Abstract
In settings of high methicillin-resistant Staphylococcus aureus (MRSA) prevalence, detection of nosocomial transmission events can be difficult without strain typing. Prospective typing of all MRSA isolates could potentially identify transmission in a timely fashion, making infection control responses to outbreaks more effective. We describe the development and evaluation of a novel 19-target binary typing system for MRSA using the multiplex-PCR/reverse line blot hybridization platform. Pulse-field gel electrophoresis (PFGE), spa typing, and phage-derived open reading frame (PDORF) typing were performed for comparison. The system was utilized to identify transmission events in three general surgical wards over a 12-month period. Initial MRSA isolates from 273 patients were differentiated into 55 unique binary types. One or more potential contacts colonized with the same MRSA strain were identified in 69 of 87 cases (79%) in which definite or possible nosocomial MRSA acquisition had occurred. The discriminatory power of the typing system was similar to that of PFGE (Simpson's index of diversity [D] = 0.994, versus 0.987) and higher than that of spa typing (D = 0.926). Strain typing reduced the total number of potential MRSA-colonized source contacts from 859 to 212 and revealed temporal clustering of transmission events. Prospective MRSA typing using this novel binary typing method can rapidly identify nosocomial transmission events, even in high-prevalence settings, which allows timely infection control interventions. The system is rapid, inexpensive, discriminatory, and suitable for routine, high-throughput use in the hospital microbiology laboratory.
INTRODUCTION
In settings of high prevalence of methicillin-resistant Staphylococcus aureus (MRSA) colonization and infection, it is difficult to determine without strain typing whether a newly identified case is the result of nosocomial acquisition. Traditionally, strain typing has been done, retrospectively, after identification of a spatiotemporal cluster of cases, but this approach may delay infection control interventions. New PCR-based high-throughput typing methods offer a rapid turnaround time, with lower costs and in many cases high discriminatory power (1, 7, 9, 15, 21–23). This makes possible the concept of prospective typing, where isolates are typed routinely and the results are examined for evidence of nosocomial transmission in near-real time, allowing faster, targeted infection control interventions. High discriminatory power, ease of interpretation, and portability of results are essential elements of such a system. spa sequence typing has been used in this context (11) but in some settings has insufficient discriminatory power to reliably discern transmission events.
We have developed a novel binary typing system specifically for prospective MRSA strain typing to detect transmission events. The most informative targets from three previous binary typing systems (toxin gene profiling [3], phage-derived open reading frame typing [13], and SCCmec subtyping [2]) were selected and incorporated into a multiplex PCR/reverse line blot (mPCR/RLB) assay platform. We describe the development and assessment of performance characteristics of the system, following established guidelines (19), and present results from 1 year of routine MRSA strain typing in a high-prevalence nosocomial setting.
MATERIALS AND METHODS
Setting.
Westmead Hospital is a 975-bed tertiary referral hospital in metropolitan Sydney, Australia. It has a relatively high prevalence of MRSA colonization and infection, with 26 inpatient and 9 health care-associated outpatient MRSA bacteremias in 2011 and an MRSA colonization rate of around 25% on point prevalence surveys of the three general surgical wards (total, 82 beds). The general surgical wards largely house patients admitted for upper gastrointestinal, colorectal, and other intra-abdominal surgeries, and include a 16-bed high-dependency unit. Prior to the study period, no MRSA screening was performed routinely except for patients admitted to “high-risk” units (intensive care, renal/urology, or hematology) or for selected elective orthopedic and cardiothoracic surgical procedures. A program of screening all surgical patients for MRSA colonization at the preadmission clinic and/or on admission to three general surgical wards was introduced in August 2010. Body sites screened were the nose, groin, and axillae, with the addition of wound or ulcer swabs where applicable. Swabs were pooled and examined for MRSA using the Staphylococcus 4 rev 1 nucleic acid detection method (AusDiagnostics, Alexandria, Australia), with positive samples confirmed by culture on Brilliance MRSA agar (Oxoid, Basingstoke, United Kingdom). Patients found to be colonized with MRSA were isolated in single rooms with contact precautions.
Isolate collections used in development and assessment of the typing method. (i) Reference isolates.
Forty-two well-characterized epidemiologically unrelated MRSA reference isolates representing the dominant hospital- and community-associated clones in Australia and a number of important international clones were used; this collection has been described in detail elsewhere (2, 3, 13) and includes two MRSA isolates with published whole-genome sequences (COL and MW2).
(ii) Clinical isolates.
Ninety-nine unselected MRSA clinical isolates from patients at two tertiary referral hospitals in Sydney between 2005 and 2008 were included.
(iii) Historical isolates.
Twenty-four historical MRSA isolates collected from patients at various Sydney hospitals from 1984 to 1997 were used.
(iv) Stability isolates.
Eight-one isolate pairs, each collected from individual patients at intervals of 1 month to 3 years, were used to assess in vivo stability; only pairs for which both isolates belonged to the same multilocus sequence type (MLST) were included.
(v) Surveillance isolates.
The first MRSA isolates from all colonized or infected patients admitted to the three general surgical wards were collected over a 12-month period from November 2009 to October 2010. Since patients transferred into these wards may have had contact with colonized patients in other surgical wards prior to transfer to the general surgical ward, isolates were also collected from patients in these wards for the duration of the study. A total of 273 isolates from surgical patients were collected, stored at −80°C, and analyzed retrospectively.
Selection of targets.
The method used for selection of targets has been described in detail elsewhere (M. V. O'Sullivan, V. Sintchenko, and G. L. Gilbert, submitted for publication). Briefly, the most discriminatory subset of 19 targets was selected from 51 used in three previously described mPCR/RLB binary typing assays for toxin genes (3), phage-derived open reading frame (PDORF) sequences (13), and SCCmec subtypes (2), using typing results of the three assays for 165 MRSA isolates (the reference isolates, clinical isolates, and historical isolates). This was achieved using specially developed software, AuSeTTS (available at http://www.cidmpublichealth.org/pages/ausetts.html). This program systematically calculates Simpson's index of diversity (D) for different combinations of genetic targets and identifies the most informative combination. A table outlining the 19 targets selected for the current binary typing assay is presented in the supplemental material.
AuSeTTS was also used to determine concordance of the binary typing result with MLST using a subset of 153 isolates for which the sequence types (STs) had been determined by standard (5) or single nucleotide polymorphism (SNP)-based (9) methods. Concordance was measured using the adjusted Wallace coefficient (AW), which estimates the probability that any two isolates that are the same using the binary typing method will also be the same using MLST (17). The correlation between binary types and MLST was subsequently utilized to predict MLST results for the “surveillance” isolates.
mPCR/RLB.
The primers and probes used in this study are listed in the supplemental material. We used previously published primers and probes (2, 3, 13), modified as necessary to avoid primer dimer formation and to produce amplicons of 100 to 200 bp which gave strong probe signals. Each target was represented by two probes on the membrane; nuc and mecA probes were used as controls. A positive-control panel, consisting of a combination of reference isolates (mu3, COL, E804531, 14176-5710, SJOG30, RDH81, and NCTC8325) known to give positive signals for all probes, and a DNA-free negative control were tested with each run.
DNA was extracted by suspending one colony of a 24-h growth of each isolate in 400 μl of molecular-grade water, which was boiled at 100°C for 10 min, frozen at −20°C, thawed, and centrifuged for 5 min at 16,100 × g. The supernatant was then used as a template in PCRs. Multiplex PCR was performed in a single tube using all 61 primers, each at a concentration of 0.25 μM, with 3.5 mM MgCl2, 0.2 mM deoxynucleoside triphosphates (dNTPs), 1× PCR buffer, and 0.03 U/μl HotStarTaq polymerase (Qiagen, Victoria, Australia). Two microliters of DNA template was used in each 30-μl reaction mixture. Cycling conditions were 95°C for 10 min of preheating and 35 cycles of 94°C for 30s, 55°C for 30s, and 72°C for 60s, with a final extension at 72°C for 10 min.
RLB membrane preparation, probe hybridization, and product detection were performed as previously described (2, 3, 10, 13, 14). A probe signal was interpreted as positive if it was at least as strong as the control probe for that target. A probe signal was interpreted as weak if the signal was present but weaker than the control probe signal. Negative probes had no signal. A target was interpreted as being present if at least one strong probe signal or two weak probe signals were present for that target. The result for a target that was identified as present was assigned a value of 1, an absent target was assigned a value of 0, and the result for all targets was concatenated into a 19-digit binary number, which was converted to a decimal number for ease of comparison. Isolates were said to be indistinguishable if the targets detected were the same, while the significance of a one-target difference between isolates was further investigated.
Other typing methods.
Pulse-field gel electrophoresis (PFGE) was performed according to the Harmony protocol (12). Interpretation was conducted using the software program Bionumerics v3.0 (Applied Maths, Sint-Martens-Latem, Belgium) with a position tolerance set at 2% and change toward the end of the fingerprint of 0.5%. Similarity was calculated using the Dice coefficient, and clustering was performed using the unweighted pair group method with arithmetic means. PDORF typing and spa sequence typing were performed and interpreted as previously described (3, 13).
Data analysis.
Simpson's index of diversity, D (8), and 95% confidence intervals (6) were calculated for all typing methods using the 42 epidemiologically unrelated reference isolates. In vivo stability of the 19 selected targets was determined and compared with spa sequence typing, PDORF typing, and PFGE using the 81 pairs of stability isolates, In addition to the standard measurement of stability as the proportion of pairs which exhibited no change between isolates (19), stability was also measured as the probability of no change in type at 6 months as determined by Kaplan-Meier survival analysis (14a). Typing of the 42 reference isolates was repeated twice, with separate hybridization membranes and using DNA extracted on two occasions to test the reproducibility of the method. Reproducibility was expressed as the percentage of all isolates tested that had the same strain type on repeat testing (19).
Identification of transmission events.
The utility of the binary typing method for detection of nosocomial transmission events was tested using isolates obtained from patients in the three general surgical wards. Patients were classified as “known carriers” (MRSA first isolated prior to or within 48 h of current hospital admission), having “possible nosocomial acquisition” (MRSA first isolated at >48 h after admission but with no previous negative MRSA screen), or having “definite nosocomial acquisition” (MRSA first isolated at >48 h after admission with a previous negative MRSA screen in the same admission). The “window period” during which acquisition was assumed to have occurred was defined as the interval between the last negative MRSA screen or admission, if there was no previous MRSA screen, and the time of first MRSA isolation. A suspected nosocomial MRSA transmission event was recorded when a patient with possible or definite nosocomial acquisition was in the same ward, during the window period, as one or more other patients with the same MRSA binary type, who were either known carriers or also in the window period at the time. These patients, who were possible sources of MRSA acquisition, were classified as “contacts” of the index case.
RESULTS
Binary assay performance.
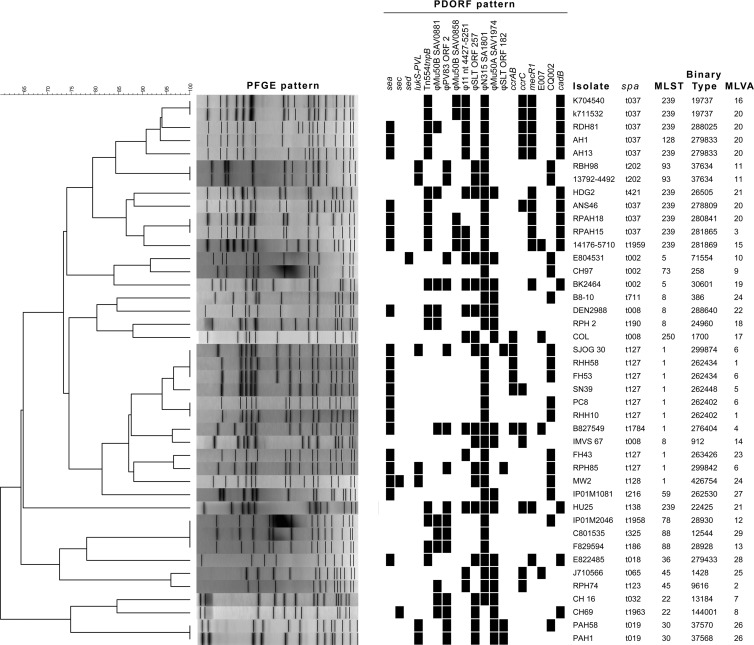
The results of comparison with other typing methods are shown in Fig. 1 and Table 1. Based on testing of 42 epidemiologically unrelated MRSA isolates (isolate group i), the discriminatory power of binary typing was similar to that of PFGE and PDORF typing but higher than that of spa typing and MLST. In five cases, isolates with indistinguishable PFGE patterns could be resolved into different binary types, mostly differing by one target (Fig. 1). Binary typing showed excellent concordance with MLST (AW, 0.993; 95% confidence interval [CI], 0.986 to 1.000). Two isolates with the same binary type belonged to different MLST types, which were MLST single-locus variants differing by a single point mutation (ST239 and ST128). Otherwise, all isolates with the same binary type also had the same MLST, indicating that binary typing may be useful for predicting MLST (but not vice versa).
Fig 1.
Typing results for the 42 reference isolates. Clustering is based on PFGE pattern. A black rectangle represents detection of the relevant binary typing target by mPCR/RLB.
Table 1.
Measures of discriminatory power and stability of typing methods assessed based on typing of 42 reference MRSA isolates (used to calculate D and no. of types) and 81 pairs of stability isolates
| Method | D (95% CI)a | No. of types | No. (%) of pairs unchangedb | % probability of no changec (95% CI) |
|---|---|---|---|---|
| MRSA binary typing | 0.994 (0.988–1.00) | 37 | 57 (70) | 68 (53–79) |
| PFGE | 0.987 (0.977–0.998) | 33 | 50 (62) | 58 (43–70) |
| PDORF | 0.971 (0.945–0.997) | 30 | 64 (79) | 71 (55–82) |
| spa typing | 0.926 (0.879–0.972) | 22 | 78 (96) | 95 (82–99) |
| MLST | 0.882 (0.823–0.941) | 15 | NAd | NA |
D, Simpson's index of diversity.
Concordant results within pairs.
Probability of same result at 6 months.
NA, not analyzed; pairs which differed by MLST clonal complex were excluded from the analysis of in vivo stability because these were assumed to represent reinfection.
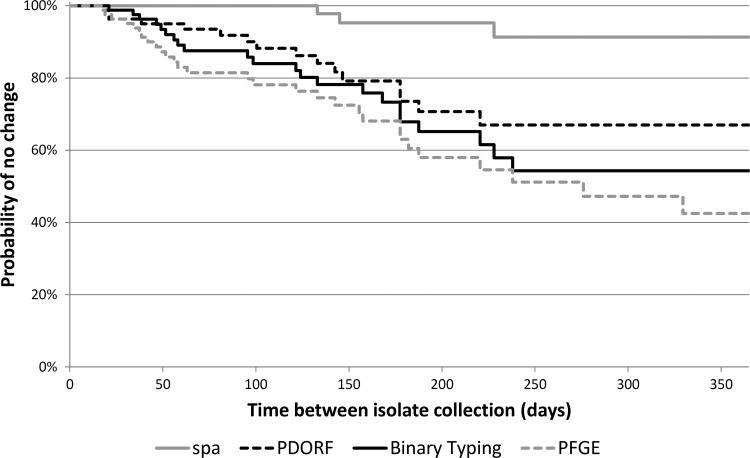
The in vivo stability of binary typing at 6 months was similar to that of PFGE and PDORF typing but lower than that of spa typing (Table 1 and Fig. 2). Sixteen of the twenty-four pairs for which the binary type changed were different by only one target. All methods had 100% typeability, and reproducibility of binary typing was 100%.
Fig 2.
Kaplan-Meier survival curves for in vivo stability of the typing methods.
Nearly 5% of probe pairs among the 5,187 surveyed for the 273 surveillance isolates gave a strong result for one probe and a negative result for the second probe. This has previously been found to be due to polymorphisms in one of the probe binding sites (13).
Identification of nosocomial transmission events.
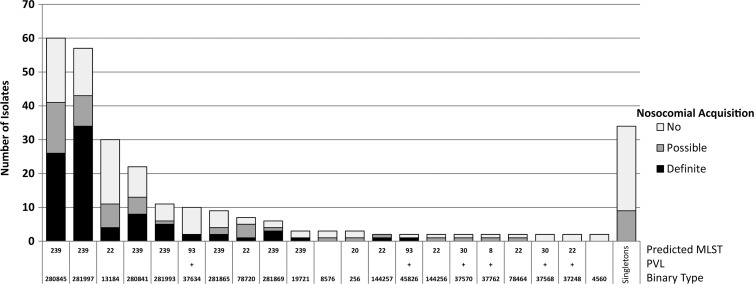
The 273 isolates from all surgical patients belonged to 55 binary types, of which 34 were represented by single isolates. There were 125 previously known MRSA carriers, whose isolates belonged to 45 binary types of which 25 were singletons. Isolates from 88 patients with definite, and 60 with possible, nosocomial acquisition belonged to 12 and 24 binary types, respectively. The distributions of binary types and predicted STs are shown in Fig. 3. The majority of patients with possible or definite nosocomial acquisition had binary types that correlated with ST239 or ST22 (also known as AUS2/3 and UK-EMRSA 15, respectively), which are the two most common nosocomial MRSA clones in Australia. Three of those with definite nosocomial acquisition had binary types consistent with ST93, or the Queensland clone, a major community-associated strain prevalent in eastern Australia, which carries the Panton-Valentine leukocidin (PVL).
Fig 3.
Distribution of 55 binary types among 273 surgical isolates. PVL, Panton-Valentine leukocidin. MLST was predicted based on the binary typing result.
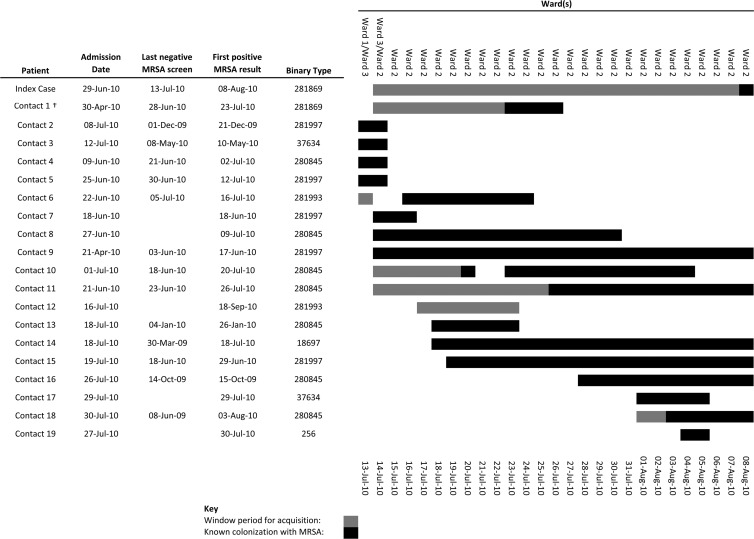
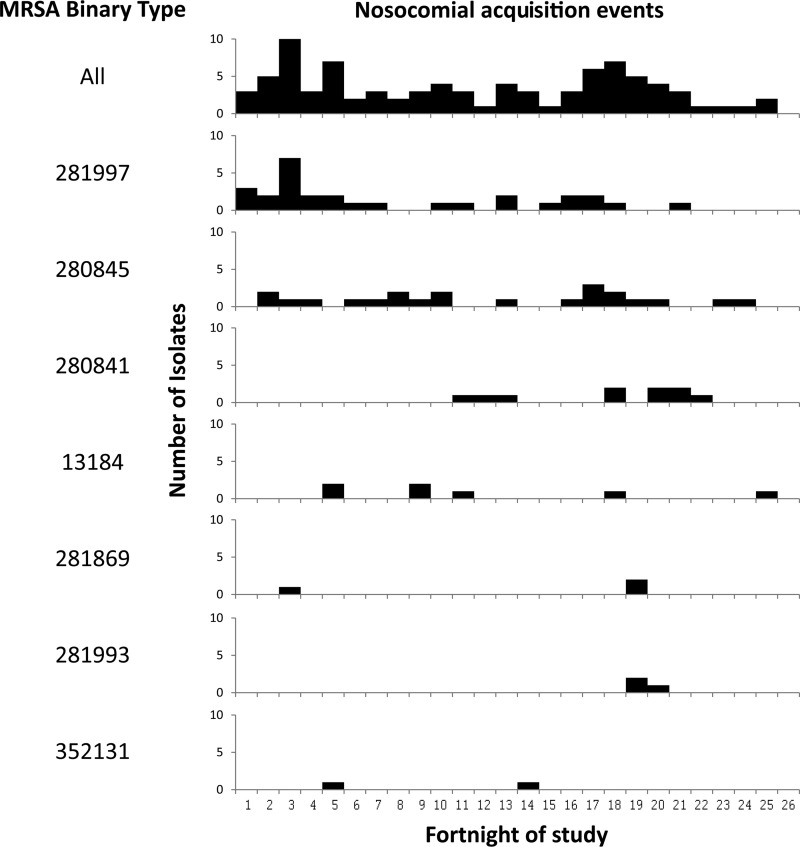
When analysis was restricted to patients who may have acquired MRSA while admitted in one of the three general surgical wards, there were 58 definite and 29 possible incidents of nosocomial acquisition over the 1-year period of the study. Based on MRSA carriage data only, all but 2 of the 87 patients who acquired MRSA had possible contact with at least 1 other MRSA carrier who was in the same ward during their window period. The number of contacts per episode of nosocomial acquisition ranged from 1 to 26 (median, 9); the total number of possible contacts for all 87 episodes was 859 (since 1 patient could be a contact for many cases of nosocomial acquisition). However, when binary typing results were used to define MRSA contacts, 69 (50 of 58 definite and 19 of 29 possible nosocomial acquisitions) cases were found to have had contact with at least 1 patient colonized with the same strain of MRSA during the window period. The number of strain-matched contacts per episode of nosocomial acquisition ranged from 1 to 11 (median, 2); there were a total of 212 contacts. Binary typing therefore excluded 647 potential MRSA contacts. Figure 4 illustrates the application of binary typing to identify the source contact for one patient, with definite nosocomial MRSA acquisition. Of 18 incidents of possible or definite nosocomial acquisition, for which no contact with an identical strain type was identified, 4 had had contact with patients colonized with single-locus variants. The temporal distribution of nosocomial transmission events in the general surgical wards by binary type is shown in Fig. 5.
Fig 4.
Case study of binary typing to identify MRSA contacts. Contacts were patients in the same ward as the index case during the latter's window period (interval between last negative screen and first MRSA isolate). The figure at the right indicates a timeline during which potential contact with the index case occurred. Only contact 1 carried MRSA with the same binary type the index case. Contacts 2 through 19 were excluded as potential sources on the basis of binary typing.
Fig 5.
Temporal distribution of nosocomial acquisition events in the three general surgical wards. The top line indicates all 87 definite or possible transmission events, regardless of MRSA type. Subsequent lines show transmission events for individual MRSA types, for which more than one transmission event occurred in the 12-month period.
DISCUSSION
PFGE has long been considered the gold standard for MRSA typing to identify nosocomial transmission events. However, it is labor-intensive and difficult to standardize between laboratories. spa sequence typing has advantages over PFGE, including faster turnaround time and lower cost; the sequence-based output makes interrun and interlaboratory comparisons straightforward. It has been used prospectively to identify nosocomial transmission events (11). However, in some settings, including our own, its discriminatory power is inadequate to identify strains within the major nosocomial MRSA clones. For example, the majority of nosocomial MRSA isolates at our hospital belong to ST239-SCCmec-III, and almost all of these harbor spa type t037 at our institution.
Binary typing using mPCR/RLB has a number of favorable characteristics for a typing method. It is inexpensive (consumables, approximately US$2 per isolate) and does not require highly specialized equipment or sequencing. The total turnaround time is approximately 10 h (including 4 h of hands-on time for DNA extraction, multiplex PCR, probe hybridization, and product detection). Up to 42 isolates can be typed on a single membrane (including controls), and multiple membranes can be processed simultaneously. It is highly reproducible, and the results, expressed in a numerical format, are portable.
We have previously developed mPCR/RLB-based MRSA binary typing assays for toxin gene profiling, SCCmec subtyping, and PDORF typing (2, 3, 13). The strain-to-strain variability of some of the targets used in these assays was exploited to produce the final binary typing method described in this article, resulting in a combined discriminatory power which is similar to that of PFGE.
Methods for binary typing of MRSA, using a variety of targets and platforms, have been published previously. van Leeuwen et al. visually identified 15 discriminatory amplicons from randomly amplified polymorphic DNA analysis, determined their sequences, and used them as the basis for a highly discriminatory binary typing system using Southern blotting of EcoRI-digested genomic DNA (21, 22). The mPCR-RLB method for binary typing in the present study has the potential advantage of higher specificity, due to PCR amplification of the binary targets with specific primers, prior to probe hybridization, using two DNA probes per binary target. DNA preparation using the current method is also more straightforward, and targets were chosen systematically (using a computer algorithm comparing all possible combinations rather than simple visual inspection). The inclusion of a gene encoding PVL in the mPCR-RLB-based assay allows identification of an important virulence factor.
Binary typing targeting five discriminatory targets, combined with detection of seven informative SNPs from housekeeping genes, which can be used to infer MLST clonal complex, has also been described using various platforms, including allele-specific PCR and matrix-assisted laser desorption–ionization time-of-flight (MALDI-TOF) mass spectroscopy, using the Sequenom MassARRAY iPLEX platform (9, 18, 20). This approach has the advantage of providing more direct inference of the MLST clonal complex but would be expected to have lower discriminatory power, lower throughput, and higher cost than the 19-target binary system presented in the current study. MALDI-TOF mass spectroscopy of bacterial protein components has also been used for typing of MRSA (16) but has limited discriminatory power.
PCR ribotyping is another rapid-PCR-based typing method, based on amplicon size variation of the multicopy 16S-23S rRNA gene spacer region, which has been applied to Staphylococcus aureus, using both gel electrophoresis (4) and capillary electrophoresis (1) platforms. Capillary electrophoresis allows very accurate size determination and identification of the presence or absence of alleles of known sizes, so results can be presented in a binary format. While this method has good discriminatory power for all Staphylococcus aureus isolates, it has lower ability than PFGE to discriminate between isolates of MRSA (4).
Measurement of stability of a new typing method is important, particularly when the targets interrogated include mobile genetic elements, or MGEs, (such as those located on integrated prophages), in order to establish interpretive criteria for relatedness of strains. While it can be difficult to exclude colonization with a second strain when collecting multiple samples over time, we feel that in vivo stability measures, using sequential isolates, are preferable to in vitro passages, in which conditions are unlikely to mimic those that promote mobilization of MGEs in vivo. Exclusion of isolate pairs which differed by MLST clonal complex would have eliminated many cases of colonization with a second strain, but it is possible that some of the isolate pairs still represent this scenario. Nevertheless, the analysis suggests that the stability of the binary typing method was similar to that of PFGE and therefore suitable for studying short-term epidemiology, applicable to identifying nosocomial transmission events.
The high concordance with MLST suggests that the method is also sufficiently stable to predict longer-term epidemiology, notwithstanding minor changes that occurred over time. While binary types correlated well with MLSTs in this study, the binary targets are independent of the housekeeping genes utilized in MLST, and concordance may not always hold. Further concordance studies with a larger collection of samples from a wider range of MLSTs are needed to explore this further. In vivo stability analysis showed that in most isolate pairs in which the binary type changed, the difference was at a single locus. Likewise, there were four cases of nosocomial acquisition, in which there were no other patients with identical strains in the same ward at the same time, but there were patients colonized with single-locus variants in the vicinity, suggesting that the latter can be interpreted as possibly related for the purpose of outbreak investigation.
While direct contact with contaminated environmental surfaces and the hands of health care workers is the main mode of acquisition of MRSA, these surfaces become contaminated from other colonized patients and health care workers. The novel binary typing method presented here was highly effective in excluding potential contact patients as nosocomial sources of MRSA acquisition; for the 87 possible or definite acquisition events studied, 859 potential source contacts (median, 9) were reduced to 212 potential source contacts (median, 2). Sources of transmission were not found using molecular typing in 18 of 87 (21%) cases. Possible explanations include contact with MRSA-colonized patients, lack of identification due to a false-negative or missed screening, contact with colonized staff (who were not screened), or environmental contamination.
The method described in this study was designed to distinguish between strains of MRSA. The discriminatory power when applied to methicillin-susceptible isolates of S. aureus (MSSA) would be expected to be lower, since six of the targets are found on the SCCmec element. However, the principles of binary typing using mPCR/RLB could be applied to MSSA, and indeed to a wide range of pathogens, with careful species-specific target selection.
In conclusion, binary typing of MRSA using mPCR/RLB can assist in the identification and monitoring of nosocomial transmission events in high-prevalence settings. This has the potential to enhance surveillance of hospital-acquired infection and enable prompt, targeted infection control interventions. Further studies evaluating the impact of prospective typing, including rapid feedback of results, on MRSA acquisition and infection rates are in progress.
Supplementary Material
ACKNOWLEDGMENTS
This project was supported by an Australian National Health and Medical Research Council (NHMRC) project grant (no. 1010452). M.V.N.O. has been supported by an NHMRC postgraduate scholarship (no. 512029).
We thank Jim Manos for providing historical isolates of MRSA.
Footnotes
Published ahead of print 15 August 2012
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1. Budding AE, et al. 2010. Binary IS typing for Staphylococcus aureus. PLoS One 5: e13671 doi:10.1371/journal.pone.0013671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cai L, et al. 2009. A new multiplex PCR-based reverse line-blot hybridization (mPCR/RLB) assay for rapid staphylococcal cassette chromosome mec (SCCmec) typing. J. Med. Microbiol. 58: 1045–1057 [DOI] [PubMed] [Google Scholar]
- 3. Cai Y, et al. 2007. Comparison of single- and multilocus sequence typing and toxin gene profiling for characterization of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 45: 3302–3308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dolzani L, Tonin E, Lagatolla C, Monti-Bragadin C. 1994. Typing of Staphylococcus aureus by amplification of the 16S-23S rRNA intergenic spacer sequences. FEMS Microbiol. Lett. 119: 167–173 [DOI] [PubMed] [Google Scholar]
- 5. Enright MC, Day NPJ, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38: 1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grundmann H, Hori S, Tanner G. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 39: 4190–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harmsen D, et al. 2003. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 41: 5442–5448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26: 2465–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huygens F, et al. 2006. Staphylococcus aureus genotyping using novel real-time PCR formats. J. Clin. Microbiol. 44: 3712–3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kong F, Gilbert GL. 2006. Multiplex PCR-based reverse line blot hybridization assay (mPCR/RLB)—a practical epidemiological and diagnostic tool. Nat. Protoc. 1: 2668–2680 [DOI] [PubMed] [Google Scholar]
- 11. Mellmann A, et al. 2006. Automated DNA sequence-based early warning system for the detection of methicillin-resistant Staphylococcus aureus outbreaks. PLoS Med. 3: e33 doi:10.1371/journal.pmed.0030033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Murchan S, et al. 2003. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 41: 1574–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Sullivan MV, Kong F, Sintchenko V, Gilbert GL. 2010. Rapid identification of methicillin-resistant Staphylococcus aureus transmission in hospitals by use of phage-derived open reading frame typing enhanced by multiplex PCR and reverse line blot assay. J. Clin. Microbiol. 48: 2741–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Sullivan MV, Zhou F, Sintchenko V, Kong F, Gilbert GL. 2011. Multiplex PCR and reverse line blot hybridization assay (mPCR/RLB). J. Vis. Exp. 54: e2781 doi:10.3791/2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14a. O'Sullivan MVN, Sintchenko V, Gilbert GL. Quantitatitve estimation of MRSA strain-typing system stability using Kaplan-Meier survival analysis. J. Clin. Microbiol., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sabat A, et al. 2003. New method for typing Staphylococcus aureus strains: multiple-locus variable-number tandem repeat analysis of polymorphism and genetic relationships of clinical isolates. J. Clin. Microbiol. 41: 1801–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schlebusch S, et al. 2010. First outbreak of PVL-positive nonmultiresistant MRSA in a neonatal ICU in Australia: comparison of MALDI-TOF and SNP-plus-binary gene typing. Eur. J. Clin. Microbiol. Infect. Dis. 29: 1311–1314 [DOI] [PubMed] [Google Scholar]
- 17. Severiano A, Pinto FR, Ramirez M, Carriço JA. 2011. Adjusted Wallace coefficient as a measure of congruence between typing methods. J. Clin. Microbiol. 49: 3997–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stephens AJ, et al. 2006. Methicillin-resistant Staphylococcus aureus genotyping using a small set of polymorphisms. J. Med. Microbiol. 55: 43–51 [DOI] [PubMed] [Google Scholar]
- 19. Struelens MJ. 1996. Consensus guidelines for appropriate use and evaluation of microbial epidemiologic typing systems. Clin. Microbiol. Infect. 2: 2–11 [DOI] [PubMed] [Google Scholar]
- 20. Syrmis MW, et al. 2011. Comparison of a multiplexed MassARRAY system with real-time allele-specific PCR technology for genotyping of methicillin-resistant Staphylococcus aureus. Clin. Microbiol. Infect. 17: 1804–1810 [DOI] [PubMed] [Google Scholar]
- 21. van Leeuwen W, et al. 2001. Binary typing of Staphylococcus aureus strains through reversed hybridization using digoxigenin-universal linkage system-labeled bacterial genomic DNA. J. Clin. Microbiol. 39: 328–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Leeuwen W, et al. 1999. Validation of binary typing for Staphylococcus aureus strains. J. Clin. Microbiol. 37: 664–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wichelhaus TA, et al. 2001. Rapid molecular typing of methicillin-resistant Staphylococcus aureus by PCR-RFLP. Infect. Control Hosp. Epidemiol. 22: 294–298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.