol/μl), 2 μl enzyme mix, 0.4 μl RNase inhibitor (20 U/μl), and 5 to 10 μl template RNA and filled up to 50 μl with H2O. The following thermal amplification cycle conditions were applied: 50°C for 30 min, followed by 94°C for 15 min, followed by 45 cycles of 94°C for 1 min, 48°C for 1 min, and 72°C for 1 min, followed by 72°C for 4 min.
Samples were prepared for sequencing by gel extraction by the QIAquick gel extraction kit or PCR purification by the MiniElute PCR purification spin kit (both from Qiagen). The sequencing reactions were performed by FIMM SeqLab (Institute for Molecular Medicine Finland, Helsinki, Finland).
Sequence analysis.
Sequence analysis was performed on a 400-nt region of class I viruses (nt 125 to 524) and on a 450-nt region (nt 275 to 724) of class II viruses of the NDV F gene. Sequences were edited using eBioX version 1.5.1 software (www.ebioinformatics.org) and compared to published strains by BLAST. Reference strains were picked based on BLAST search results as well as from representatives for different lineages. The alignments and phylogenetic analyses were conducted using MEGA software version 5.0 (38). The trees were generated by using the neighbor-joining algorithm, and alignments were bootstrapped 1,000 times.
Assessment of pathogenicity.
The F gene sequences of our strains were analyzed for pathogenic determinants in the amino acid sequences of the fusion protein cleavage sites (amino acids 112 to 117), shown in Tables 2 and 3.
Table 2.
Finnish NDV strains derived through active surveillance
| No. | GenBank accession no. | Isolate | Yr | Class | Genotype | F cleavage siteb | CT valued | Host |
|---|---|---|---|---|---|---|---|---|
| 1 | EU493453 | 12136a | 2006 | II | I | GKQGRL | Not tested | Eurasian teal (Anas crecca) |
| 2 | EU493451 | 12104a | 2006 | I | 2 | ERQERL | Not tested | Eurasian teal (Anas crecca) |
| 3 | EU493454 | 13193a | 2006 | I | 2 | ERQERL | Not tested | Common pochard (Aythya ferina) |
| 4 | EU493452 | 12119a | 2006 | I | 2 | ERQERL | Not tested | Eurasian teal (Anas crecca) |
| 5 | EU493450 | 12074a | 2006 | II | I | GKQGRL | Not tested | Eurasian teal (Anas crecca) |
| 6 | JX844028 | 13111 | 2008 | I | New genotype | ERQERL | Not tested | Eurasian teal (Anas crecca) |
| 7 | JX844037 | 8789 | 2009 | II | I | GKQGRL | Not tested | Mallard (Anas platyrhynchos) |
| 8 | JX844038 | 8791 | 2009 | II | I | GKQGRL | Not tested | Mallard (Anas platyrhynchos) |
| 9 | JX844039 | 8803 | 2009 | II | I | GKQGRL | Not tested | Mallard (Anas platyrhynchos) |
| 10 | JX844040 | 9067 | 2009 | II | I | c | Not tested | Eurasian wigeon (Anas penelope) |
| 11 | JX844041 | 10666 | 2009 | II | I | GKQGRL | Not tested | Eurasian teal (Anas crecca) |
| 12 | JX844042 | 10668 | 2009 | II | I | GKQGRL | Not tested | Eurasian teal (Anas crecca) |
| 13 | JX844043 | 8625 | 2010 | II | I | GKQGRL | 35.7 | Eurasian wigeon (Anas penelope) |
| 14 | JX844044 | 8633 | 2010 | II | I | GKQGRL | 30.0 | Eurasian teal (Anas crecca) |
| 15 | JX844045 | 8637 | 2010 | II | I | GKQGRL | 35.9 | Eurasian teal (Anas crecca) |
| 16 | JX844046 | 8641 | 2010 | II | I | GKQGRL | 39.0 | Northern shoveler (Anas clypeata) |
| 17 | JX844047 | 8645 | 2010 | II | I | GKQGRL | 34.6 | Eurasian wigeon (Anas penelope) |
| 18 | JX844048 | 8768 | 2010 | II | I | EKQGRL | 21.8 | Mallard (Anas platyrhynchos) |
| 19 | JX844049 | 8776 | 2010 | II | I | EKQGRL | 35.4 | Eurasian teal (Anas crecca) |
| 20 | c | 8778 | 2010 | II | I | EKQGRL | 32.2 | Eurasian teal (Anas crecca) |
| 21 | c | 9125 | 2010 | II | I | GKQGRL | 38.2 | Mallard (Anas platyrhynchos) |
| 22 | JX844050 | 9127 | 2010 | II | I | GKQGRL | 37.0 | Mallard (Anas platyrhynchos) |
| 23 | JX844051 | 9129 | 2010 | II | I | GKQGRL | 25.9 | Mallard (Anas platyrhynchos) |
| 24 | JX844052 | 9143 | 2010 | II | I | GKQGRL | 26.1 | Mallard (Anas platyrhynchos) |
| 25 | JX844030 | 9147 | 2010 | I | 2 | ERQERL | 41.2 | Mallard (Anas platyrhynchos) |
| 26 | JX844053 | 9149 | 2010 | II | I | GKQGRL | 30.3 | Mallard (Anas platyrhynchos) |
| 27 | JX844054 | 9167 | 2010 | II | I | GKQGRL | 27.4 | Mallard (Anas platyrhynchos) |
| 28 | c | 9171 | 2010 | c | c | c | 35.3 | Mallard (Anas platyrhynchos) |
| 29 | c | 9185 | 2010 | II | I | GKQGRL | 35.7 | Eurasian teal (Anas crecca) |
| 30 | JX844055 | 9197 | 2010 | II | I | GKQGRL | 25.5 | Eurasian teal (Anas crecca) |
| 31 | JX844056 | 9360 | 2010 | II | I | GKQGRL | 33.4 | Mallard (Anas platyrhynchos) |
| 32 | JX844057 | 10539 | 2010 | II | I | EKQGRL | 27.0 | Eurasian teal (Anas crecca) |
| 33 | JX844058 | 10541 | 2010 | II | I | EKQGRL | 32.0 | Mallard (Anas platyrhynchos) |
| 34 | JX844059 | 12376 | 2010 | II | I | GKQGRL | 30.2 | Eurasian teal (Anas crecca) |
| 35 | JX844060 | 13349 | 2010 | II | I | GKQGRL | 35.3 | Mallard (Anas platyrhynchos) |
| 36 | JX844029 | 13355 | 2010 | I | 2 | ERQERL | 38.8 | Mallard (Anas platyrhynchos) |
| 37 | JX844061 | 13357 | 2010 | II | I | GKQGRL | 32.9 | Mallard (Anas platyrhynchos) |
| 38 | JX844062 | 15549 | 2010 | II | I | GKQGRL | 32.2 | Mallard (Anas platyrhynchos) |
| 39 | JX844063 | 15551 | 2010 | II | I | GKQGRL | 32.0 | Eurasian teal (Anas crecca) |
Published by Lindh et al. (27).
Amino acids 112 to 117.
Not enough sequence obtained.
Analyzed by L gene real-time RT-PCR.
Table 3.
Finnish NDV strains derived from outbreaks and through passive surveillance
| No. | GenBank accession no. | Isolate | Yr | Class | Genotype | F cleavage siteb | ICPI | Host |
|---|---|---|---|---|---|---|---|---|
| 1 | AY034794 | Fin-69a | 1969 | II | IV | RRQRRF | NTd | Williow grouse (Lagopus lagopus) |
| 2 | AY034795 | Fin-70a | 1970 | II | IV | RRQRRF | NT | Chicken (Gallus gallus) |
| 3 | AY034796 | Fin-92a | 1992 | II | VI | RRQKRF | 1.4 | Feral pigeon (Columba livia) |
| 4 | AY034797 | Fin-96aa | 1996 | II | VI | RRKKRF | 1.32 | Feral pigeon (Columba livia) |
| 5 | AY034798 | Fin-96ba | 1996 | II | VII | RRQRRF | 1.38 | Goosander (Mergus merganser) |
| 6 | AY034799 | Fin-96ca | 1996 | II | VI | RRKKRF | 1.4 | Feral pigeon (Columba livia) |
| 7 | AY034800 | Fin-96da | 1996 | II | VI | RRKKRF | 1.4 | Feral pigeon (Columba livia) |
| 8 | AY034801 | Fin-97a | 1997 | I | 2 | ERQERL | 0.17 | Mallard (Anas platyrhynchos) |
| 9 | JX844031 | 7775 | 2003 | II | I | GKQGRL | 0.5 | Pheasant (Phasianus colchius) |
| 10 | JX844032 | 8036 | 2003 | II | I | GKQGRL | 0.5 | Pheasant (Phasianus colchius) |
| 11 | JX844033 | 5789 | 2004 | II | VII | RRQRRF | 1.6 | Turkey (Melagris) |
| 12 | JX844034 | 6985 | 2008 | II | VI | RRQKRF | NT | Domestic pigeon (Columba livia domestica) |
| 13 | JX844035 | 17557 | 2008 | II | VI | RRQKRF | NT | Domestic pigeon (Columba livia domestica) |
| 14 | JX844036 | 15475 | 2009 | II | VI | KRQKRF | NT | Feral pigeon (Columbia livia) |
| 15 | c | 4913 | 2010 | II | Not typed | c | NT | Razorbill (Alca torda) |
Published by Huovilainen et al. (18).
Amino acids 112 to 117.
Not enough sequence obtained.
NT, test not done.
The outbreak-associated strains were sent to The Animal Health and Veterinary Laboratories Agency (AHVLA; formerly VLA, Surrey, United Kingdom), where ICPI tests were carried out.
GenBank accession numbers.
GenBank accession numbers of the Finnish strains described in this study are designated in Tables 2 and 3.
RESULTS
Prevalence and sampled species.
In total, 715 birds were sampled (Table 4), most of which were wild waterfowl. The most prevalent species sampled for active surveillance were mallard (Anas platyrhynchos) (53%) and Eurasian teal (Anas crecca) (22%), as they are the most common bagged duck species and nest in high numbers in our study areas (16, 22). The samples were derived from the whole country, except from the northernmost part of Finland.
All together, 39 NDV strains were found in the active surveillance study. F gene RT-PCR (used for samples collected prior to 2010) or real-time L gene RT-PCR (samples from 2010) positive samples were confirmed to be NDV by either sequencing and/or by isolation in embryonated eggs with hemagglutination activity. All samples which yielded cycle threshold (CT) values of <45 in the real-time RT-PCR assay were considered potentially positive and were subject to further verification by the other methods mentioned above (Table 4). Most (30/32) samples that were confirmed class II positive in this study had CT values under 37, which was used as a threshold by Fuller et al. (13), while our class I viruses yielded CT values over 41 (Table 2). The high CT values of two class II viruses were probably due to poor sample quality, whereas the class I viruses with high CT values were readily detected by the conventional RT-PCR.
Table 4.
Active surveillance sample size and number of NDV positives per year
| Yr | No. of samples | No. of PCR positives | No. of isolations | Class (no. of NDV-positive samples in class) |
|---|---|---|---|---|
| 2006 | 115 | 5a | 4 | I (3) |
| II (2) | ||||
| 2008 | 182 | 1a | 1 | I (1) |
| 2009 | 189 | 6a | 6 | II (6) |
| 2010 | 229 | 27b | 5c | I (2) |
| II (24) | ||||
| Untyped (1) | ||||
| Total | 715 | 39 (5.5%) | 16 (>41%) | I (6), II (32) |
Screened by F gene RT PCR.
Screened by L gene real-time RT-PCR.
Isolation attempted from only a part of the samples.
The NDV-positive active surveillance samples (Table 2) derived most often from Eurasian teals (10.3% of sampled teals, statistically significantly more often than from other sampled species in this study; P = 0.0092), mallards (4.8% of sampled mallards), Eurasian wigeons (Anas penelope; 5% of sampled wigeons), a Northern shoveler (Anas clypeata), and a common pochard (Aythya ferina). The yearly prevalence of NDV among wild waterfowl varied between 0.5% in 2008 to 11.8% in 2010. In passive surveillance, up to 100 samples were collected per year, and only one NDV-positive sample was detected during the study period. This passive surveillance sample was from a dead razorbill (Alca torda), which was initially suspected to be infected with AIV but found positive for NDV. All the birds sampled for active surveillance appeared clinically healthy, although five birds (four Eurasian teals and a northern shoveler) were infected by both lentogenic NDV and low-pathogenic avian influenza virus. No velogenic strains were found from the active surveillance samples.
Characterization of the detected NDV strains.
Sequence data of the F gene cleavage site was gained from all but three of the PCR-positive active surveillance samples, probably because of poor sample quality. All the waterfowl-derived strains expressed the lentogenic fusion protein cleavage site motifs: GKQGRL, EKQGRL, or ERQERL (Table 2). Outbreak-associated isolates from 2004 and 2008 and the feral pigeon strain from 2009 possessed cleavage site motifs typical for velogenic strains: turkey/Finland/5789/2004, RRQRRF; domestic pigeon/Finland/17557/2008, RRQKRF; feral pigeon/Finland/15475/2009, KRQKRF. Pheasant/Finland/8036/2003 had the lentogenic motif GKQGRL (Table 3).
The wild waterfowl-derived viruses were all lentogenic viruses of class I or class II genotype I. The 31 class II wild-waterfowl-derived samples, collected during 2006, 2009, and 2010, were at least 96.5% identical, but most were over 98% identical at the nucleotide level (comparison of the 450-nt region). On a few occasions, fully identical viruses were isolated 4 years apart from the same location. As many of our class II strains analyzed in this study are very similar, a few representatives were selected for phylogenetic purposes in Fig. 1, while the relationships between all Finnish strains are depicted in Fig. 2.
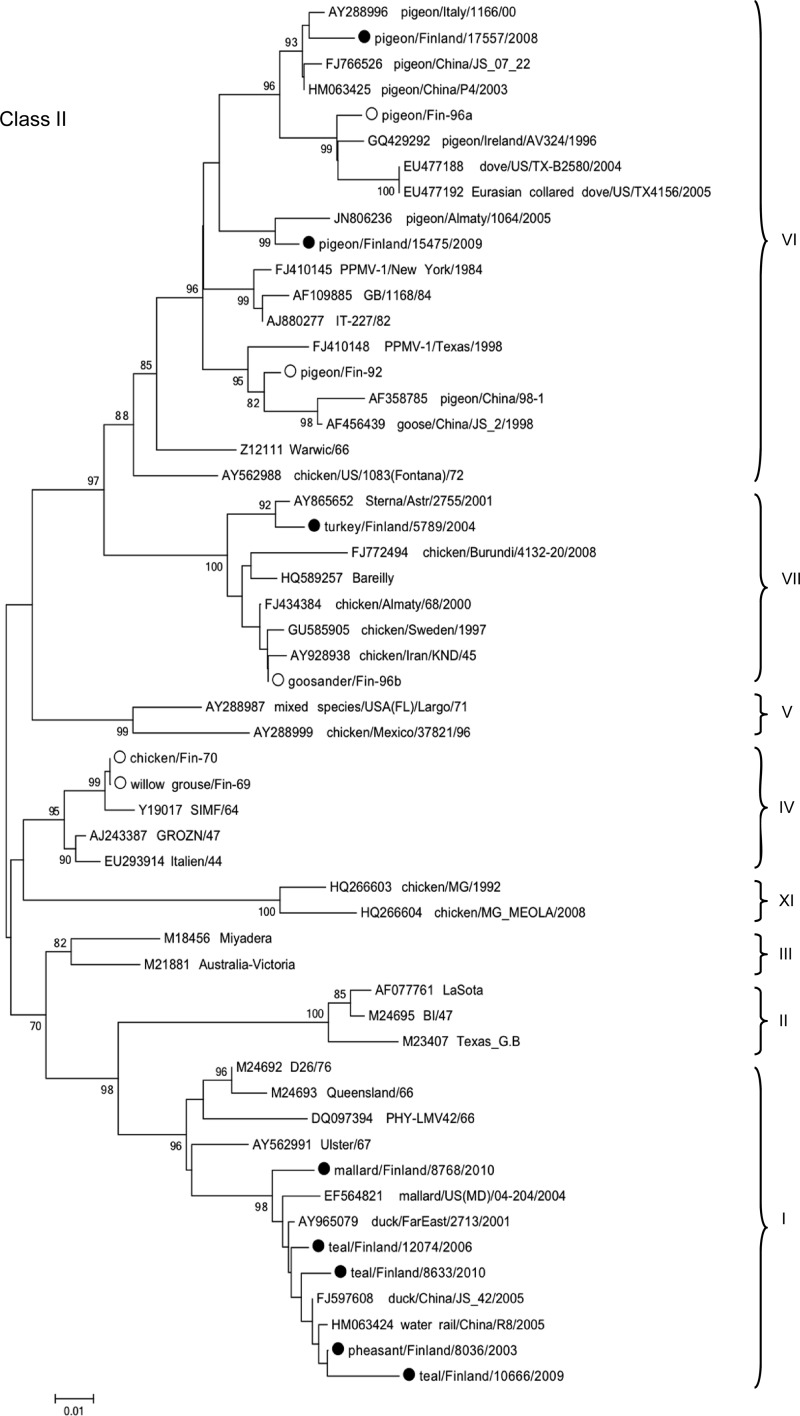
Fig 1.
Class II phylogeny. Phylogenetic analysis of the F gene (450 nt, positions 275 to 724) of Finnish class II strains. Finnish strains described in this study are marked by black dots, and previous Finnish strains are marked by white dots. Highly similar strains were left out and are depicted in Fig. 2. The tree was generated by a neighbor-joining algorithm, and alignments were bootstrapped 1,000 times. Bootstrap values of >70 are shown. The strain names were edited to include origin of isolates where needed. Genotypes are marked on the right.
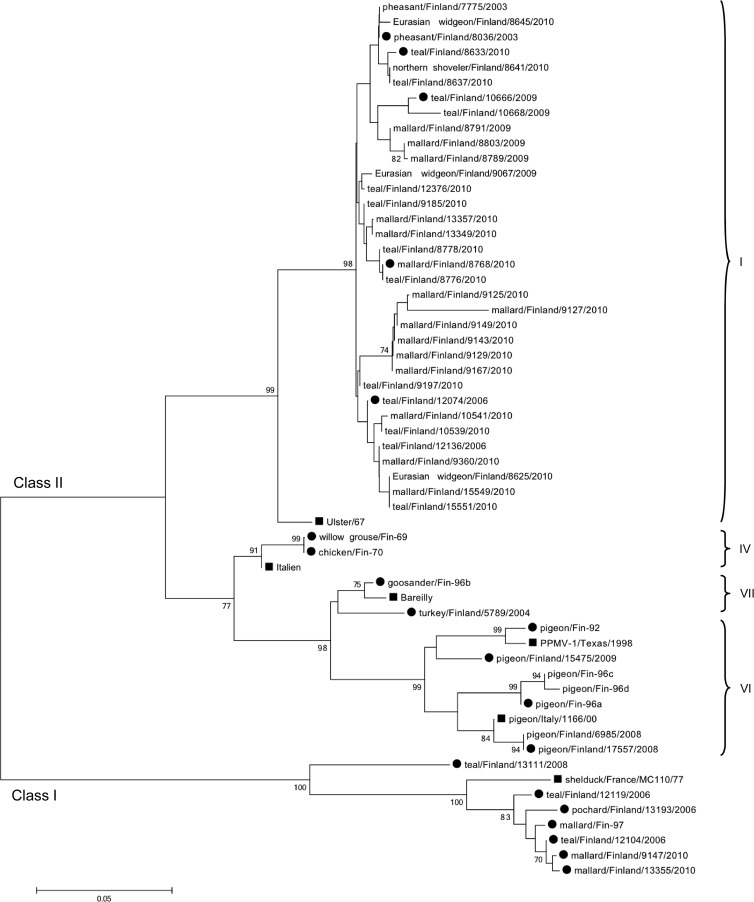
Fig 2.
Phylogenetic tree of all Finnish NDV strains. Phylogenetic analysis of the F gene (275 nt; positions 304 to 578) of all Finnish NDV strains. Finnish strains used for phylogeny in Fig. 1 are marked by black dots, and indicator strains for genotypes are marked by black squares. The tree was generated by a neighbor-joining algorithm, and alignments were bootstrapped 1,000 times. Bootstrap values of >70 are shown. The strain names were edited to include origin of isolates where needed. Genotypes are marked on the right. GenBank numbers for all the Finnish strains are found in Tables 2 and 3.
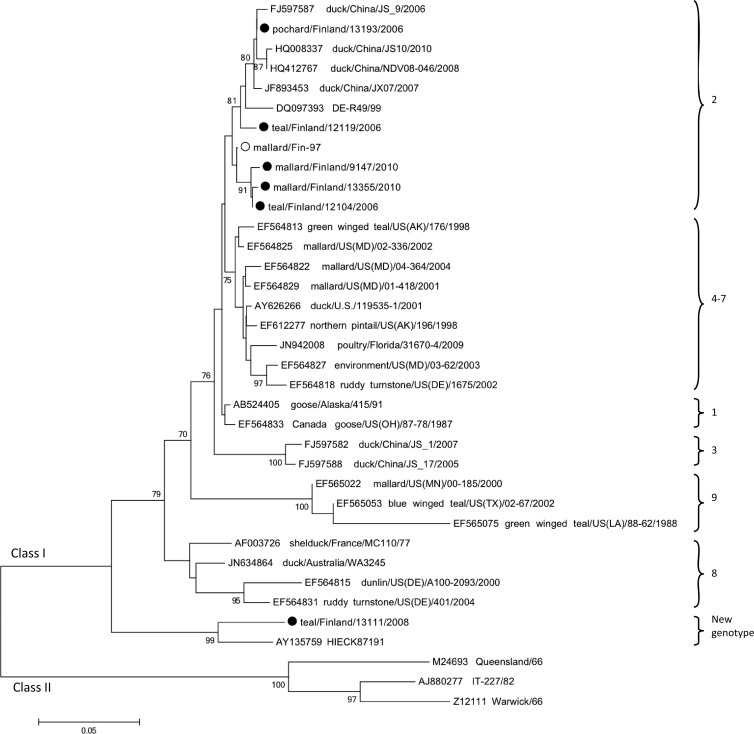
Our class I strains (collected in 1997, 2006, 2008, 2009, and 2010) were 97.5% to 99.5% identical and clustered phylogenetically with strains of the class I genotype 2 (by analysis of 400 nt of the F gene; Fig. 3). An exception was the strain teal/Finland/13111/2008, which differs by >13% of the other Finnish strains. Teal/Finland/13111/2008 is only up to 88% identical at the nucleotide level with other class I viruses, with a single exception: strain HIECK87191 (or Espey, AY135759) isolated from a chicken in Ireland in 1987 (3). By phylogenetic analysis, these two strains cluster independently from all other published strains and form a new genotype, which based on the grouping made by Kim et al. (23) would form a 10th genotype. The amino acid sequence of teal/Finland/13111/2008 and HIECK87191 diverges by 7% or more from other class I strains. Interestingly, teal/Finland/13111/2008 has a highly variable 20-amino-acid motif, 9YPCILTLTSAVVTLTLMTG28, in the N-terminal region of the fusion protein, preceding the cleavage site (Fig. 4). A BLAST search of this nucleotide and amino acid sequence identified a homologous domain solely in strain HIECK87191. The distinct N-terminal sequence was left out from phylogenetic analyses and alignments in order to not confer with the results.
Fig 3.
Class I phylogeny. Phylogenetic analysis of the F gene (400 nt; positions 125 to 524) of Finnish class I strains. The Finnish strains described in this study are marked by black dots and a previous isolate by a white dot. The tree was generated by a neighbor-joining algorithm, and alignments were bootstrapped 1,000 times. Bootstrap values of >70 are shown. The strain names were edited to include origin of isolates where needed. Genotypes (according to Kim et al. [24]) are marked on the right.
Fig 4.
Alignment of the fusion protein amino acids 1 to 40. Amino acid sequence of the fusion protein, showing the distinct motif of teal/Finland/13111/2008 and HIECK87191 at positions 9 to 28. The region is distinct between class I and class II genotypes. GenBank numbers are found in Fig. 1 and 3.
Of the outbreak-associated strains, strain pheasant/Finland/8036/03 was found highly identical with some of our wild waterfowl-derived strains, differing by as little as 0.6%. It also clustered phylogenetically with strains from ducks and wild birds in China and Russia, which shared 99% sequence identity (Fig. 1).
Strain turkey/Finland/5789/04 clustered with the genotype VIIb viruses and was most closely related (99% identical) to a strain isolated from a shorebird (little tern; Sterna albifrons; GenBank accession no. AY865652) in Russia 3 years earlier. It also clustered phylogenetically with an earlier Finnish isolate (Fin-96; 97% nucleotide identity) and poultry-derived isolates from Iran (1995), Sweden (1997), and Russia (2000) (96 to 97% nucleotide identities) (Fig. 1).
Domestic pigeon/Finland/17557/2008, isolated during a recent outbreak among hobby pigeons, and feral pigeon/Finland/15475/2009 clustered phylogenetically together with genotype VIb strains. The domestic pigeon isolates were genetically close to strain P4 and other Chinese PPMV-1 strains (up to 99% nucleotide identity), while the feral pigeon isolate was 98% identical with a Kazakhstanian strain but reached only 96% identity with all other strains of clade VIb (Fig. 1).
DISCUSSION
NDV is a common pathogen of birds, and infections have been reported in at least 236 species (21). Czeglédi and colleagues (10) speculated that class I and class II genotype I are ancestral representatives of NDV maintained by their natural hosts, the wild waterfowl. The recent genotypes, including most velogenic strains, are thought to have arisen in the 20th century through the involvement of a secondary host, the chickens. Because of the economic impact of NDV on the poultry industry, most of the literature is focused on strains derived from commercial flocks. Less is known about the NDV strains circulating in the nature, their evolution, and the role of different host species.
Studies of NDV among wild birds in North America and China have recently been published (9, 23). Similar information on prevalence and molecular epidemiology of NDV in wild birds in Europe has been scarce, although outbreaks among poultry are frequently reported. In our study, the yearly prevalence of NDV ranged from 0.5% to 11.8%. The highest prevalence was recorded in 2010 when a new real-time RT-PCR method was introduced. A similar, around 10% yearly prevalence of AIV has been recorded in our laboratory (27). Although sampling procedures varied slightly from year to year and relied on voluntary collectors, isolation and sequencing success was high for PCR-positive samples.
Most of the strains from waterfowl in Northern Europe found during this study were class II genotype I viruses. Our strains appear highly related to Chinese strains that appear endemic in both live-bird markets and in wild-waterfowl populations (9, 28). These two areas are connected by the Eurasian migratory flyway. The genotype I viruses isolated between 2006 and 2010 from four main sampling sites situated 150 to 500 km apart showed overall very little variation. The strains were highly identical and showed rather site-specific than species-specific similarities, manifested as specific nucleotide signatures at given locations. This is not unexpected, since mallards and Eurasian teals are nesting in the same areas all over Finland. The observed geographic clustering might, however, be related to sample relatedness in terms of sampling time and host population.
Class II genotype I viruses are generally lentogenic, but a virulent strain that was the causative agent of a disease outbreak in Australia in 1998 is thought to have arisen from a lentogenic progenitor (15). The change from lentogenic NDV to velogenic NDV during favorable conditions, e.g., replication in dense poultry populations, continues to be a major concern. Factors favoring evolution of virulence are (i) the requirement of only a few mutations in the F gene and (ii) the circulation of a high load of diverse lentogenic NDVs in the wild-bird reservoir (33). Some of the genotype I derivatives, e.g., Ulster2C/67 and Queensland/V4, are used as live vaccines in many countries. Billions of doses of these low-virulence vaccines are used annually, some of which leak into the environment and might be encountered in wild birds.
Six of 39 wild-bird-derived strains were class I NDV. Notably, teal//Finland/13111/2008 and HIECK87191 strains both differ by >12% from all other published class I NDV strains and harbor a distinct N-terminal motif. Phylogenetic analysis further defines these two strains as the sole members of a distinct genotype of NDV, provisionally named here class I genotype 10. Class I viruses have previously been found in waterfowl and live-bird markets. Although generally regarded as lentogenic strains, a class I strain has at least on one occasion been associated with a velogenic outbreak among poultry (5). Shengqing et al. and Tsunekuni et al. assessed the potential of wild-waterfowl-derived lentogenic class I NDV to become velogenic by serial passaging of goose/Alaska415/91. They showed that during 14 passages in chicken, seven amino acid substitutions arose, leading to a shift to a highly virulent variant (37, 41).
Class I viruses have been neglected in many screening studies, mainly because of poor detection rates by conventional RT-PCR-based screening assays, designed for class II NDV (23). Kim et al. calculated that a commonly used M gene assay (43) probe site diverged from class I nucleotide sequences by at least 25%, failing to detect them (24). The F gene RT-PCR protocols used in Finland were updated to include class I viruses after the first Finnish class I virus isolation in 1997 (18). The use of a rapid real-time multiplex RT-PCR assay for screening clinical samples is much welcomed in diagnostic laboratories. However, attention is needed to reassure that also more divergent strains are detected, e.g., by verifying inconclusive results by isolation attempts or by complementing PCR assays. It should be kept in mind that a sole CT value does not provide sufficient diagnostic value and that the conventional methods are needed to assess other properties of the detected strains. The real-time RT-PCR assay used here seems to have an increased sensitivity compared to our previous methods, judged by the higher number NDV PCR positives in 2010, but as noted by the developers, this method is less sensitive for the detection of class I viruses, as demonstrated by higher CT values (13).
Finland is located on the Western European flyway, and a big proportion of hatch-year dabbling ducks wintering in West and Central Europe have been found to originate in Southern Finland (16). Interestingly, while a clear phylogenetic separation can be seen between North American and Eurasian AIV strains, our wild-waterfowl-derived strains were closely related to strains that were isolated on the other side of the Atlantic Ocean. This might reflect the involvement of different host species, with different migratory patterns, than the common hosts of AIV. Trade of birds might also play a part in the transatlantic exchange of strains. There are no duck species which regularly migrate across the Atlantic sea, although many ducks, including American green-winged teal (Anas carolensis), stray to the European side of the Atlantic, where they share the same wintering grounds with Eurasian teals. These two species are able to reproduce together and could serve as the bridge between North American and European NDV. This would further indicate that lentogenic NDV has only very mild or no effect on the fitness of its waterfowl hosts, who are able to migrate across the Atlantic Ocean while infected. The prevalence of NDV in Eurasian teal was found to be significantly high in this study, which could further indicate that this species has a pronounced role in the ecology of NDV in Northern Europe.
Pheasants are susceptible to all strains of NDV, usually introduced by strains prevalent in poultry and wild birds (1). Isolates from the outbreak among farmed pheasants in 2003 are highly similar (over 99% nucleotide identity) to genotype I viruses that have repeatedly been detected among waterfowl populations in Finland since 2006 and can be considered endemic. The pheasants were most likely infected through contact with wild birds while sharing common grounds during part of their rearing or through trade with another backyard-poultry holding where the reared birds were exposed to wild birds.
During the outbreak in a turkey farm in 2004, similar outbreaks coincided in other Nordic countries. While velogenic strains generally cause acute fatal infections of chickens of all age groups, this turkey farm showed no symptoms of disease. The only published strain with high similarity (99%) to our outbreak-derived virus is a velogenic isolate from a little stern in Russia. A previous, closely related wild-bird-derived strain, goosander/Fin-96b, was isolated concurrently with outbreaks among poultry in Sweden and Norway and outbreaks in the British Isles in 1996 to 1997. Genetic analyses were consistent with the theory of viral spread by migratory birds, although a role of trade of birds or related products could not be ruled out (4, 26). Apparently, endemic genotype VIIb viruses are regularly introduced to domestic birds where they cause outbreaks. Our isolate (turkey/Finland/5789/04) clustered together with mainly poultry-derived strains, but this is probably a bias caused by the trend to sample and publish sequences of poultry origin.
Epidemiological investigations did not reveal the source of the outbreak among hobby pigeons in 2008. Although import was suspected to be the primary source, genetic analyses of the viruses isolated during the outbreak did not give any further evidence of this. Whether the introduction happened through trade of birds or through unidentified contacts with wild birds remains unsolved, as the closest matches were only up to 98% identical. Although the focus is often on protecting poultry populations, transmission of pathogens can also occur in the opposite direction, from domestic birds to wild birds. This should be noted in order to reduce the spread of PPMV-1 strains.
The Finnish Food Safety Authority has evaluated the effects of vaccinations for the Finnish poultry industry in 2005 (11). The conclusion was that vaccination of poultry should remain banned and that NDV should continuously be prevented in Finland by efficient use of biosecurity measures and effective laboratory diagnostics. An exception to our nonvaccinating policy is racing pigeons participating in shows and races, where vaccinations with a killed virus are required.
In conclusion, our study shows that lentogenic NDVs of class I and class II genotype I are endemic among clinically healthy wild waterfowl in Finland. Because Central and Northwestern Europe are connected by migratory routes of the species analyzed here, our data should apply to Europe in general. Efforts are needed to restrict contacts between wild birds and poultry, as these two host systems appear to have a continuous exchange of NDVs. Import, trade, and bird shows are other events that easily allow the spread of new virulent strains to susceptible populations, unless strict control measures are applied. Identification of new strains escaping conventional diagnostic assays and vaccines underlines the need to continuously update our surveillance systems in terms of diagnostic assays, biosecurity, and research, in order to protect both poultry and wild birds.
ACKNOWLEDGMENTS
We gratefully acknowledge all the volunteer hunters for providing samples for this study and the Finnish Game and Fisheries Research Institute for their support. Hannu Pöysä, Jorma Autioniemi, Jorma Korhonen, Petri Timonen, and Einari Väyrynen are especially acknowledged for their contribution to the sample collection. We also thank technical staff members, especially Merja Hautala and Mia Biström (BVDc), for their contribution in the laboratory work. Tarja Sironen is thanked for the expertise she provided in phylogenic analyses.
The Animal Health and Veterinary Laboratories Agency (AHVLA; formerly VLA) is acknowledged for assessing the intracerebral pathogenicity indexes (ICPI) for strains described in our study.
The research was funded by the Finnish Ministry of Agriculture and Forestry, Helsinki Biomedical Graduate School (HBGS), and Victoriastiftelsen.
Footnotes
Published ahead of print 12 September 2012
REFERENCES
- 1. Aldous EW, Alexander DJ. 2008. Newcastle disease in pheasants (Phasianus colchicus): a review. Vet. J. 175: 181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aldous EW, Fuller CM, Mynn JK, Alexander DJ. 2004. A molecular epidemiological investigation of isolates of the variant avian paramyxovirus type 1 virus (PPMV-1) responsible for the 1978 to present panzootic in pigeons. Avian Pathol. 33: 258–269 [DOI] [PubMed] [Google Scholar]
- 3. Aldous EW, Mynn JK, Banks J, Alexander DJ. 2003. A molecular epidemiological study of avian paramyxovirus type 1 (Newcastle disease virus) isolates by phylogenetic analysis of a partial nucleotide sequence of the fusion protein gene. Avian Pathol. 32: 239–256 [DOI] [PubMed] [Google Scholar]
- 4. Alexander DJ, et al. 1999. Antigenic and genetic characterisation of Newcastle disease viruses isolated from outbreaks in domestic fowl and turkeys in Great Britain during 1997. Vet. Rec. 145: 417–421 [DOI] [PubMed] [Google Scholar]
- 5. Alexander DJ, et al. 1992. Characterisation of an antigenically unusual virus responsible for two outbreaks of Newcastle disease in the Republic of Ireland in 1990. Vet. Rec. 130: 65–68 [DOI] [PubMed] [Google Scholar]
- 6. Alexander DJ, et al. 1997. Antigenic diversity and similarities detected in avian paramyxovirus type 1 (Newcastle disease virus) isolates using monoclonal antibodies. Avian Pathol. 26: 399–418 [DOI] [PubMed] [Google Scholar]
- 7. Ballagi-Pordany A, Wehmann E, Herczeg J, Belak S, Lomniczi B. 1996. Identification and grouping of Newcastle disease virus strains by restriction site analysis of a region from the F gene. Arch. Virol. 141: 243–261 [DOI] [PubMed] [Google Scholar]
- 8. Briand FX, Henry A, Massin P, Jestin V. 2012. Complete genome sequence of a novel avian paramyxovirus. J. Virol. 86: 7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai S, et al. 2011. Genetic characterization and evolutionary analysis of 4 Newcastle disease virus isolate full genomes from waterbirds in South China during 2003–2007. Vet. Microbiol. 152: 46–54 [DOI] [PubMed] [Google Scholar]
- 10. Czegledi A, et al. 2006. Third genome size category of avian paramyxovirus serotype 1 (Newcastle disease virus) and evolutionary implications. Virus Res. 120: 36–48 [DOI] [PubMed] [Google Scholar]
- 11. Ek-Kommonen C, Jakava-Viljanen M, Perko-Mäkelä P, Rosengren H, Rossow L. 2005. Impact of vaccination for Newcastle disease in Finland—a report. Nat. Vet. Food Res. Inst. Pub. ISSN 1458–6878. [Google Scholar]
- 12. EU 1992. Community measures for the control of Newcastle disease. European Union law. Council directive 92/66/EEC. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31992L0066:EN:NOT
- 13. Fuller CM, Brodd L, Irvine RM, Alexander DJ, Aldous EW. 2010. Development of an L gene real-time reverse-transcription PCR assay for the detection of avian paramyxovirus type 1 RNA in clinical samples. Arch. Virol. 155: 817–823 [DOI] [PubMed] [Google Scholar]
- 14. Gould AR, et al. 2003. Newcastle disease virus fusion and haemagglutinin-neuraminidase gene motifs as markers for viral lineage. Avian Pathol. 32: 361–373 [DOI] [PubMed] [Google Scholar]
- 15. Gould AR, et al. 2001. Virulent Newcastle disease in Australia: molecular epidemiological analysis of viruses isolated prior to and during the outbreaks of 1998-2000. Virus Res. 77: 51–60 [DOI] [PubMed] [Google Scholar]
- 16. Gunnarsson G, et al. 2012. Disease dynamics and bird migration—linking mallards Anas platyrhynchos and subtype diversity of the influenza A virus in time and space. PLoS One. 7: e35679 doi:10.1371/journal.pone.0035679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Herczeg J, et al. 1999. Two novel genetic groups (VIIb and VIII) responsible for recent Newcastle disease outbreaks in Southern Africa, one (VIIb) of which reached Southern Europe. Arch. Virol. 144: 2087–2099 [DOI] [PubMed] [Google Scholar]
- 18. Huovilainen A, Ek-Kommone C, Manvell R, Kinnunen L. 2001. Phylogenetic analysis of avian paramyxovirus 1 strains isolated in Finland. Arch. Virol. 146: 1775–1785 [DOI] [PubMed] [Google Scholar]
- 19. ICTV. King A, Lefkowitz E, Adams MI, Carstens EB. 2012. Virus taxonomy: ninth report of the International Committee on Taxonomy of Viruses. Academic Press, London, United Kingdom [Google Scholar]
- 20. Kaleta EF, Alexander DJ, Russell PH. 1985. The first isolation of the avian PMV-1 virus responsible for the current panzootic in pigeons? Avian Pathol. 14: 553–557 [DOI] [PubMed] [Google Scholar]
- 21. Kaleta EF, Baldauf C. 1988. Newcastle disease in free-living and pet birds, p 197–246 In Alexander DJ. (ed), Newcastle disease. Kluwer Academic Publishers, Boston, MA [Google Scholar]
- 22. Kauppinen J, Väänänen VM. 1999. Factors affecting changes in waterfowl populations in eutrophic wetlands in the Finnish lake district. Wildl. Biol. 7: 73–81 [Google Scholar]
- 23. Kim LM, et al. 2007. Phylogenetic diversity among low-virulence Newcastle disease viruses from waterfowl and shorebirds and comparison of genotype distributions to those of poultry-origin isolates. J. Virol. 81: 12641–12653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim LM, King DJ, Suarez DL, Wong CW, Afonso CL. 2007. Characterization of class I Newcastle disease virus isolates from Hong Kong live bird markets and detection using real-time reverse transcription-PCR. J. Clin. Microbiol. 45: 1310–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kommers GD, King DJ, Seal BS, Brown CC. 2003. Virulence of six heterogeneous-origin Newcastle disease virus isolates before and after sequential passages in domestic chickens. Avian Pathol. 32: 81–93 [DOI] [PubMed] [Google Scholar]
- 26. Linde AM, et al. 2010. Complete genome characterisation of a Newcastle disease virus isolated during an outbreak in Sweden in 1997. Virus Genes 41: 165–173 [DOI] [PubMed] [Google Scholar]
- 27. Lindh E, et al. 2008. Orthomyxo-, paramyxo- and flavivirus infections in wild waterfowl in Finland Virol. J. 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu X, et al. 2009. Surveillance for avirulent Newcastle disease viruses in domestic ducks (Anas platyrhynchos and Cairina moschata) at live bird markets in Eastern China and characterization of the viruses isolated. Avian Pathol. 38: 377–391 [DOI] [PubMed] [Google Scholar]
- 29. Liu XF, Wan HQ, Ni XX, Wu YT, Liu WB. 2003. Pathotypical and genotypical characterization of strains of Newcastle disease virus isolated from outbreaks in chicken and goose flocks in some regions of China during 1985–2001. Arch. Virol. 148: 1387–1403 [DOI] [PubMed] [Google Scholar]
- 30. Lomniczi B, et al. 1998. Newcastle disease outbreaks in recent years in Western Europe were caused by an old (VI) and a novel genotype (VII). Arch. Virol. 143: 49–64 [DOI] [PubMed] [Google Scholar]
- 31. Maminiaina OF, et al. 2010. Newcastle disease virus in Madagascar: identification of an original genotype possibly deriving from a died out ancestor of genotype IV. PLoS One 5: e13987 doi:10.1371/journal.pone.0013987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller PJ, et al. 2010. Evidence for a new avian paramyxovirus serotype 10 detected in rockhopper penguins from the Falkland Islands. J. Virol. 84: 11496–11504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller PJ, Decanini EL, Afonso CL. 2010. Newcastle disease: evolution of genotypes and the related diagnostic challenges. Infect. Genet. Evol. 10: 26–35 [DOI] [PubMed] [Google Scholar]
- 34. Nagai Y, Klenk HD, Rott R. 1976. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology 72: 494–508 [DOI] [PubMed] [Google Scholar]
- 35. Seal BS, King DJ, Bennett JD. 1995. Characterization of Newcastle disease virus isolates by reverse transcription PCR coupled to direct nucleotide sequencing and development of sequence database for pathotype prediction and molecular epidemiological analysis. J. Clin. Microbiol. 33: 2624–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Senne DA, et al. 1996. Survey of the hemagglutinin (HA) cleavage site sequence of H5 and H7 avian influenza viruses: amino acid sequence at the HA cleavage site as a marker of pathogenicity potential. Avian Dis. 40: 425–437 [PubMed] [Google Scholar]
- 37. Shengqing Y, et al. 2002. Generation of velogenic Newcastle disease viruses from a nonpathogenic waterfowl isolate by passaging in chickens. Virology 301: 206–211 [DOI] [PubMed] [Google Scholar]
- 38. Tamura K, et al. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Toyoda T, et al. 1987. Structural comparison of the cleavage-activation site of the fusion glycoprotein between virulent and avirulent strains of Newcastle disease virus. Virology 158: 242–247 [DOI] [PubMed] [Google Scholar]
- 40. Tsai HJ, et al. 2004. Antigenic and genotypical characterization of Newcastle disease viruses isolated in Taiwan between 1969 and 1996. Vet. Microbiol. 104: 19–30 [DOI] [PubMed] [Google Scholar]
- 41. Tsunekuni R, Ito H, Otsuki K, Kida H, Ito T. 2010. Genetic comparisons between lentogenic Newcastle disease virus isolated from waterfowl and velogenic variants. Virus Genes 40: 252–255 [DOI] [PubMed] [Google Scholar]
- 42. Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56: 152–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wise MG, et al. 2004. Development of a real-time reverse-transcription PCR for detection of Newcastle disease virus RNA in clinical samples. J. Clin. Microbiol. 42: 329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]