Abstract
A multiplex, real-time TaqMan assay was designed to identify clinical isolates carrying plasmid-mediated ampC genes. The specificity and sensitivity of this assay were 100% when testing characterized AmpC/non-AmpC-producing isolates and randomly selected clinical isolates. This is a rapid assay that can be performed in a clinical microbiology laboratory.
TEXT
Antibiotic resistance is a global health crisis. There are at least two approaches to address this problem. One way is the design and use of novel therapeutic drug classes to treat infections caused by resistant pathogens. A second approach is the development and implementation of novel surveillance techniques in which to identify not only the pathogen but the resistance mechanisms employed by these organisms. Implementation of molecular-based surveillance techniques is the most immediate response to this crisis and will increase the speed and accuracy of detecting resistance, which is important for both infection control and therapeutic options in hospital and community settings.
The most common Gram-negative resistance mechanism associated with β-lactams is the production of inactivating β-lactamases, including extended-spectrum β-lactamases (ESBLs), plasmid-mediated AmpCs, and the Klebsiella pneumoniae carbapenemases (KPCs) (6, 12, 13, 14). K. pneumoniae, Escherichia coli, and Salmonella spp. are the most common organisms that produce plasmid-mediated AmpCs. Genes encoding AmpCs are derived from the chromosomal ampC genes of various members of the Enterobacteriaceae family, including Enterobacter cloacae and Enterobacter asburiae, Citrobacter freundii, Morganella morganii, Aeromonas sobria, Aeromonas hydrophila, and Hafnia alvei (1, 3, 4, 5, 7, 8, 9, 10, 14, 16).
Production of plasmid-mediated AmpCs in Gram-negative organisms is clinically important because of their ability to confer resistance to broad-spectrum penicillins, broad/extended-spectrum cephalosporins, monobactams, and the cephamycins (3, 4, 5, 9, 14). In addition, the presence of plasmid-mediated AmpCs can mask the phenotypic detection of ESBLs and KPC-producing organisms, which can hinder surveillance and infection control practices (8, 11, 13, 14, 17). An additional concern is that plasmid-mediated AmpCs are frequently associated with false susceptibility to the cephalosporins in routine susceptibility testing, which increases the risk of therapeutic failure (14). However, given these concerns, there are no guidelines set forth by the CLSI to help clinical microbiologists identify these types of organisms.
Modifications to a previously designed endpoint AmpC multiplex PCR has allowed us to develop a real-time multiplex PCR assay using TaqMan probes for the detection of plasmid-mediated AmpC β-lactamase genes, which allows ease of implementation into the clinical laboratory (9). The primers and TaqMan probes used for amplification were designed with Beacon Designer 7 software and presented in Table 1. BLAST analysis using sequences submitted to GenBank was used to evaluate the ability of the primer/probe combinations to anneal to target gene variants. All of the primer/probe sequences annealed with 100% specificity to the target gene variants listed in Table 1. TaqMan probes specific for each ampC product and ribosomal DNA were labeled 5′ with 6-carboxyfluorescein (FAM) and hexachlorofluorescein (HEX) fluorescent dyes, respectively. Fluorophores attached 3′ included black hole quencher-1 and Iowa black FQ, respectively. Real-time multiplex PCR was performed using the Rotor-Gene Q (Qiagen, Valencia, CA) system with fluorescence acquisition in the green channel to detect ampC amplification (FAM) and in the yellow channel to detect 16S ribosomal DNA amplification (HEX) as a control for DNA integrity.
Table 1.
Primers and TaqMan probe sequences used for amplification
| Target(s)a | Primer | Sequence (5′ to 3′) | Expected amplicon size (bp) | Nucleotide positions | GenBank accession no. |
|---|---|---|---|---|---|
| MOX-1 to MOX-7, CMY1, CMY8 to CMY11 | MOX-F1 4P1 | AGACCCTGTTCGAGATAG | 148 | 242–259 | AF373217 |
| MOX-R1 4P1 | ATGGTGATGCTGTCAAAG | 389–372 | |||
| MOX-Taqprobe 4P1 | 5′-6-FAM-CGTGAGCAAGACCCTGACTG-3′ BHQ 1 | 264–283 | |||
| FOX-1 to FOX-8 | FOX-F1 4P1 | ACATATTTCAACTATGGGGTT | 148 | 881–898 | X77455 |
| FOX-R1 4P1 | TTGTCATCCAGCTCAAAG | 1026–1009 | |||
| FOX1-Taqprobe 4P1 | 5′-6-FAM-TGACCGCAGCATAGGCAC-3′ BHQ 1 | 1001–984 | |||
| CMY2, 4, 6, 7, 14–16, 18, 22, 25–44, 49, 53–56, 59 | CMY2-F1 4P1 | TCCAGCGTTATTGATATGG | 147 | 733–751 | HM565135 |
| CMY2-R1 4P1 | CATCTCCCAGCCTAATCC | 879–862 | |||
| CMY2-Taqprobe 4P1 | 5′-6-FAM-ACATATCGCCAATACGCCAGT-3′ BHQ 1 | 856–836 | |||
| DHA-1, DHA-6, DHA-7 | DHA-F3 4P1 | TTATCTCACACCTTTATTACTG | 139 | 469–490 | EF078892 |
| DHA-R3 4P1 | TATCTTTTGAGGCGGATT | 607–590 | |||
| DHA3-Taqprobe 4P1 | 5′-6-FAM-CCGTAAGATTCCGCATCAAGC-3′ BHQ 1 | 584–564 | |||
| ACT-1, ACT-2, ACT-5, ACT-8, MIR-1 to MIR-4 | ACT-F1 4P1 | GTGGCGGTGATTTATGAG | 125 | 178–195 | U58495 |
| ACT-R1 4P1 | CCGGTGAAGGTTTTACTT | 302–285 | |||
| ACT-Taqprobe 4P1 | 5′-6-FAM-CAGCCGCACTACTTCACCT-3′ BHQ 1 | 199–217 | |||
| ACC-1 | ACC-F2 4P1 | CGCTGATGCAGAAGAATA | 86 | 771–788 | AJ133121 |
| ACC-R2 4P1 | CGCTAACCCATAGTTATAAATG | 856–835 | |||
| ACC2-Taqprobe 4P1 | 5′-6-FAM-TCACTGCGACCGACATACCG-3′ BHQ 1 | 815–796 | |||
| 16S rRNA in E. coli, Klebsiella spp., and Salmonella spp. | 16srRNAEcKp-F1 | GAGAGGATGACCAGCCACAC | 55 | ||
| 16srRNAEcKp-R1 | CGCCATTGTGCAATATTCC | ||||
| 16srRNAEcKp-probe | 5′-HEX-TGAGACACGGTCCAGACTCCTACGG-3′ Iowa Black FQ |
Plasmid-mediated ampC genes detected.
Test or positive-control organisms were cultured as previously described (15). Total DNA was extracted from an overnight culture using the DNeasy blood and tissue kit (Qiagen). Multiplex PCR was performed using a 50-μl final reaction volume. Each PCR mixture contained a 1× final concentration of QuantiTect multiplex buffer (Qiagen); 100 μM primers CMY2-F1 4P1, CMY2-R1 4P1, ACT-F1 4P1, ACT-R1 4P1, DHA-F3 4P1, DHA-R3 4P1, MOX-F1 4P1, MOX-R1 4P1, ACC-F2 4P1, ACC-R2 4P1, 16srRNAEcKp-F1, and 16srRNAEcKp-R1; 7.5 μM CMY2-Taqprobe 4P1 and FOX1-Taqprobe 4P1; 5 μM ACT-Taqprobe 4P1; 1.25 μM DHA3-Taqprobe 4P1; 1 μM 16srRNAEcKp-probe; 0.625 μM MOX-Taqprobe 4P1 and ACC2-Taqprobe 4P1 (Table 1). Template DNA (2 μl of eluate, ∼250 ng) was added to 48 μl of the master mix. The PCR conditions consisted of an initial denaturation step at 95°C for 15 min for HotStar Taq polymerase activation. Two-step cycling conditions followed and included 40 cycles of denaturation at 95°C for 1 min and primer/probe binding and primer extension at 55°C for 1 min. No template controls contained sterile nanopure water in place of template DNA.
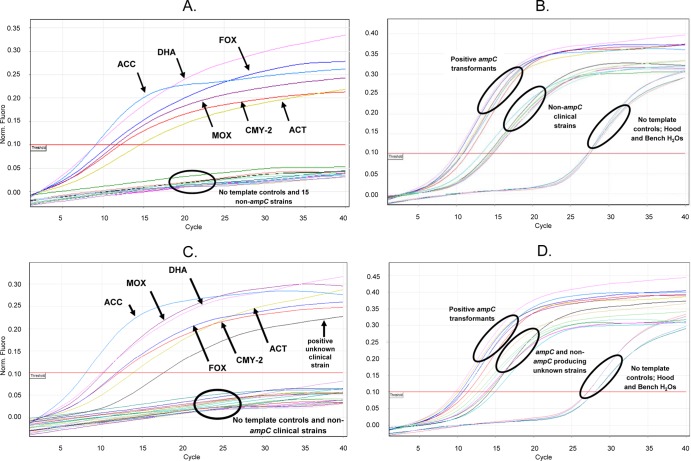
The ability of the seven designed primer/probe combinations to anneal to target genes was tested first using a constructed panel of 6 E. coli transformants housing each plasmid-mediated ampC target and the conditions described above. Each sigmoidal curve in Fig. 1A represents amplification of one of the 6 families of plasmid-mediated ampC genes detected by fluorescence of FAM. To confirm that a specific primer pair amplified only one target of the predicted size, a confirmatory agarose gel was used to visualize the multiplex PCR products (data not shown). The internal control (55 bp) amplified in all isolates regardless of the presence of an ampC gene (Fig. 1B and D). The specificity of the AmpC primer/probe pairs and internal control for all transformants tested was 100%. The designed primer/probe combinations for this real-time assay did not cross-hybridize with the chromosomal ampC gene in the tested E. coli transformants (1, 2, 3, 9, 15). This assay did detect primer dimer formation in the no-template controls of which fluorescence was observed at 26 to 28 cycles (Fig. 1B and D).
Fig 1.
Evaluation of ampC TaqMan multiplex PCR assay. (A and B) Detection and FAM fluorescence of AmpC transformant controls (A) and HEX fluorescence of the 16S ribosomal internal control (B) for AmpC transformants and non-AmpC-producing clinical strains tested in panel A. (C and D) FAM fluorescence of positive AmpC transformant controls and an unknown clinical isolate from CUMC (C) and HEX fluorescence of the 16S ribosomal internal control for those strains tested in panel C (D). Arrows indicate the specific ampC transformant control or unknown clinical isolate detected; circles indicate the group of strains tested or no template controls.
Previously characterized strains listed in Table 2 carrying plasmid-mediated ampC genes were tested and positively identified using the optimized multiplex real-time PCR assay. Five AmpC-producing Salmonella strains were also tested in this assay (data not shown). An additional panel of 109 ampC-negative but KPC (n = 14)-, CTX-M (n = 52)-, and TEM/SHV (n = 43)-producing strains plus five ampC-negative Salmonella strains were evaluated. No FAM fluorescence was detected in the ampC-negative strains. However, the HEX-labeled probe for ribosomal gene detection was positive for PCR amplification in all the strains tested, indicating that the DNA extracted was of good quality and capable of being amplified and detected (Fig. 1B and D). The specificity and sensitivity of this assay when evaluating the characterized non-AmpC- and AmpC-producing isolates were 100%. These data were validated using the endpoint AmpC multiplex PCR described as the gold standard for plasmid-mediated ampC detection (9, 15). Using the endpoint assay, the AmpC-producing strains amplified the expected ampC gene, whereas the strains producing other β-lactamases were negative.
Table 2.
Previously characterized strains containing a plasmid-mediated AmpC β-lactamase
| Strain no. | Organism | AmpC type |
|---|---|---|
| Vitek901664 | K. pneumoniae | FOX |
| HVAMC39 | K. pneumoniae | ACT-1 |
| 01HNH5 | K. pneumoniae | ACT-1 |
| V110977 | K. pneumoniae | CMY-2-likea |
| Ecoli226 | E. coli | CMY-2-likea |
| V110963 | E. coli | CMY-2-likea |
| Misc 345 | E. coli | CMY-2 |
| 01CSHS31 | E. coli | CMY-2-likea |
| Ecoli264 | E. coli | CMY-2-likea |
| UMJMH14 | K. pneumoniae | DHA-1 |
| Misc340 | K. pneumoniae | FOX-1 |
| CCF52 | K. pneumoniae | FOX-5 |
| MHM2 | K. pneumoniae | FOX-5 |
| NSLIJ26 | K. pneumoniae | FOX-5 |
| UL3 | K. pneumoniae | FOX-5 |
| UMJMH21 | K. pneumoniae | FOX-5 |
| UMM4 | K. pneumoniae | FOX-5 |
| UN47 | K. pneumoniae | FOX-5 |
| 01CMH13 | K. pneumoniae | FOX-5 |
| 01VUMM451 | K. pneumoniae | DHA-1-likea |
| Sal100 | Salmonella spp. | CMY-2 |
| Sal358 | Salmonella spp. | CMY-2 |
| Sal362 | Salmonella spp. | CMY-2 |
| Sal365 | Salmonella spp. | CMY-2 |
| Sal377 | Salmonella spp. | CMY-2 |
| Misc 304 | K. pneumoniae | MIR-1 |
Not sequenced.
A total of 120 clinical isolates comprised of K. pneumoniae, Klebsiella oxytoca, and E. coli isolates were randomly collected from the clinical laboratory at Creighton University Medical Center (CUMC) and evaluated for susceptibility using disc diffusion and tested for the presence of plasmid-mediated ampC genes using this real-time assay (see Table S1 in the supplemental material). Of the 120 unknown isolates, 4% (5/120) were identified as plasmid-mediated AmpC producers. Three of five positive AmpC-producing isolates exhibited resistance to the third-generation cephalosporins but remained susceptible to cefepime. The remaining two ampC-positive isolates displayed susceptibility to the cephalosporins. A representation of a positive clinical isolate is shown in Fig. 1C. These studies were also validated using the endpoint AmpC multiplex assay to confirm the presence or absence of an ampC gene.
Currently, there is no recommendation by the CLSI for the detection of plasmid-mediated AmpC-producing strains. A recently written review by George Jacoby indicates the gold standard for detecting these types of isolates is the endpoint ampC multiplex developed by our laboratory in 2002 (9). Data from the literature indicates a clinical responsibility to identify patients infected by organisms that produce a plasmid-mediated AmpC (13, 14). The real-time TaqMan multiplex PCR assay described in this report provides a fast and easy-to-use tool to screen for plasmid-mediated AmpC genes: a resistance mechanism that can be difficult to discern phenotypically and can result in a poor clinical outcome when undetected.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kenneth Thomson and Stephen Cavalieri for discussions regarding this study. We also thank the Clinical Microbiology Laboratory at Creighton University and the Center for Research in Anti-Infectives and Biotechnology (C.R.A.B.) for helping compile a panel of characterized negative and positive AmpC-producing clinical isolates.
This work was supported by an investigator initiation funding from Becton, Dickinson GeneOhm Diagnostics.
Footnotes
Published ahead of print 15 August 2012
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Bauernfeind A, Chong Y, Lee K. 1998. Plasmid-encoded AmpC beta-lactamases: how far have we gone 10 years after the discovery? Yonsei Med. J. 39:520–525 [DOI] [PubMed] [Google Scholar]
- 2. Black JA, Moland ES, Thomson KS. 2005. AmpC disk test for the detection of plasmid-mediated AmpC beta-lactamases in Enterobacteriaceae lacking chromosomal AmpC beta-lactamases. J. Clin. Microbiol. 43:3110–3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradford P, et al. 1997. Imipenem resistance in Klebsiella pneumoniae is associated with the combination of ACT-1, a plasmid-mediated AmpC β-lactamase, and the loss of an outer membrane protein. Antimicrob. Agents Chemother. 41:563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coudron P. 2005. Inhibitor-based methods for detection of plasmid-mediated AmpC β-lactamases in Klebsiella spp., Escherichia coli, and Proteus mirabilis. J. Clin. Microbiol. 43:4163–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coudron P, Moland ES, Thomson KS. 2000. Occurrence and detection of AmpC beta-lactamases among Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates at a veterans medical center. J. Clin. Microbiol. 38:1791–1795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elemam A, Rahimian J, Mandell W. 2009. Infection with panresistant Klebsiella pneumoniae: a report of 2 cases and a brief review of the literature. Clin. Infect. Dis. 49:271–274 [DOI] [PubMed] [Google Scholar]
- 7. Hanson ND, Sanders CC. 1999. Regulation of inducible AmpC beta-lactamase expression among Enterobacteriaceae. Curr. Pharm. Des. 5:881–894 [PubMed] [Google Scholar]
- 8. Hemalatha V, Padma M, Uma Sekar Vinodh TM, Arunkumar AS. 2007. Detection of AmpC beta lactamases production in Escherichia coli and Klebsiella by an inhibitor based method. Indian J. Med. Res. 126:220–223 [PubMed] [Google Scholar]
- 9. Jacoby GA. 2009. AmpC β-lactamases. Clin. Microbiol. Rev. 22:161–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kang CI, et al. 2004. Cefepime and the inoculum effect in tests with Klebsiella pneumoniae producing plasmid-mediated AmpC-type β-lactamase. J. Antimicrob. Chemother. 54:1130–1133 [DOI] [PubMed] [Google Scholar]
- 11. Netzel TC, et al. 2007. The AmpC inhibitor, Syn2190, can be used to reveal extended-spectrum β-lactamases in Escherichia coli. Diagn. Microbiol. Infect. Dis. 58:345–348 [DOI] [PubMed] [Google Scholar]
- 12. Owens RC, Jr, et al. 2011. Community transmission in the United States of a CTX-M-15 producing sequence type ST131 Escherichia coli strain resulting in death. J. Clin. Microbiol. 49:3406–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pai H, et al. 2004. Epidemiology and clinical features of bloodstream infections caused by AmpC-type beta-lactamase-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:3720–3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Park YS, et al. 2009. Risk factors and clinical features of infections caused by plasmid-mediated AmpC beta-lactamase-producing Enterobacteriaceae. Int. J. Antimicrob. Agents 34:38–43 [DOI] [PubMed] [Google Scholar]
- 15. Perez-Perez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rand KH, et al. 2011. Clinical detection of AmpC β-lactamase: does it affect patient outcome? Am. J. Clin. Pathol. 135:572–576 [DOI] [PubMed] [Google Scholar]
- 17. Tenover et al. 2009. Identification of plasmid-mediated AmpC beta-lactamases in Escherichia coli, Klebsiella spp., and Proteus species can potentially improve reporting of cephalosporin susceptibility testing results. J. Clin. Microbiol. 47:294–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.