Abstract
Mixed infections with different Leishmania species could explain differences in the clinical courses of these infections. On identification of Leishmania parasites from Iranian patients with mucosal leishmaniasis (ML), a patient with both oral and nasal lesions was found to be concomitantly infected with Leishmania tropica and L. major. Mixed infection was identified by PCR amplification of Leishmania kinetoplast DNA on scraping of cytological smears and histopathological sections. L. major and L. tropica were isolated from the nasal and oral lesions, respectively. These species were also confirmed by immunohistochemistry. This seems to be the first reported case of concurrent ML infection with two Leishmania species. It indicates that, at least in this patient, previous infection with one of these Leishmania species did not protect against infection with the other. This result has important implications for the development of vaccines against leishmaniases and implies careful attention in the treatment of this infectious disease.
CASE REPORT
A 34-year-old immunocompetent male patient presented with lesions of the mucous membranes of the nose and mouth. The patient was from Fars Province, southern Iran. He presented with a 7- and 5-month history of intranasal and oral lesions, respectively. No scar or other lesion was found in other parts of the body. On examination, there were multiple tiny erythematous lesions, varying in size from 0.1 to 0.3 cm in diameter. The nasal pyramid was edematous, and bloody crusts were observed on the inferior conchae, septum, and floor of the nasal fossa. The nasal lesions were located in the intranasal portion in the mucous membrane over the turbinates, far from the cutaneous lesions. Clinically, diffuse yellowish white erosions with grayish fibrinous membranes were seen on a reddish edematous background on the involved oral mucosa (Fig. 1).
Fig 1.
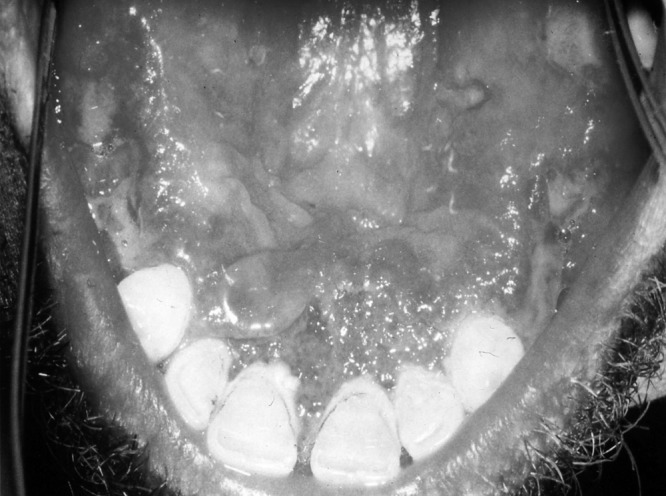
Diffuse erosions with pseudomembranes on edematous background on oral mucosa are visible.
His blood biochemistry and complete blood count were within the reference range. His hemoglobin level was 13.6 g/dl, his total leukocyte count was 6,600/mm3, his serum creatinine level was 0.8 mg/dl, and his blood urea nitrogen level was 18 mg/dl. Serological studies for human immunodeficiency virus and hepatitis B and C viruses were negative.
Tissue samples from the lesions were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at a 5-μm thickness, and stained with hematoxylin and eosin.
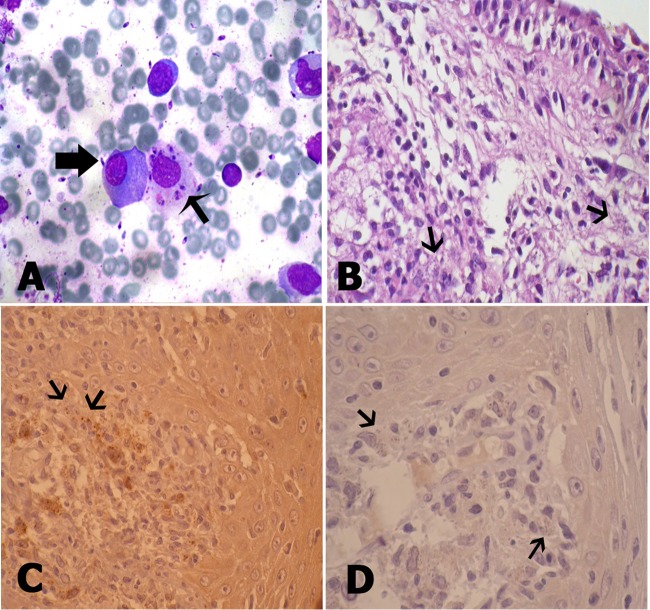
Cytologic smears were prepared by scraping of the oral lesions with a scalpel. In addition, exfoliative cytology from the nasal lesions was performed by washing the nasal cavity as previously noted (7). Multiple smears were made on slides and were both air dried and alcohol fixed and then stained by the Wright method. Review of the cytologic smears and histologic sections was conducted blindly by three pathologists. Microscopic examination showed the amastigote forms of Leishmania (Fig. 2A and B).
Fig 2.
(A) Plasma cell (thick arrow) and macrophage loaded with Leishmania bodies associated with free Leishmania bodies (thin arrow) (Wright stain; magnification, ×200). (B) Massive infiltration of macrophages loaded with Leishmania bodies in the submucosal area of the respiratory epithelium (arrows) (hematoxylin and eosin staining; magnification, ×200). (C) Oral lesions showing immunoreactivity to L. tropica-specific MAb (arrows) (IHC; magnification, ×100). (D) Negative IHC staining of oral lesions for L. major (arrows) (magnification, ×100).
The antibodies IS2-2B4 (A11; specific for L. tropica) and XLVI-5B8-B3 (T1; specific for L. major) were kindly provided by the Special Programme for Research and Training in Tropical Disease, WHO, and used as primary monoclonal antibodies (MAbs).
Sections 3 μm thick were used for immunohistochemical (IHC) analysis. The slides were deparaffinized in xylol, rehydrated, and treated with 3% hydrogen peroxide solution for 10 min at room temperature to quench endogenous peroxides. Antigen retrieval was conducted by microwave pretreatment (a power level of 100 for 10 min and then a power level of 20 for 20 min) using a 10-mmol/liter concentration of citrate buffer (pH 6.0). The primary antibody was applied for 1 h (diluted 1:200). Detection of the immunoreaction was achieved. The detection system used was Envision+ (DakoCytomation), and development was done with diaminobenzidine (DakoCytomation). 3,3′-Diaminobenzidine–hydrogen peroxide was used as the chromogen, and hematoxylin was used as the counterstain. The nasal lesions showed immunoreactivity to the L. major-specific MAb, and the oral lesions showed immunoreactivity to the L. tropica-specific MAb (Fig. 2C), while IHC staining of the nasal lesions with the L. tropica MAb and IHC staining of the oral lesions for L. major were negative (Fig. 2D).
To identify the Leishmania-specific DNA, the entire smear was scraped off the slide with a sterile scalpel and the phenol-chloroform-isoamyl alcohol extraction method was used as previously described to extract the DNAs (23). The DNA samples were dissolved in 50 μl of deionized distilled water and stored at 4°C. Variable segments of the minicircles of the kinetoplast DNA from the Leishmania species present in the smear scrapings were amplified by two nested PCR rounds. The primers for the first round were CSB1XR (ATT TTT CGC GAT TTT CGC AGA ACG) and CSB2XF (CGA GTA GCA GAA ACT CCC GTT CA), and those used for the second round were LiR (TCG CAG AAC GCC CCT) and 13Z (ACT GGG GGT TGG TGT AAA ATAG) (23). The products of the second-round PCR were loaded onto a 1.5% agarose gel. As positive controls, the DNA extracted from promastigote cultures of the reference strains of L. infantum (MCAN/IR/97/LON490) were run on each gel. Extravasation cysts (oral mucoceles) from 10 patients were used as negative controls. Negative controls in which ultrapure water replaced the template DNA were also run. A 560-bp fragment of L. major-specific kinetoplast minicircle DNA was amplified from the nasal lesions by the second-round PCR assay, whereas a 750-bp fragment of L. tropica-specific kinetoplast minicircle DNA was amplified from the oral lesions by the second-round PCR assay (Fig. 3).
Fig 3.
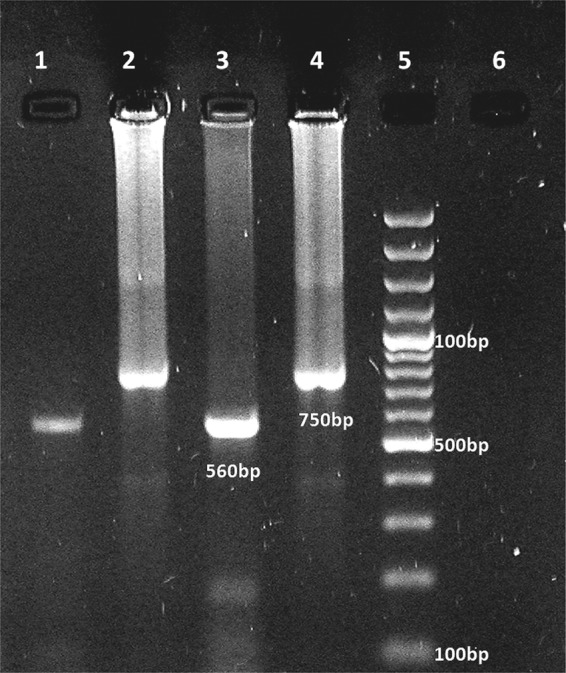
Electrophoresis of products of nested-PCR-based amplification of DNA extracted from stained smears. The six lanes contained the products from reference strains of L. tropica (lane 4) and L. major (lane 3), a negative-control test sample (lane 6), oral and nasal lesions due to L. tropica and L. major (lanes 1 and 2, respectively), and a molecular size ladder (lane 5).
The patient was treated by intravenous infusion of amphotericin B at 1 mg/kg/day for 14 days, and resolution of the lesions started 1 week after the treatment started.
Ethics statement.
The Ethics Committee of the Faculty of Medical School, University of Shiraz Medical Sciences and the Institutional Review Board of the Dr. Daneshbod Laboratory approved this study, and we obtained written informed consent from the patient.
Mucosal leishmaniasis (ML) is a rare disease in the world, even in areas where it is endemic, such as Iran (6, 25). The importance of ML is due to the severity of its clinical lesions, poor response to traditional antimony therapy, and destruction of the nasal architecture with gross facial alterations (17). ML is a form of tegumentary leishmaniasis that has been shown to be associated with L. braziliensis and L. panamensis and less frequently with L. amazonensis, although it has been reported in infections caused by other New-World Leishmania species, such as L. guyanensis (13). A few patients in the Old World with ML infections caused by L. infantum, L. tropica, and L. major have been described (16, 18, 21, 25). The patient in the present study had nasal lesions caused by L. major, but L. tropica was isolated from his oral lesions too. The association between ML and previous or active skin lesions is widely accepted, as both forms can originate from a single species (13). It has also been demonstrated that localization of the parasites in the mucous membranes of the nasal, oral, and pharyngeal areas occurs as a result of migration of Leishmania via the lymphatic system or because of hematogenous dissemination of amastigotes from the skin of 5% of patients with cutaneous leishmaniasis (CL) (14). Oral involvement is unusual, and in most cases it becomes evident several years after the resolution of the original cutaneous lesions (22).
Sporadic L. major and L. tropica infections have occasionally been reported in patients with ML in Afghanistan, Saudi Arabia, and Sudan (5, 9, 11). Fars Province, a region in southern Iran, is a classical focus of CL, and the previous studies have consistently documented the etiologic agents to be L. tropica and L. major in urban and rural areas, respectively (4, 12). Mixed infections with different Leishmania species could explain differences between the clinical courses of these infections, as well as resistant cases (1). We have presented the first report of coinfection with L. major and L. tropica isolated from a patient with ML. In the sub-Andean region of Bolivia, coinfection with L. amazonensis and L. infantum/L. chagasi has been identified in a patient with diffuse CL (20). In the suburban district of Campo Grande, Municipality of Rio de Janeiro, Brazil, L. donovani and L. braziliensis have been isolated from the bone marrow and forehead of a patient with concurrent asymptomatic visceral leishmaniasis (VL) and typical CL (24). There are data indicating that concomitant natural infection with L. donovani and L. major has occurred in humans with CL and VL (2, 15). Mixed infections have also been observed in sand flies and dogs (8, 10). Antoniou et al. indicated that the VL form may occur because of mixed infection with different strains of L. infantum (3). Such reservoirs are exposed to large numbers of sand fly bites, which increases the possibility of infection with different strains or species of the parasite. Moreover, mixed infections of the same macrophage with different species of Leishmania have been shown to be experimentally possible (1). On the basis of PCR and IHC results, concomitant or mixed mucosal infection with two Leishmania species can occur in immunocompetent subjects. This seems to be the first described case of concurrent or mixed ML infection with L. major and L. tropica. L. major was isolated from the nasal lesion that occurred 2 months earlier than the oral lesion from which L. tropica was isolated, and this can indicate that, at least in this patient, a previous infection with L. major did not protect against L. tropica. On the other hand, it has previously been reported that L. tropica primary infection was not efficient in reducing the parasite load of the spleen in the secondary L. major infection (19). This result has important implications for the development of vaccines against leishmaniases and emphasizes attention to the diagnosis and treatment of mixed ML infections.
ACKNOWLEDGMENTS
We thank the authorities of the Veterinary School, Shiraz University and Medical School, Shiraz University of Medical Sciences, and the Institute of Experimental Pathology, University of Muenster, for their support.
We thank M. Davarmanesh, M. M. Davarpanah, K. Daneshbod, M. Kalantari, and J. Brosius, T. Rozhdestvensky, and G. Randau, Institute of Experimental Pathology, University of Muenster, for their help and advice.
Footnotes
Published ahead of print 12 September 2012
REFERENCES
- 1. Abdullah SM, Flath B, Presber W. 1998. Mixed infection of human U-937 cells by two different species of Leishmania. Am. J. Trop. Med. Hyg. 59: 182–188 [DOI] [PubMed] [Google Scholar]
- 2. al-Diwany LJ, Alawkati NA, Atia M, Rassam MB. 1995. Concomitant natural infection with L. donovani and L. major: a case report from Iraq. Soz. Praventivmed. 40: 234–238 [DOI] [PubMed] [Google Scholar]
- 3. Antoniou M, Doulgerakis C, Pratlong F, Dedet JP, Tselentis Y. 2004. Short report: treatment failure due to mixed infection by different strains of the parasite Leishmania infantum. Am. J. Trop. Med. Hyg. 71: 71–72 [PubMed] [Google Scholar]
- 4. Asgari Q, et al. 2007. Zoonotic cutaneous leishmaniasis in Shiraz, southern Iran: a molecular, isoenzyme and morphologic approach. J. Res. Med. Sci. 12: 7–15 [Google Scholar]
- 5. Benmously-Mlika R, et al. 2008. Primary Leishmania infantum MON-80 endonasal leishmaniasis in Tunisia. Ann. Dermatol. Venereol. 135: 389–392 [DOI] [PubMed] [Google Scholar]
- 6. Daneshbod Y, et al. 2011. Clinical, histopathologic, and cytologic diagnosis of mucosal leishmaniasis and literature review. Arch. Pathol. Lab. Med. 135: 478–482 [DOI] [PubMed] [Google Scholar]
- 7. Daneshbod Y, Khademi B, Kadivar M, Ganjei-Azar P. 2008. Fine needle aspiration of salivary gland lesions with multinucleated giant cells. Acta Cytol. 52: 671–680 [DOI] [PubMed] [Google Scholar]
- 8. de Fátima Madeira M, et al. 2006. Post mortem parasitological evaluation of dogs seroreactive for Leishmania from Rio de Janeiro, Brazil. Vet. Parasitol. 138: 366–370 [DOI] [PubMed] [Google Scholar]
- 9. El Fékih N, et al. 2008. Mucosal leishmaniasis by contiguity with a skin lesion: another case report from Tunisia. Med. Trop. 68: 634–636. (In French.) [PubMed] [Google Scholar]
- 10. el Sawaf BM, El-Sattar SA, Shehata MG, Lane RP, Morsy TA. 1994. Reduced longevity and fecundity in Leishmania-infected sand flies. Am. J. Trop. Med. Hyg. 51: 767–770 [DOI] [PubMed] [Google Scholar]
- 11. García de Marcos JA, et al. 2007. Localized leishmaniasis of the oral mucosa. A report of three cases. Med. Oral Patol. Cir. Bucal 12: E281–E286 [PubMed] [Google Scholar]
- 12. Ghasemian M, Maraghi S, Samarbafzadeh AR, Jelowdar A, Kalantari M. 2011. The PCR-based detection and identification of the parasites causing human cutaneous leishmaniasis in the Iranian city of Ahvaz. Ann. Trop. Med. Parasitol. 105: 209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guerra JA, et al. 2011. Mucosal leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl. Trop. Dis. 5: e980 doi:10.1371/journal.pntd.0000980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herwaldt BL. 1999. Leishmaniasis. Lancet 354: 1191–1199 [DOI] [PubMed] [Google Scholar]
- 15. Ibrahim ME, Smyth AJ, Ali MH, Barker DC, Kharazmi A. 1994. The polymerase chain reaction can reveal the occurrence of naturally mixed infections with Leishmania parasites. Acta Trop. 57: 327–332 [DOI] [PubMed] [Google Scholar]
- 16. Kharfi M, Fazaa B, Chaker E, Kamoun MR. 2003. Mucosal localization of leishmaniasis in Tunisia: 5 cases. Ann. Dermatol. Venereol. 130: 27–30 [PubMed] [Google Scholar]
- 17. Lessa MM, et al. 2007. Mucosal leishmaniasis: epidemiological and clinical aspects. Braz. J. Otorhinolaryngol. 73: 843–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahdi M, et al. 2005. Sudanese mucosal leishmaniasis: isolation of a parasite within the Leishmania donovani complex that differs genotypically from L. donovani causing classical visceral leishmaniasis. Infect. Genet. Evol. 5: 29–33 [DOI] [PubMed] [Google Scholar]
- 19. Mahmoudzadeh-Niknam H. 2004. Induction of partial protection against Leishmania major in BALB/c mice by Leishmania tropica. Scand. J. Lab. Anim. Sci. 31: 201–207 [Google Scholar]
- 20. Martinez E, et al. 2002. Co-infection by Leishmania amazonensis and L. infantum/L. chagasi in a case of diffuse cutaneous leishmaniasis in Bolivia. Trans. R. Soc. Trop. Med. Hyg. 96: 529–532 [DOI] [PubMed] [Google Scholar]
- 21. Morsy TA, et al. 1995. Mucosal leishmaniasis caused by Leishmania tropica in Saudi Arabia. J. Egypt. Soc. Parasitol. 25: 73–79 [PubMed] [Google Scholar]
- 22. Motta ACF, et al. 2007. Oral leishmaniasis: a clinicopathological study of 11 cases. Oral Dis. 13: 335–340 [DOI] [PubMed] [Google Scholar]
- 23. Noyes HA, Reyburn H, Bailey JW, Smith D. 1998. A nested PCR-based schizodeme method for identifying Leishmania kinetoplast minicircle classes directly from clinical samples and its application to the study of the epidemiology of Leishmania tropica in Pakistan. J. Clin. Microbiol. 36: 2877–2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oliveira Neto MP, et al. 1986. Concurrent human infection with Leishmania donovani and Leishmania braziliensis braziliensis. Ann. Trop. Med. Parasitol. 80: 587–592 [DOI] [PubMed] [Google Scholar]
- 25. Shirian S, Oryan A, Hatam GR, Daneshbod Y, Daneshbod K. 2012. Molecular diagnosis and species identification of mucosal leishmaniasis in Iran, and correlation with cytological findings. Acta Cytol. 56: 304–309 [DOI] [PubMed] [Google Scholar]