Abstract
Enteroviruses have been reported in encephalitis cases. However, clinical and epidemiological characteristics of enteroviruses in encephalitis are not fully established. We prospectively investigated 204 children with encephalitis over a period of 2 years (2009 to 2010) for enterovirus. Enterovirus was detected in 45 specimens (22.1%); of these, 40 were typed by seminested reverse transcription-PCR (RT-PCR) and sequencing of the VP1 gene. Molecular typing of enterovirus revealed the predominance of echovirus 21 associated with an epidemic during the rainy seasons of 2010 and the circulation of echovirus 1, coxsackievirus B1, enterovirus 75, enterovirus 76, coxsackievirus B5, and echovirus 19. The nucleotide divergence among echovirus 21 strains was 0 to 2% at the nucleotide level. This study suggests that enterovirus is an important cause of encephalitis in children from India. To our knowledge, this is the first report of echovirus 21 in encephalitis cases worldwide.
INTRODUCTION
Enteroviruses (EVs) are RNA viruses in the family Picornaviridae comprising more than 100 serotypes that are divided into four species, human enteroviruses A to D (9). The clinical manifestations of EVs range from conjunctivitis, respiratory tract infection, myocarditis, meningitis, encephalitis, and neonatal sepsis, like illness (1, 26). Encephalitis is a rare presentation of EV infection, but many EV serotypes (coxsackievirus [CV] A9, A10, and B5, echovirus [ECV] 4, 5, 9, 11, 19, and 30, and EV 71, 75, 76 and 89) have been reported in encephalitis cases from different parts of the world (2, 4, 12, 17, 18, 27).
Although EVs are ubiquitous, some serotypes may be predominating in a particular area and others introduced periodically, causing epidemics. Continuous epidemiological surveillance is essential for identification of new serotypes or variants responsible for an outbreak and their disease pattern (1). Classical methods for EV serotype identification require virus isolation in cell cultures and then neutralization with type-specific antisera. However, due to the poor sensitivity of virus isolation over PCR and the emergence of untypeable strains, several methods have been developed for enterovirus typing in the direct clinical specimen by amplification and sequencing of the structural VP1 capsid region (15, 23–25).
Encephalitis is a significant cause of morbidity and mortality in children each year in Uttar Pradesh, India's so-called northern state. Japanese encephalitis virus (JEV), dengue virus, chikungunya virus, and EV have been reported in encephalitis cases from India (12, 13, 16, 20, 21, 27). Due to the large number of EV serotypes and their diverse clinical presentations (19), it is difficult to distinguish EV in encephalitis cases from other viruses, which is necessary for timely supportive therapy to avoid neurological sequelae (7). In this study, 204 children with clinical diagnosis of encephalitis were prospectively investigated for viral agents. EV (22.1%) was the main etiologic agent. We observed an epidemic during the rainy seasons of 2010 associated with ECV 21.
MATERIALS AND METHODS
Patients and clinical specimens.
Children (age, ≤15 years) with clinical symptoms of encephalitis admitted to Chhatrapati Shahuji Maharaj Medical University and Sanjay Gandhi Postgraduate Institute of Medical Sciences, Lucknow, Uttar Pradesh, India, were studied over a period of 2 years between January 2009 and December 2010. Cerebrospinal fluid (CSF) was collected at the time of admission. Demographic information, clinical features, and routine chemistry laboratory data were collected on case record forms at enrolment and during follow up. A case of encephalitis was defined as fever with altered sensorium lasting more than 24 h with ≥1 of the following symptoms: seizures and CSF pleocytosis, with neuroimaging findings indicating parenchymal involvement. Written informed consent was received from patients' parents or legal guardians. This study was approved by the Institutional Ethical Board of both institutions (reference no. XXXVII-ECM/A-P12, A-04 PGI/EMP/IEC/46/25.07.2009).
Nucleic acid extraction and virus detection.
RNA was extracted from CSF samples using a viral RNA isolation minikit (Qiagen, Hilden, Germany) per the manufacturer's protocol. Detection of EV, JEV, dengue virus, and chikungunya virus was performed using Geno-Sen's Rotor Gene quantitative real-time PCR kit (Genome Diagnostics, India), which is specific for each virus in a Rotor-Gene 6000 real-time instrument (Corbett Research, Victoria, Australia) according to the manufacturer's protocol. Immunoglobulin M capture enzyme-linked immunosorbent assay (ELISA) was also used to detect JEV antibodies in CSF samples.
EV typing and phylogenetic analysis.
EV real-time PCR-positive CSF samples were typed by seminested reverse transcriptase PCR (RT-PCR) amplification of a partial VP1 region according to Nix et al. (23). The amplicons were purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany) and sequenced on the ABI Prism automated sequencer by Vimta Labs, India, with the AN88 and AN89 primers. GenBank basic local alignment search tool (BLAST) searches (http://www.ncbi.nlm.nih.gov/Blast) were performed for serotype identification. Strains having ≥75% similarity at the nucleotide level were considered to have the same serotype. Clustal W (www.ebi.ac.uk/clustalw) was used for multiple-sequence alignment. The phylogenetic tree was inferred by using the Kimura 2-parameter algorithm and the neighbor-joining algorithm with 1,000 bootstrap replicates in MEGA 4.0.2 software (www.megasoftware.net) (28).
Statistical analysis.
Chi-square test, Fisher exact test, and independent-sample t test were used to compare data between groups of patients when appropriate by using either SPSS for Windows version 16 (SPSS Inc., Chicago, IL) or Fisher exact test using an online application (http://in-silico.net/tools/statistics/fisher_exact_test).
Sequence accession numbers.
The GenBank accession numbers for the partial VP1 sequences of EV strains identified in this study are JQ740110 to JQ740149.
RESULTS
A total of 204 children were enrolled during the study period. From 204 CSF samples, 45 (22.1%) were positive for EV, 35 (17.1%) for JEV, 17 (7.3%) for dengue virus, and 9 (4.4%) for chikungunya virus. Five cases of dual infection were observed during the study, out of which three were coinfected with JEV and EV and two with dengue and EV.
The clinical features and laboratory findings of children with encephalitis associated with EV (n = 32) and other viruses (n = 44) are shown in Table 1. There were no significant differences between enterovirus-associated encephalitis (EVAE) and other-virus-associated encephalitis (OVAE) groups in demographic features except for sex. Fever was the most common clinical feature present in all (100%) encephalitis patients associated with EV and other viruses. Levels of personality change (43.7% versus 18.2%; P < 0.021), rashes (28.1% versus 0%; P < 0.023), and diarrhea (25% versus 4.5%; P < 0.036) were significantly higher in EVAE than in OVAE. However, neck stiffness (43.7% versus 75%; P < 0.008) was significantly less common in EVAE.
Table 1.
Comparative demographic and clinical features of encephalitis patients associated with enterovirus and other virus (Japanese encephalitis virus, dengue virus, and chikungunya virus) in the present studya
| Variable | Result for patients with: |
P value | |
|---|---|---|---|
| EVAE (n = 32) | OVAE (n = 44) | ||
| Demographics | |||
| Age (yr, means ± SD) | 5.8 ± 3.4 | 5.2 ± 3.5 | NS |
| Sex (no. [%]) | |||
| Male | 18 (56.2) | 35 (79.5) | 0.043 |
| Female | 14 (43.8) | 9 (20.5) | |
| Clinical symptoms (no. [%]) | |||
| Fever | 32 (100) | 44 (100) | NS |
| Headache | 20 (62.5) | 35 (79.5) | NS |
| Neck stiffness | 14 (43.7) | 33 (75) | 0.008 |
| Altered sensorium | 26 (81.2) | 24 (54.5) | NS |
| Convulsion | 25 (78.1) | 28 (63.6) | NS |
| Coma | 5 (15.6) | 15 (34.1) | NS |
| Personality change | 14 (43.7) | 8 (18.2) | 0.021 |
| Myalgia | 3 (9.4) | 5 (11.4) | NS |
| Seizure | 10 (31.2) | 17 (38.6) | NS |
| Focal neurologic finding | 9 (28.1) | 4 (9.1) | NS |
| Abdominal pain | 1 (3.1) | 4 (9.1) | NS |
| Vomiting | 12 (37.5) | 17 (38.6) | NS |
| Rash | 3 (9.4) | 3 (6.81) | 0.023 |
| Diarrhea | 8 (25) | 2 (4.5) | 0.036 |
| Hepatomegaly | 2 (6.2) | 8 (18.2) | NS |
| Death | 12 (37.5) | 6 (13.6) | |
| Laboratory findings (no. [%]) | |||
| CSF pleocytosis | 26 (81.2) | 37 (84.1) | NS |
| Elevated CSF protein | 25 (78.1) | 31 (70.5) | NS |
SD, standard deviations; NS, not significant; EVAE, enterovirus-associated encephalitis; OVAE, other-virus-associated encephalitis.
Out of 45 EV real-time PCR-positive CSF samples, 40 (88.9%) were typed by seminested RT-PCR and sequencing of the VP1 gene. Molecular typing of EV revealed seven different serotypes corresponding to ECV 21 (n = 14), ECV 1 (n = 7), CV B1 (n = 6), EV 75 (n = 5), EV 76 (n = 4), CV B5, and ECV 19 (n = 2 each). Five samples did not amplify, perhaps due to low virus load in CSF samples or failure of PCR primers. All EV strains from this study met the serotype identification criteria for homologous serotypes, including at least 75% nucleotide or 88% amino acid identity in the VP1 region (24). The nucleotide divergence range for each serotype (CV B1, CV B5, ECV 1, 19, and 21, EV 75, and EV 76) from the respective reference serotype was 0 to 12%, 11%, 1 to 22%, 11%, 0 to 2%, 6 to 9%, and 14 to 25%, respectively.
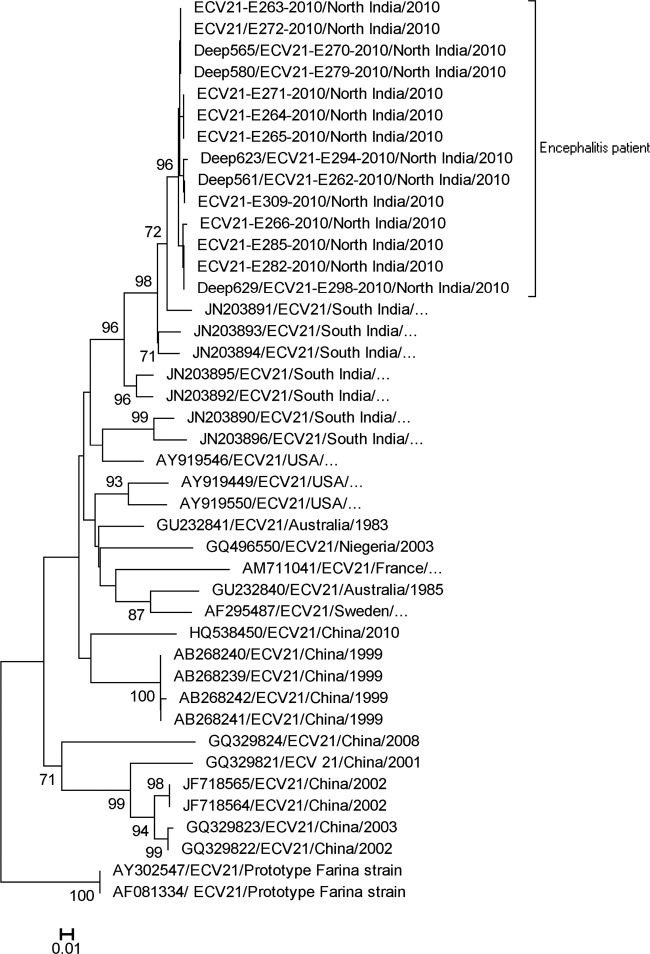
Annual distribution of EV serotypes was observed throughout the study with detection of EV 76 (37.5%) in 2009, while ECV 21 was detected only in 2010 (Table 2). During this study, an epidemic of ECV 21 was observed in 2010 (Fig. 1). Phylogenetic analysis of ECV 21 sequences from the present study with all available sequences shows the emergence of a new genotype more closely related to strains from the United States (87.3% identity), Australia (86.2% identity), Sweden (85.5% identity), Nigeria (84.5% identity), and France (82.1% identity) than to the Asian strain (83.9% identity) (Fig. 2).
Table 2.
Enterovirus serotypes detected over a 2-year period in the present study
| Enterovirus type | No. (% of total) detected in: |
|
|---|---|---|
| 2009 | 2010 | |
| Echovirus 21 | 0 | 14 (51.8) |
| Echovirus 1 | 3 (23.1) | 4 (14.8) |
| Coxsackievirus B1 | 2 (15.4) | 4 (14.8) |
| Enterovirus 75 | 2 (15.4) | 3 (11.1) |
| Enterovirus 76 | 4 (30.8) | 0 |
| Coxsackievirus B5 | 1 (7.7) | 1 (3.7) |
| Echovirus 19 | 1 (7.7) | 1 (3.7) |
Fig 1.
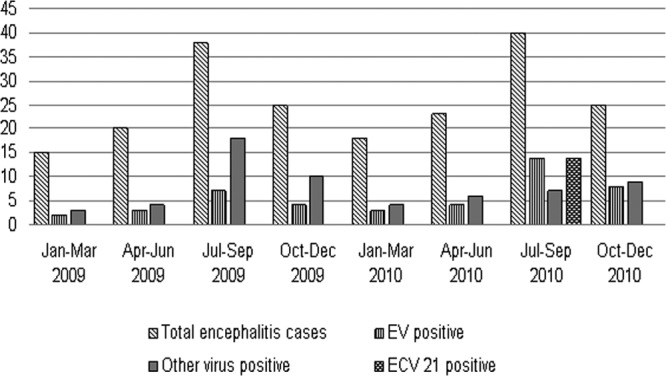
Seasonal distribution of encephalitis cases associated with enterovirus and other viruses (Japanese encephalitis virus, dengue virus, and chikungunya virus).
Fig 2.
Phylogenetic analysis of partial VP1 region of echovirus 21 nucleotide sequences. The tree was constructed by using the neighbor-joining method and Kimura 2-parameter model. Significance of phylogenies was investigated by bootstrap analysis with 1,000 pseudoreplicate data sets. Bootstrap values of >70% are indicated on the tree. The bracket on the right indicates echovirus 21 strains from encephalitis patients in this study. All echovirus 21 sequences are named by using the conventional GenBank accession numbers (published sequences) or sample name (sequences from this study), country of origin, and year of isolation (ellipses indicate no information about the year of isolation). The scale bar indicates nucleotide substitutions per site.
DISCUSSION
More than 100 viruses can cause encephalitis that varies by distribution and occurrence in different geographical regions (22). This study shows that EV was the most common etiologic agent in children with encephalitis from the Uttar Pradesh state of India. This finding may be overstated because the diagnosis was defined only in 51% of cases, which might be due to identification criteria specific to previously reported etiologic agents in the studied area. However, the prevalence of EV in encephalitis cases (22.1%) in this study is similar to earlier reports from Kuwait and European countries (2, 3, 11).
Our study shows that EV-associated encephalitis patients present high degrees of altered sensorium and mortality compared to previous reports (4, 5, 10). Recently, a study from south Vietnam also reported severe outcomes in EV-associated encephalitis in younger children (14). This finding is similar to an earlier report which shows that some virulent EV serotypes prevailing in a particular area may be responsible for severe infection (6, 8).
During this study, ECV 21 was the main serotype that occurred in 2010 in the form of an epidemic. Retrospective analysis of ECV 21 showed that it had circulated sporadically in Australia, the United States, Nigeria, France, Sweden, China, and south India. The clinical manifestations of ECV 21 range from asymptomatic infection to respiratory infection, acute flaccid paralysis, and aseptic meningitis (8). To our knowledge, this is the first report of ECV 21 in encephalitis cases worldwide. The highest identity of Indian ECV 21 strains and U.S. isolates compared to other worldwide strains suggests their importation into these highly populated areas. However, further study based on complete genomes and animal models are required to study their evolution and pathogenesis due to increased evidence of recombination within enterovirus B species (19).
In conclusion, EVs are a significant cause of encephalitis in children from northern India. To our knowledge, this is the first report of CV B1, ECV 1, and ECV 21 serotypes in encephalitis cases from India. Molecular typing of EVs is useful for correlating clinical symptoms with a virus type, characterizing emerging serotypes and their epidemiological investigation, and developing vaccines for encephalitis.
ACKNOWLEDGMENTS
We thank the children and parents for participating in the study and Prashant Jauhari for the clinical history of patients.
This study was supported by a grant-in-aid from the Indian Council of Medical Research, Government of India, New Delhi (ref no. 5/8/7/23/2007-ECD-I).
Footnotes
Published ahead of print 15 August 2012
REFERENCES
- 1. Cherry JD. 1998. Enteroviruses: coxsackieviruses, echoviruses and polioviruses, p 1787–1839 In Feigin RD, Cherry JD. (ed), Textbook of pediatric infectious diseases. Saunders, Philadelphia, PA [Google Scholar]
- 2. Dalwai A, Ahmad S, Pacsa A, Al-Nakib W. 2009. Echovirus type 9 is an important cause of viral encephalitis among infants and young children in Kuwait. J. Clin. Virol. 44: 48–51 [DOI] [PubMed] [Google Scholar]
- 3. Fowler A, Stodberg T, Eriksson M, Wickstrom R. 2008. Childhood encephalitis in Sweden: etiology, clinical presentation and outcome. Eur. J. Paediatr. Neurol. 12: 484–490 [DOI] [PubMed] [Google Scholar]
- 4. Fowlkes AL, et al. 2008. Enterovirus-associated encephalitis in the California encephalitis project, 1998–2005. J. Infect. Dis. 198: 1685–1691 [DOI] [PubMed] [Google Scholar]
- 5. Glaser CA, et al. 2006. Beyond viruses: clinical profiles and etiologies associated with encephalitis. Clin. Infect. Dis. 43: 1565–1577 [DOI] [PubMed] [Google Scholar]
- 6. Hsiung GD, Wang JR. 2000. Enterovirus infections with special reference to enterovirus 71. J. Microbiol. Immunol. Infect. 33: 1–8 [PubMed] [Google Scholar]
- 7. Kennedy PG. 2004. Viral encephalitis: causes, differential diagnosis, and management. J. Neurol. Neurosurg. Psychiatry 75: 10–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khetsuriani N, LaMonte-Fowlkes A, Oberste S, Pallansch MA. 2006. Enterovirus surveillance–United States, 1970–2005. MMWR Surveill. Summ. 55: 1–20 [PubMed] [Google Scholar]
- 9. Knowles NJ, et al. 2011. Picornaviridae, p 855–880 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses Elsevier, San Diego, CA [Google Scholar]
- 10. Kolski H, et al. 1998. Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994–1995. Clin. Infect. Dis. 26: 398–409 [DOI] [PubMed] [Google Scholar]
- 11. Koskiniemi M, et al. 1997. Epidemiology of encephalitis in children. A prospective multicentre study. Eur. J. Pediatr. 156: 541–545 [DOI] [PubMed] [Google Scholar]
- 12. Kumar A, et al. 2011. An epidemic of encephalitis associated with human enterovirus B in Uttar Pradesh, India, 2008. J. Clin. Virol. 51: 142–145 [DOI] [PubMed] [Google Scholar]
- 13. Kumar R, et al. 2008. Dengue encephalopathy in children in northern India: clinical features and comparison with nondengue. J. Neurol. Sci. 269: 41–48 [DOI] [PubMed] [Google Scholar]
- 14. Le VT, et al. 2010. Viral etiology of encephalitis in children in southern Vietnam: results of a one-year prospective descriptive study. PLoS Negl. Trop. Dis. 26: e854 doi:10.1371/journal.pntd.0000854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leitch EC, et al. 2009. Direct identification of human enterovirus serotypes in cerebrospinal fluid by amplification and sequencing of the VP1 region. J. Clin. Virol. 44: 119–124 [DOI] [PubMed] [Google Scholar]
- 16. Lewthwaite P, et al. 2009. Chikungunya virus and central nervous system infections in children, India. Emerg. Infect. Dis. 15: 329–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewthwaite P, et al. 2010. Enterovirus 75 encephalitis in children, southern India. Emerg. Infect. Dis. 16: 780–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin TY, Twu SJ, Ho MS, Chang LY, Lee CY. 2003. Enterovirus 71 outbreaks, Taiwan: occurrence and recognition. Emerg. Infect. Dis. 9: 291–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lukashev AN. 2005. Role of recombination in evolution of enteroviruses. Rev. Med. Virol. 15: 157–167 [DOI] [PubMed] [Google Scholar]
- 20. Mathur A, et al. 1982. Japanese encephalitis epidemic in Uttar Pradesh, India during 1978. Indian J. Med. Res. 75: 161–169 [PubMed] [Google Scholar]
- 21. Misra UK, Kalita J, Syam UK, Dhole TN. 2006. Neurological manifestations of dengue viral infection. J. Neurol. Sci. 244: 117–122 [DOI] [PubMed] [Google Scholar]
- 22. Misra UK, Chong TT, Kalita J. 2008. Viral encephalitis and epilepsy. Epilepsia 49: 13–18 [DOI] [PubMed] [Google Scholar]
- 23. Nix WA, Oberste MS, Pallansch MA. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J. Clin. Microbiol. 44: 2698–2704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oberste MS, Maher K, Kilpatrick DR, Pallansch MA. 1999. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 73: 1941–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oberste MS, et al. 1999. Typing of human enterovirus by partial sequencing of VP1. J. Clin. Microbiol. 37: 1288–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pallansch MA, Roos RP. 2001. Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses, p 723–775 In Knipe DM, et al. (ed), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 27. Sapkal GN, et al. 2009. Enteroviruses in patients with acute encephalitis, Uttar Pradesh, India. Emerg. Infect. Dis. 15: 295–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tamura K, Dudley J, Nei M, Kuma S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4. Mol. Biol. Evol. 24: 1596–1599 [DOI] [PubMed] [Google Scholar]