Abstract
Recombinant soluble trimeric influenza A virus (IAV) hemagglutinin (sHA3) has proven an effective vaccine antigen against IAV. Here, we investigate to what extent the glycosylation status of the sHA3 glycoprotein affects its immunogenicity. Different glycosylation forms of subtype H5 trimeric HA protein (sH53) were produced by expression in insect cells and different mammalian cells in the absence and presence of inhibitors of N-glycan-modifying enzymes or by enzymatic removal of the oligosaccharides. The following sH53 preparations were evaluated: (i) HA proteins carrying complex glycans produced in HEK293T cells; (ii) HA proteins carrying Man9GlcNAc2 moieties, expressed in HEK293T cells treated with kifunensine; (iii) HA proteins containing Man5GlcNAc2 moieties derived from HEK293S GnTI(−) cells; (iv) insect cell-produced HA proteins carrying paucimannosidic N-glycans; and (v) HEK293S GnTI(−) cell-produced HA proteins treated with endoglycosidase H, thus carrying side chains composed of only a single N-acetylglucosamine each. The different HA glycosylation states were confirmed by comparative electrophoretic analysis and by mass spectrometric analysis of released glycans. The immunogenicity of the HA preparations was studied in chickens and mice. The results demonstrate that HA proteins carrying terminal mannose moieties induce significantly lower hemagglutination inhibition antibody titers than HA proteins carrying complex glycans or single N-acetylglucosamine side chains. However, the glycosylation state of the HA proteins did not affect the breadth of the antibody response as measured by an HA1 antigen microarray. We conclude that the glycosylation state of recombinant antigens is a factor of significant importance when developing glycoprotein-based vaccines, such as recombinant HA proteins.
INTRODUCTION
Influenza A viruses (IAVs) are important human and animal pathogens. Because of their wide spread and their presence in animal reservoirs, eradication of IAV is not possible. Vaccination provides the most effective way to control IAVs and to protect against IAV infection and disease. Despite the efficacy of the approved influenza vaccines, issues related to, among other things, their production and safety call for alternative approaches, as has been reviewed elsewhere (12, 28).
As protection against influenza virus infection and disease correlates with serum anti-hemagglutinin (HA) (23) antibody levels, recombinant HA proteins may prove to be attractive vaccine antigens. Recombinant HA glycoproteins can be produced using safe, quality controlled, and scalable conditions (10) without the need for virus cultivation. Furthermore, adverse reactions may be limited, as the HA preparations can be highly purified and do not contain egg contaminants. In addition, recombinant HA proteins can be manufactured with short lead times, allowing an accelerated response to emerging IAV strains.
Expression of recombinant HA proteins in higher eukaryotic expression systems is likely to result in superior antigens compared to other expression systems, as folding and trimerization of the HA protein are known to depend on multiple co- and posttranslational modifications, including glycosylation and disulfide bond formation. Consistently, mammalian-cell-derived HA trimers have been shown to induce higher levels of neutralizing antibodies than their monomeric counterparts (37). We and others have previously demonstrated the efficacy of recombinant trimeric HA protein preparations from insect or mammalian cells to protect against IAV infection and disease (4, 5, 17, 34, 36).
N-linked glycosylation is essential for proper folding, oligomerization, and transport of the HA protein (13). In addition, glycosylation may affect cleavage of the HA protein (8) and HA-receptor binding and fusion (21, 22). Loss of N-glycan sites neighboring the receptor-binding site or enzymatic truncation of the N-glycan structures were both shown to result in increased affinity of HA for receptors (21). We and others recently showed that HA proteins that differ only in their glycosylation status, as a result of different production or processing conditions, possess different receptor fine specificities (9, 34). Furthermore, HA-linked oligosaccharides may function in masking antigenic regions, while they may also serve as a target for recognition of IAV by the innate immune system (25, 31). Antibodies raised against HA protein bearing only a single N-acetylglucosamine (GlcNAc) at each glycosylation site were reported to show better binding affinity and IAV neutralization activity than antibodies raised against HA containing complex glycans (34).
In view of the apparent importance of glycosylation for HA structure, functioning, and antigenicity, we decided to compare the immune responses induced by recombinant soluble trimeric HA proteins of the H5 subtype (sH53) that differ in their glycosylation states. The results show that HA proteins carrying N-glycans with terminal mannoses induce lower antibody titers than those carrying complex N-glycans or single GlcNAc residues. HA proteins carrying single GlcNAc side chains induced similar or higher antibody responses, depending on the specific H5 protein used. The HA glycosylation state did not appear to affect the breadth of the antibody response, as similar reactivities were observed against different HA1 domains, regardless of the glycosylation state of the immunogens used.
MATERIALS AND METHODS
Genes and expression vectors.
cDNA clones corresponding to residues 18 to 523 (H3 numbering) of HA from A/reassortant/NIBRG-14 (Viet Nam/1194/2004 × Puerto Rico/8/1934) (H5N1) (GenBank accession no. ACU65077.1) and A/Mallard/Denmark/64650/03 (H5N7) (GenBank accession no. AAT07996.1) were synthesized using human-preferred codons by GenScript USA Inc. H5 derived from the H5N7 virus (designated sH53N7) lacks a glycosylation site on the globular head and has 94% sequence identity to H5 derived from H5N1 virus (designated sH53N1). Cloning of the H5 sequences into the appropriate expression vector was done as described previously (5). Briefly, cDNA was cloned into the pCD5 expression vector for efficient expression in mammalian cells or into the pMT-Bip vector (Invitrogen) for expression in Schneider S2 cells. The HA-encoding cDNA was cloned in frame with DNA sequences coding for a signal sequence, an artificial GCN4 isoleucine zipper trimerization motif (RMKQIEDKIEEIESKQKKIENEIARIKK), and Strep-tag II (WSHPQFEK; IBA, Germany).
Protein expression and purification.
pCD5 expression vectors containing the HA ectodomain-encoding sequences were transfected into HEK293T and HEK293S GnTI(−) cells (26) as described previously (5). HA proteins secreted by the cells were purified using Strep-Tactin Sepharose beads according to the manufacturer's instructions (IBA, Germany). When indicated, HA trimers bound to Strep-Tactin beads were treated with endoglycosidase H (EndoH) for 3 h at 37°C (2 μU/ml), followed by three washing steps prior to elution of the protein from the beads. Drosophila Schneider S2 cells were cotransfected with pMT-Bip vector encoding the HA ectodomain and pCoBlast using Cellfectin in a 19:1 ratio, and stable cell lines were selected according to the manufacturer's protocols (Invitrogen) as described previously (9). HA proteins were purified as described above. HA protein expression and secretion were confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by Western blotting using a mouse anti-Strep-tag antibody (IBA, Germany). The concentration of purified protein was determined by using a Nanodrop 1000 spectrophotometer (Isogen Life Sciences) according to the manufacturer's instructions.
Characterization of recombinant HA.
Oligomerization of the sH53 proteins was confirmed by Blue Native PAGE as described previously (9). For dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN)-binding experiments, 96-well Nunc MaxiSorp plates were coated with 5 μg/ml HA for 16 h at 4°C in PBS. The plates were blocked with a solution of 3% bovine serum albumin (BSA) and 0.1% Tween 20 in phosphate-buffered saline (PBS) for 2 h at room temperature (RT), followed by three washes with 0.05% Tween 20 in PBS. Subsequently, serially diluted DC-SIGN–Fc (R&D Systems) was added for 2 h at RT, followed by three washes with 0.05% Tween 20 in PBS. Horseradish peroxidase (HRP)-labeled goat anti-human IgG was added for 1 h at RT at a 1:1,000 dilution, followed by three washes with 0.05% Tween 20 in PBS. Peroxidase activity was visualized using tetramethylbenzidine substrate (BioFX) and an enzyme-linked immunosorbent assay (ELISA) reader (EL-808 [BioTEK]), reading the optical density (OD) at 450 nm. The sialic acid-binding activity of the sH53 protein preparations was assessed by using a fetuin solid-phase assay as described previously (9).
Glycan release, purification, labeling, and mass spectrometry.
The sH53 proteins were treated with the glycosidase PNGaseF (NEB) according to the manufacturer's protocol. The N-glycan mixture released by the PNGaseF treatment was applied to a C18 RP cartridge (500 mg; JT Baker, Phillipsburg, NJ). The combined flowthrough and wash fractions (2 ml 10% acetonitril [AcN] and 4 ml water) of these cartridges were subsequently applied to carbon cartridges (150 mg Carbograph; Grace, Deerfield, IL). After a wash with 6 ml water, glycans were eluted with 3 ml 25% AcN and 3 ml 50% AcN containing 0.1% trifluoroacetic acid (TFA). The purified N-glycans were subsequently labeled with the fluorophore 2-aminobenzoic acid (2-AA), as described elsewhere (27). The labeled glycans in 75% AcN were loaded on Biogel P10 (Bio-Rad Hercules, CA) conditioned with 80% AcN. After a wash with 80% AcN, the glycans were eluted with water and analyzed with an Ultraflex II matrix-assisted laser desorption ionization–time-of-flight (MALDI-TOF) mass spectrometer (Bruker Daltonics, Bremen, Germany) operating in the negative-ion reflectron mode. 2,5-Dihydroxybenzoic acid (DHB; Bruker Daltonics, Bremen, Germany) was used as a matrix. When indicated, 2-AA-labeled glycans (4 μl) were treated with beta-galactosidase from jackbean (45 mU; Prozyme, Hayward, CA) in 60 μl 250 mM sodium citrate buffer for 24 h at 37°C. The digestion products were purified using a Ziptip C18 column (Millipore, Billerica, MA) following the manufacturer's instructions. Glycans were eluted directly to a MALDI target plate with 10 mg/ml DHB in 50% AcN containing 0.1% TFA and analyzed in the negative-ion reflectron mode.
Immunization experiments.
Animal studies were conducted at the Central Veterinary Institute (CVI), Lelystad, The Netherlands, and at the Central Laboratory Animal Research Facility (CLARF) Utrecht after approval by the appropriate animal ethics committees. Thirty 1-day-old layer hens (white Leghorn) were purchased from a local breeder. The chickens had been vaccinated against Newcastle disease virus and infectious bronchitis virus at the age of 1 day, according to the farm's routine, and were raised in the CVI's animal facility. At the age of 6 weeks, the birds were allocated to 3 experimental groups of 10 birds each. The animals were immunized twice (on days 0 and 21) by intramuscular (i.m.) injection of 2 μg sH53 antigen adjuvanted with Stimune (Prionic). As a control, one group was mock vaccinated twice (on days 0 and 21) with PBS in Stimune. Blood was taken before the second vaccination and 3 weeks after the second vaccination. For the mouse experiments, female 9-week-old C57BL/6 mice obtained from Charles River Laboratories were immunized twice (on days 0 and 21) by i.m. injection (0.05 ml) of 4 μg sH53 antigen adjuvanted with Stimune. Six animals per group were used. As a control, in each experiment, one group of mice was mock vaccinated twice. Blood was taken before and 3 weeks after the second vaccination.
HI assay.
Vibrio cholerae neuraminidase (VCNA)-treated and heat-inactivated immune sera from mice and chicken blood samples were tested for hemagglutination inhibition (HI) activity with 8 or 4 hemagglutinating units (HAU) of homologous antibody-complexed sH53 produced in HEK293S GnTI(−) cells as described previously (5). To this end, the recombinant proteins were precomplexed with anti-Strep tag and anti-mouse antibodies as described previously (5), mixed with limiting dilutions of mouse sera, and incubated with 0.5% chicken red blood cells in PBS containing 1% BSA. Antibody titers were expressed as the reciprocal of the highest serum dilution showing HI.
ELISA.
IgG1 antibody titers against sH53 produced in HEK293T cells were determined by using a sH53-specific ELISA. To this end, 96-well Nunc MaxiSorp plates were coated with 100 μl of 2-μg/ml sH53 per well and incubated with limiting dilutions of mouse sera. After extensive washing, the plates were incubated with goat-anti-IgG1 mouse antibodies conjugated with HRP. Peroxidase activity was visualized using tetramethylbenzidine substrate (bioFX) and an ELISA reader (EL-808 [BioTek]), reading the OD at 450 nm. Wells coated with purified mouse IgG1 were used to determine the amount of sH53-specific IgG1 present in the sera.
Suppression of pDC function.
Human plasmacytoid dendritic cells (pDCs) were isolated from buffy coats obtained from the New York Blood Center. pDCs were purified from human peripheral blood mononuclear cells (PBMC) using a CD304 (BDCA-4) Microbead Kit (Miltenyi Biotec). The purity of the enriched pDC population was >97%, as assessed by CD123 and BDCA-2 staining. Freshly isolated pDCs were treated with 10 μg/ml of sH53 in the presence of endotoxin-free recombinant CpG-A (ODN 2216; Invivogen) at 500 ng/ml for 18 h. Recombinant HIV-1 gp120 and HIV-1 p24 proteins (ImmunoDiagnostics Inc.) served as positive and negative controls, respectively. Culture supernatants were collected for quantification of alpha interferon (IFN-α) by ELISA, according to the manufacturer's instructions (PBL Biomedical Laboratories).
HA1 antigen microarray.
An HA1 antigen microarray was done as described previously (15). Briefly, commercially available HA1 proteins of H1, H2, H3, and H5 influenza A viruses produced in HEK293 cells and purified by His tag purification (Immune Technology, New York, NY) (see Fig. 7 for strain information on the HA1 antigens used) were spotted on 16-pad nitrocellulose-coated slides (Oncyte Avid; Grace Bio-Labs, Bend, OR). The slides were treated with Blotto blocking buffer to avoid nonspecific binding to the nitrocellulose surface (Thermo Fisher Scientific Inc., Rockford, MA), washed, and incubated with serial 4-fold dilutions of serum ranging from 1:20 to 1:327,680 in Blotto containing 0.1% Surfact-Amps 20 (Thermo Fisher Scientific Inc.). After washing, goat anti-mouse IgG (Fc fragment specific) conjugated with Dylight 649 fluorescent dye (Jackson Immuno Research, West Grove, PA) was added. The slides were scanned using a ScanArray Gx Plus microarray scanner (PerkinElmer), and titers were defined as the interpolated serum concentration that provoked a response halfway on a concentration-response curve between the minimum and maximum signals, as described previously (15). Titers lower than the smallest serum dilution (1:20) were set to 20.
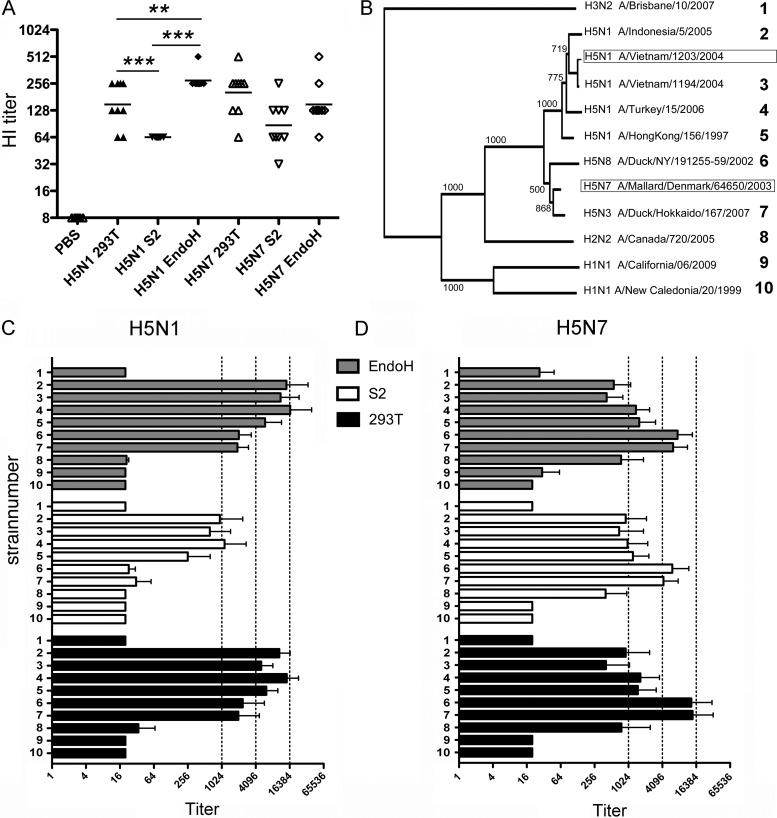
Fig 7.
Profiling HA-glycan-dependent induction of antibodies by using HA1 microarrays. (A) Mice (nine per group) were immunized twice with sH53N1 or sH53N7 protein preparations produced in HEK293T cells (293T) or insect S2 cells (S2) or with HEK293S GnTI(−) cell-produced proteins treated with EndoH (EndoH). Blood samples were taken 3 weeks after the second immunization. HI titers in serum against 4 HAU of sH53 for each mouse are shown, corresponding to the immunogen used. The horizontal lines represent the geometric means per group. Significant differences between groups are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) An unrooted protein tree (neighbor joining) was generated from a sequence alignment (ClustalX 1.8; standard settings) of the HA1 domains, comprising the sequence from the signal cleavage site to the HA1-HA2 proteolytic cleavage site of the 12 indicated virus strains (numbers on right). Bootstrap values (1,000 replicates) are indicated on the branches. The HA proteins that were used as immunogens are boxed; the HA1 domains of the other HA proteins were spotted on the HA microarray. (C and D) A panel of recombinant HA1 domains of HA proteins derived from different viruses (see panel B for strain information) spotted in an HA1 antigen microarray format was probed with sera of mice immunized with sH53N1 (C) or sH53N7 (D). The titers were defined as the interpolated serum concentration that provoked a response halfway on a concentration-response curve between the minimum and maximum signals. Titers lower than the smallest serum dilution (1:20) were set to 20. The error bars indicate standard deviations.
Statistical analysis.
Significance among animal groups was analyzed by Student's t test or by one-way analysis of variance (ANOVA) and Tukey test after ANOVA. Differences were considered significant at a P value of <0.05.
RESULTS
HA-glycan-dependent induction of antibodies against sH53N1.
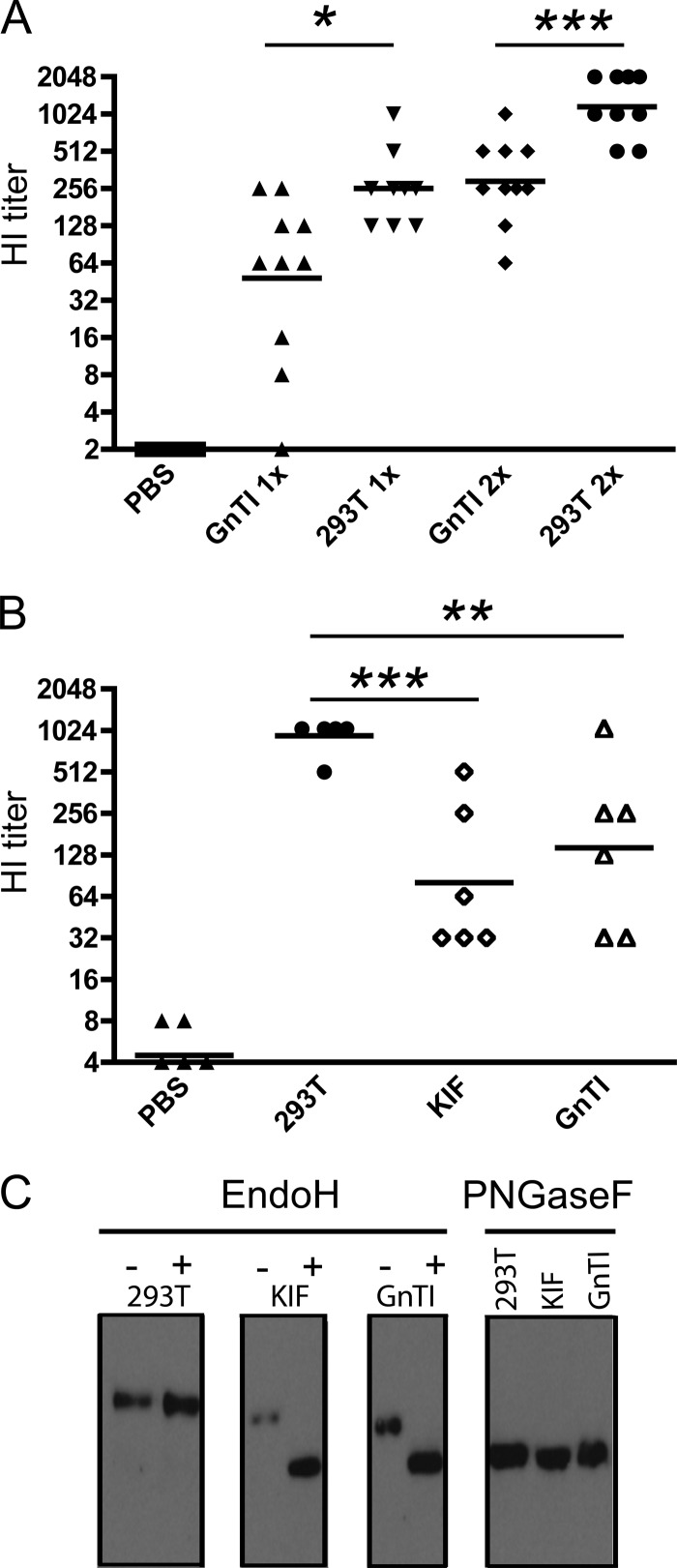
Previously, we showed that recombinant soluble trimeric HA proteins can protect chickens, mice, pigs, and ferrets against a (lethal) challenge with the homologous IAV. The recombinant proteins used to vaccinate the animals were produced in HEK293T cells (4, 17) or in HEK293S GnTI(−) cells, which are deficient in N-acetylglucosaminyltransferase I (GnTI) activity, resulting in immature HA glycoproteins containing Man5GlNAc2 glycans (5, 26). During one of these vaccination-challenge experiments in which chickens were vaccinated with 2 μg sH53N1 produced in HEK293S GnTI(−) cells (5), a parallel experiment was performed in which other chickens were vaccinated twice with the same amount of recombinant protein produced in HEK293T cells. These recombinant proteins differ only in their glycosylation states. While HEK293T cell-produced HA protein carries complex glycans, the HA proteins made in the HEK293S GnTI(−) cells carry high-mannose oligosaccharides. While none of the chickens that were vaccinated twice with 2 μg sH53N1 died or showed symptoms indicative of influenza-related disease after challenge by intranasal/intratracheal inoculation of a lethal dose of A/Vietnam/1194/04 virus (reference 5 and data not shown), differences were observed in HI antibody titers, depending on the cells from which the recombinant proteins were derived (Fig. 1A). Although all immunized animals developed appreciable HA antibody titers both after the first vaccination and after the boost, HI titers were significantly higher after vaccination with HEK293T cell-derived sH53N1 than after vaccination with HEK293S GnTI(−) cell-derived HA protein. The mock-immunized chickens had an HI titer below the detection limit.
Fig 1.
HA-glycan-dependent induction of antibodies against sH53N1. (A) Chickens (10 per group) were immunized twice with purified sH53N1 protein preparations produced in HEK293T cells (293T) or HEK293S GnTI(−) cells (GnTI). As a control, chickens were mock treated (PBS). Blood samples were taken 3 weeks after the first and after the second vaccinations. HI titers against 8 HAU sH53N1 in serum for each bird are shown. (B) Mice (6 per group) were immunized twice with sH53N1 protein preparations produced in HEK293T cells (293T), in HEK293T cells treated with 100 μM KIF (KIF), or in HEK293S GnTI(−) cells (GnTI). Blood samples were taken 3 weeks after the second immunization. HI titers against 8 HAU sH53N1 in serum for each mouse are shown. (A and B) The horizontal lines represent the geometric means per group. Significant differences between groups (panel A, Student's t test; panel B, ANOVA) are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) Purified sH53N1 protein preparations were analyzed by SDS-PAGE, followed by Western blotting. Where indicated, the proteins were treated with EndoH or PNGaseF prior to electrophoresis. The recombinant proteins were detected using a mouse anti-Strep tag antibody (29).
To confirm and extend these observations, we switched to another animal model. Thus, mice were immunized twice with 4 μg of purified sH53N1 produced in HEK293T or in HEK293S GnTI(−) cells. In addition, another group was taken along, in which mice were immunized with sH53N1 produced in HEK293T cells in the presence of 100 μM kifunensine (KIF). KIF is a potent and selective inhibitor of class I α-mannosidases (11). Both the HEK293S GnTI(−) cell-produced HA protein and the HA protein produced in HEK293T cells in the presence of KIF carry high-mannose glycans containing either 5 or 9 mannoses, respectively. Three weeks after the second immunization, blood samples were taken and HA antibody titers in the serum were determined. The results are shown in Fig. 1B. Again, the HA proteins expressed in HEK293T cells in the absence of KIF and thus containing complex glycans induced the highest HI antibody titer. The mice immunized with the protein preparations containing the high-mannose glycans displayed significantly lower HI antibody titers. No appreciable HI antibody titers were detected after mock immunization. HI antibody titers measured in mice were previously shown to correlate with protection from lethal influenza virus infection (5).
To confirm their different glycosylation states, the protein preparations were subjected to gel electrophoresis (Fig. 1C). The sH53N1 proteins carrying the high-mannose glycans migrated somewhat faster in the gel than the HEK293T cell-produced HA, with the HEK293S GnTI(−) cell-produced HA protein, which carries the smallest N-glycan side chains, demonstrating the highest electrophoretic mobility. As expected, only the proteins with high-mannose oligosaccharides were sensitive to EndoH, in agreement with the known specificity of this endoglycosidase (18). When the HA proteins derived from the different expression systems were treated with PNGaseF, an enzyme that removes all N-linked glycans, they migrated with similar mobilities in the gel, consistent with the HA proteins having identical protein backbones and only differing in their N-linked glycosylation.
HA-glycan-dependent induction of antibodies against sH53N7.
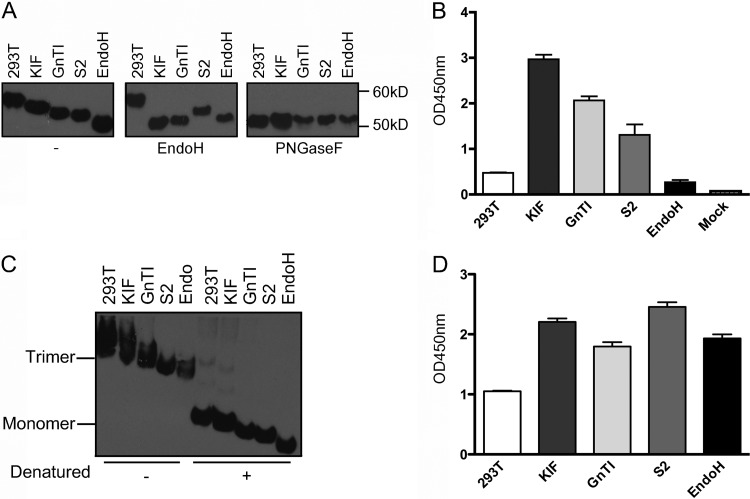
Next, we investigated whether the detrimental effect of terminal mannose moieties on the immune response was related to the specific HA or whether it could also be observed by using a different HA protein. Therefore, soluble trimeric H5 from a different H5 virus, H5N7 (sH53N7), was prepared. This H5 protein carries one oligosaccharide side chain less than sH53N1 (Fig. 2). In addition, we expanded our set of protein preparations by including HEK293S GnTI(−) cell-produced HA protein treated with EndoH, which results in an HA protein that carries a monosaccharide GlcNAc side chain, and HA protein produced in S2 insect cells, which carries paucimannosidic N-glycans with 3 mannose moieties (14). The glycoproteins were analyzed by gel electrophoresis, as shown in Fig. 3. The electrophoretic mobilities of the glycoproteins differed according to their differential glycosylation. Thus, while HEK293T cell-produced HA displayed the lowest electrophoretic mobility, consistent with sH53N7 carrying large, complex glycans, the highest mobility was observed for the EndoH-treated protein, which carries only single GlcNAc residues. The terminal-mannose-containing HA proteins migrated in line with the sizes of their glycans [9, 5, and 3 mannoses for HEK293T cell-produced HA in the presence of KIF, for HEK293S GnTI(−) cell-produced HA, and for S2 cell-produced HA, respectively (14)]. Of these HA glycoproteins, the H5 protein produced in insect cells was resistant to treatment with EndoH, as EndoH removes only N-linked sugars containing more than three mannose moieties (18). When the HA proteins were deglycosylated using PNGaseF, they all comigrated in the gel, consistent with having identical polypeptide backbones irrespective of the cell type from which they originated.
Fig 2.
Structure representation of H5 derived from H5N1 with complex N-glycans attached at its N-glycosylation sites. The protein structure (black) was created with Protein Data Bank ID code 2IBX, and the N-linked glycans (gray) were modeled with Glyprot (3). For comparison, a model (labeled H5N1) is shown in which the N-linked glycan side chain that is missing in H5 from H5N7, compared to HA derived from H5N1, has been deleted.
Fig 3.
Analysis of different sH53N7 preparations. sH53N7 proteins produced in HEK293T cells (293T), HEK293T cells treated with KIF (KIF), or HEK293S GnTI(−) cells (GnTI) or in insect S2 cells (S2), and HEK293S GnTI(−) cell-produced sH53N7 treated with EndoH (EndoH) were purified and analyzed. (A) The HA proteins were analyzed by SDS-PAGE, followed by Western blotting. The proteins were detected using a mouse anti-Strep tag antibody (29). Where indicated, samples were treated with PNGaseF or EndoH prior to electrophoresis. (B) Binding of Fc-tagged DC-SIGN (2.5 μg/well) to wells coated with the different HA preparations (5 μg/well) or with no HA protein (Mock). Binding of DC-SIGN was detected using HRP-labeled goat-anti-human IgG. (C) Blue Native-PAGE analysis of the HA protein preparations. The positions in the gel of the trimeric and monomeric protein species are indicated, as well as samples that were denatured by heating them for 1 min at 95°C prior to electrophoresis. (D) sH53 proteins were complexed with HRP-conjugated mouse antibody directed against the Strep tag prior to their application in a fetuin binding assay. HA-fetuin binding is shown at an HA concentration of 2.5 μg/ml. Standard deviations are indicated by the error bars.
To further confirm the different glycosylation states of our H5 preparations, we analyzed the interaction of sH53N7 with DC-SIGN, which is known to interact with carbohydrates carrying terminal mannose moieties (35). After 96-well plates were coated with HA, binding of DC-SIGN to HA was determined using an ELISA-type assay. While no appreciable DC-SIGN binding was observed with HA carrying complex glycans or single GlcNAc residues, binding to HA proteins carrying terminal mannoses was readily observed (Fig. 3B). The extent of binding of DC-SIGN to the different terminal mannose-containing HA preparations correlated with published data (32).
As mammalian-cell-derived HA trimers were found to induce higher levels of neutralizing antibodies than similarly produced monomeric HA protein (37), we also analyzed the oligomeric nature of the purified HA proteins produced by the different expression systems by using Blue Native PAGE. As shown in Fig. 3C, all proteins migrated at the high position in the gel that corresponds to their trimeric nature (5). When the H5 preparations were heat denatured, the initially trimeric HA species dissociated into monomers. Subsequently, we demonstrated that the sH53N7 proteins are biologically active, as the different H5 preparations displayed efficient binding to the sialylated glycoprotein fetuin (Fig. 3D), although some glycosylation-dependent differences were observed, in agreement with our previous study (9).
Mass spectrometry.
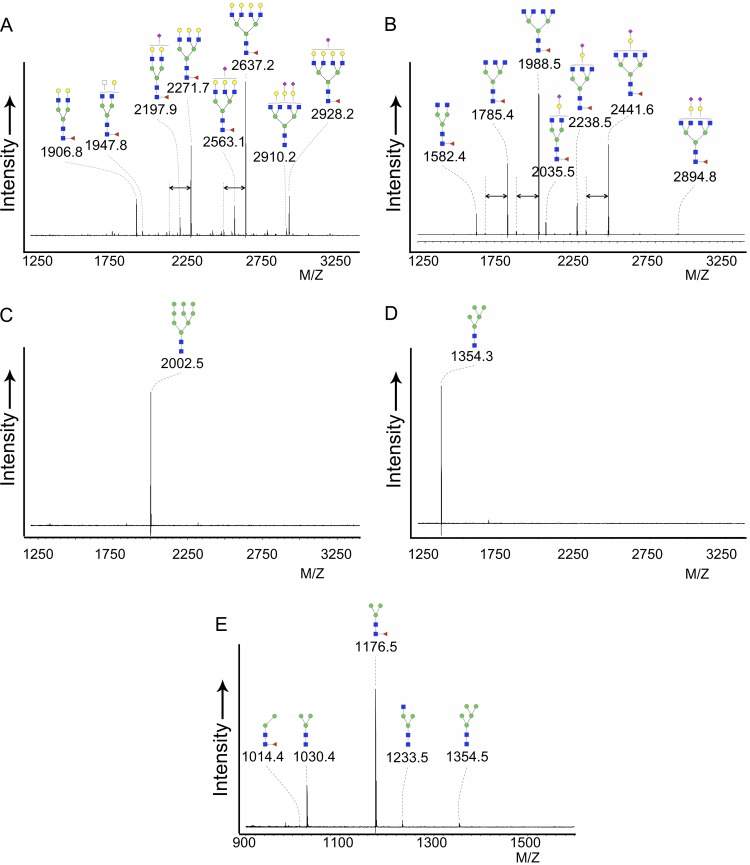
Finally, the oligosaccharides attached to the sH53N7 proteins were analyzed using mass spectrometry. To this end, glycans were enzymatically released from the glycoprotein using PNGaseF and analyzed by MALDI-TOF mass spectrometry after purification and labeling with 2-AA. To each of the peaks within the resulting spectra, a monosaccharide composition was assigned based on the monoisotopic mass, from which a putative structure was deduced, as indicated in Fig. 4. The glycans derived from HEK293T cell-produced HA (Fig. 4A) gave rise to a relatively complex mass spectrum, with major signals suggesting the presence of di-, tri- and tetra-antennary complex-type glycans with N-acetyllactosamine [Gal(β1-4)GlcNAc(β1-)] antennae. Signals representing extensions of these glycans with one or more N-acetylneuraminic acids and/or carrying a fucose at the reducing-end GlcNAc were also observed. These putative assignments were supported by β-galactosidase treatment, which led to the loss of all nonsialylated galactoses, thereby confirming the number of antennae in each glycan and the absence of possible linear N-acetyllactosamine repeats (Fig. 4B). For example, the AA-labeled fucosylated tetra-antennary glycan with the composition Hex7HexNAc6Fuc1 (Hex, hexose; HexNAc, N-acetylhexosamine; Fuc, fucose) observed at m/z 2,637.2 [M-H]− (Fig. 4A) disappeared after β-galactosidase treatment, giving rise to a new signal at m/z 1,988.5 (Fig. 4B) representing the Hex3HexNAc6Fuc1 species formed by the loss of four galactose residues from the four N-acetyllactosamine antennae in the nontreated glycan.
Fig 4.
Mass spectrometric glycan analysis of different sH53N7 preparations. Shown are MALDI-TOF mass spectra of the N-glycans of purified sH53N7 protein preparations produced in HEK293T cells (A and B), HEK293T cells treated with KIF (C), HEK293S GnTI(−) cells (D), and insect S2 cells (E). N-glycans were released by PNGaseF, labeled with anthranilic acid, and analyzed in negative-ion reflectron mode. All signals were labeled with monoisotopic masses, and the structures were deduced from the composition indicated by this mass. In the case of the HEK293T sample (A), only the 10 signals with the highest abundance were labeled with assigned structures; these structures were confirmed by beta-galactosidase treatment of the HEK293T sample (B), and in each case, the number of terminal galactose residues present is indicated. Compared to the spectrum before treatment (A), a clear loss of all galactoses not carrying N-acetylneuraminic acid is visible, indicating a high abundance of terminal galactose. The double-headed arrows indicate a difference in fucose content. Red triangles, fucose; purple diamonds, N-acetylneuraminic acid; yellow circles, galactose; blue squares, N-acetylglucosamine; green circles, mannose; white square, N-acetylhexosamine.
The spectrum of the released N-glycans of HA produced in HEK293T cells treated with KIF (Fig. 4C) showed only one peak at m/z 2,002.5 [M-H]−, which corresponded to the high-mannose N-glycan carrying 9 mannoses (Hex9HexNAc2), in line with the inhibitory effect of KIF on glycan processing. The spectrum of the HEK293S GnTI(−) cell-produced HA also gave only one high-abundance signal at m/z 1,354.3 [M-H]− with the composition Hex5HexNAc2, corresponding to a high-mannose peak carrying 5 mannose moieties (Fig. 4D). This is in agreement with expectations, since the absence of GnTI activity prevents the addition of GlcNAc to the α1-3-linked core mannose, thereby blocking further processing of the glycan. The majority of the N-glycans released from S2 cell-produced HA were of the paucimannosidic type (Fig. 4E). Major signals with the compositions Hex3HexNAc2Fuc1 and Hex3HexNAc2 were observed at m/z 1,176.5 [M-H]− and m/z 1,030.4 [M-H]−, respectively, indicating the presence of the trimannosyl core glycan with or without a fucose linked to the innermost GlcNAc residue. In addition, some minor signals were observed at m/z 1,014.4 [M-H]− (Hex2HexNAc2Fuc1), at m/z 1,233.5 [M-H]− (Hex3HexNAc3), and at m/z 1,354.5 [M-H]− (Hex5HexNAc2). This observation is in line with previously reported N-glycan structures on recombinant proteins expressed in S2 cells (14).
Induction of antibodies against sH53N7.
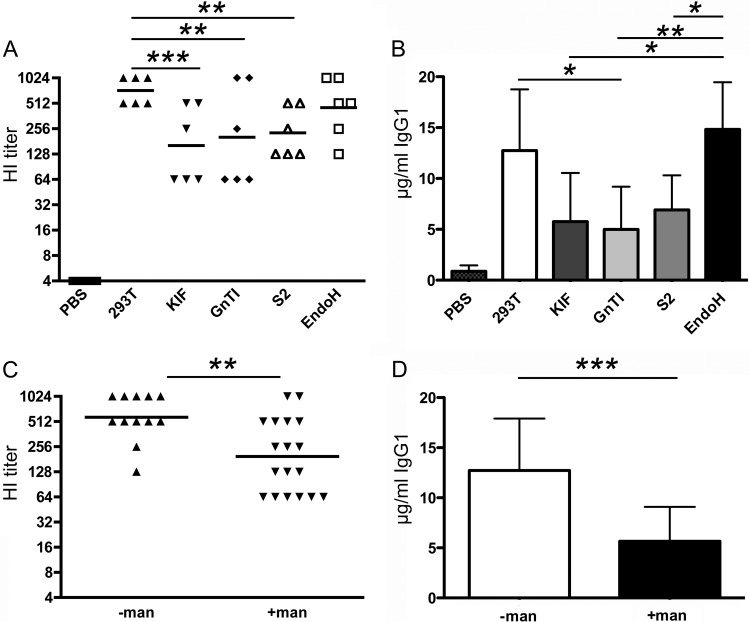
After having analyzed the glycosylation states of the different sH53N7 protein preparations, the HA proteins were tested for the ability to induce HI antibodies. To this end, mice were immunized twice with the different HA preparations, after which the HI serum antibody titers were determined. The results are shown in Fig. 5. In agreement with the previous experiment, mice vaccinated with HA proteins carrying terminal mannose moieties displayed significantly lower HI antibody titers than mice immunized with HA proteins carrying complex glycans. No apparent differences were observed in the HI antibody titers induced by HA proteins containing glycans with 9, 5, or fewer mannose moieties. Removal of the high-mannose glycans from the HA proteins produced in HEK293S GnTI(−) cells by EndoH treatment appeared to result in higher HI antibody titers, although this difference was not significant. Very similar results were observed when HA-specific IgG1 levels were determined using ELISA (Fig. 5B). When the data were pooled based on the absence (HEK293T cell-produced HA and EndoH-treated HA) or presence [HA produced in HEK293T cells treated with KIF, HEK293S GnTI(−) cell-produced HA, and S2 cell-produced HA] of terminal mannose moieties, again, significantly lower HI antibody titers and HA-specific IgG1 levels were observed for the mice immunized with HA proteins carrying mannose moieties (Fig. 5C and D).
Fig 5.
HA-glycan-dependent induction of antibodies against sH53N7. (A) Mice (six per group) were immunized twice with sH53N7 protein preparations produced in HEK293T cells (293T), HEK293T cells treated with KIF (KIF), HEK293S GnTI(−) cells (GnTI), or insect S2 cells or with HEK293S GnTI(−) cell-produced sH53N7 treated with EndoH (EndoH). Blood samples were taken 3 weeks after the second immunization. HI titers against 4 HAU sH53N7 in serum for each mouse are shown. (B) HA-specific IgG1 levels (μg per ml serum) were determined by ELISA. (C) Graph displaying the data shown in panel A pooled on the basis of the absence [−man; HEK293T cell-produced HA and EndoH-treated HA produced in HEK293S GnTI(−) cells] or presence [+man; HA produced in HEK293T cells treated with KIF, HEK293S GnTI(−) cell-produced HA, and S2 cell-produced HA] of terminal mannose residues in the sH53N7 protein preparations for which data are shown. (D) Similar to panel C, but showing the HA-specific IgG1 levels. The horizontal lines in the scatter dot plots represent geometric means per group. Significant differences between groups (panels A and B, ANOVA; panels C and D, Student's t test) are indicated as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Terminal-mannose-containing H5 proteins impair CpG-induced IFN-α production by pDCs.
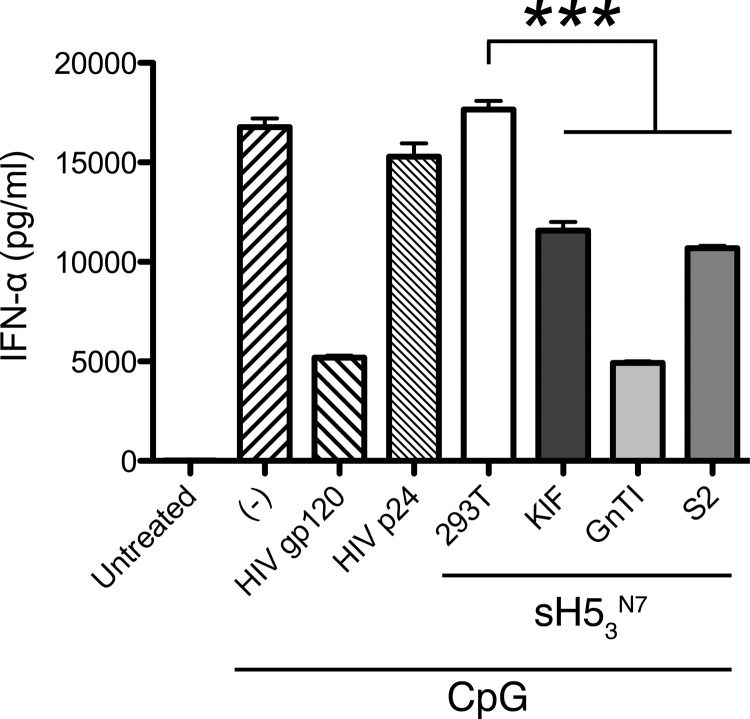
Mannoses on HIV gp120 have been reported to induce immunosuppression (1, 3), and it has been suggested that this is in part caused by suppression of dendritic cell (DC) function (19, 30). To examine the effects of terminal-mannose-containing HAs on DC function, we treated pDCs with sH53N7 proteins produced in HEK293T, KIF-treated HEK293T, HEK293S GnTI(−), and S2 cells in the presence of the TLR9 agonist CpG. As previously described, HIV gp120 suppressed IFN-α release by pDCs upon CpG stimulation, presumably through binding to the C-type lectin receptor BDCA-2 (19) (Fig. 6). HAs produced from KIF-treated HEK293T, GnTI(−), and S2 cells also significantly inhibited CpG-induced IFN-α release, by 32%, 71%, and 36%, respectively (Fig. 6). In contrast, HA derived from HEK293T cells and HIV-1 p24 Gag protein had no inhibitory effect. These data suggest that HA proteins carrying terminal mannoses, but not those carrying complex glycans, may suppress DC function.
Fig 6.
Inhibition of CpG-induced IFN-α production by HA. Freshly isolated pDCs were treated with sH53N7, produced in HEK293T, KIF-treated HEK293T, HEK293S GnTI(−), and S2 cells, in the presence of CpG. The culture supernatants were collected after 18 h for IFN-α quantification by ELISA. The data shown are from one representative experiment out of four independent experiments with pDCs from four different donors. Significant differences between groups treated with the different HA protein preparations are indicated: ***, P < 0.001.
Comparison of HA-glycan-dependent induction of antibodies against sH53N1 and sH53N7.
Wang and coworkers recently reported that antibodies raised against H5 protein bearing single N-linked GlcNAc side chains showed stronger binding to different native HA proteins and stronger virus neutralization than sera raised against H5 proteins carrying complex glycans (34). As the HI serum titers of mice immunized with sH53N7 preparations with complex glycans were quite similar to those obtained with preparations with single GlcNAc side chains (Fig. 5A), we performed a new experiment in which we analyzed antisera raised against differently glycosylated sH53N7 and sH53N1 preparations. To this end, mice were immunized twice with sH53N1 or sH53N7 preparations that carry either complex (HA produced in 293T cells) or paucimannose (HA produced in insect cells) glycans or single N-linked GlcNAc side chains [EndoH-treated samples from GnTI(−) cells], after which the HI serum antibody titers were determined using the homologous sH53N1 or sH53N7 protein. In agreement with the previous experiments, lower HI titers were obtained with sera from mice vaccinated with the HA proteins carrying paucimannose glycans than from sera from mice vaccinated with the HA proteins carrying complex glycans, although the differences were significant only for the sH53N1 preparations (Fig. 7A). Immunization of mice with HA proteins carrying single GlcNAc residues resulted in HI titers that were comparable to (sH53N7) or somewhat higher than (sH53N1) those induced by their counterparts carrying complex glycans.
Finally, we analyzed whether the antisera raised against the different H5 preparations differed in their reactivities against IAVs of various serotypes. The sera were tested using a recently developed HA1 protein array (15) against a panel of HA1 domains. In agreement with the evolutionary relationship between the HA proteins on the array and the HA proteins used for immunization (Fig. 7B), sera raised against sH53N1 exhibited higher reactivity against HA1 proteins derived from other H5N1 viruses than against those from H5N3 or H5N8 virus (Fig. 7C), while they reacted poorly with the HA1 proteins from the H1, H2, and H3 viruses. The pattern of reactivities of the sera raised against the sH53N7 proteins was different. While these sera again reacted preferentially against the HA1 proteins from the H5 viruses, particularly the H5N8 and H5N3 viruses, they also displayed reactivity against the HA1 domains of the H2 subtype. The results, furthermore, show that the breadth of the reactivity of the sera was not affected by the glycosylation states of the immunogens used. The sera raised against the insect cell-derived sH53N1 appeared somewhat less broadly reactive than the sera raised against the other sH53N1 preparations; however, this is probably explained by the lower antibody response induced in mice immunized with the insect cell sH53N1 (Fig. 7A). Very similar reactivity profiles were observed regardless of whether mice were immunized with sH53N7 or sH53N1 preparations with side chains consisting of complex glycans or single GlcNAc residues (Fig. 7C).
DISCUSSION
Recombinant soluble trimeric HA proteins provide an attractive new means of vaccination against IAV. Glycosylation, an important factor in the folding and (antigenic) structure of the HA protein, as well as in interaction with lectins of the immune system, is known to be affected by the specific expression platform used. Hence, it was of interest to study whether and to what extent the immunogenicity of recombinant soluble HA proteins is influenced by their glycosylation state. In this study, we utilized three different cell lines and different glycan-modifying enzymes/drugs to produce different sets of soluble trimeric H5 antigens that differ only in their glycosylation states. HA proteins produced in HEK293T cells were shown to contain different complex-type glycans. When the HA-producing HEK293T cells were treated with KIF, the HA proteins carried only high-mannose N-glycans with 9 mannoses. HA proteins produced in HEK293S GnTI(−) cells were found to contain only high-mannose glycans containing 5 mannose moieties, while insect S2 cell-produced HA contained different types of paucimannosidic glycans. Treatment of HA protein produced in HEK293S GnTI(−) cells with EndoH resulted in HA proteins carrying single GlcNAc residues. Chickens or mice immunized with these different purified HA preparations displayed different levels of HI antibody titers, indicating that the glycosylation state of recombinant HA antigens indeed affects their immunogenicity and is a factor of significant importance when developing vaccines based on recombinant HA proteins and probably more general vaccines based on glycoprotein antigens.
Our results indicate that HA proteins carrying terminal mannose moieties induce lower HI antibody titers in chickens and mice than HA proteins carrying complex glycans. In a previous study, Wei and coworkers reported that recombinant HA proteins produced in mammalian cells are comparably or slightly more effective in eliciting neutralizing antibodies than their insect cell-derived counterparts (36). However, the immunogenicities of their different HA preparations were not compared directly. Furthermore, a different adjuvant, mouse strain, and HA dose were used than in our study. Consistent with our results, increased immunogenicity has also been observed for HIV gp120 when terminal mannose moieties, which are abundantly present on gp120 proteins expressed in mammalian cells, are removed (1). In another study, the antibody response against gp120 was shown to be enhanced by occluding the mannose moieties on gp120 with griffithsin (2). It was demonstrated that the terminal mannose moieties of the N-linked glycans of HIV gp120 induce immunosuppressive responses in dendritic cells, which correlated with DC-SIGN expression on these cells (30). As the HA preparations carrying terminal mannose moieties exhibited efficient binding to DC-SIGN in in vitro experiments while these protein preparations also suppressed CpG-induced IFN-α release, a similar mechanism may apply for soluble trimeric HA antigen preparations. Alternatively, a role for other mannose-binding lectins, for example, in clearance of antigens with terminal-mannose-containing glycans, cannot be excluded (16).
The acquisition of N-linked glycosylation sites near antigenic sites may sterically impair antibody binding to these sites (glycan shielding) (31). The HIV gp160 protein provides a clear example of hyperglycosylation as an effective immune escape mechanism (24). Glycan shielding of epitopes has also been demonstrated for HA (6, 20, 31). However, in contrast to the HIV gp160 protein, the HA proteins are not hyperglycosylated (7, 33). This is probably due to interference of HA glycosylation with receptor binding (9, 21, 34), thereby creating a fitness barrier to accumulating glycosylation sites and providing a ready explanation for the paucity of N-linked glycan side chains compared, for example, with HIV gp160 (6). However, Wang and coworkers (34) recently reported that antisera raised against H5 protein derived from an H5N1 virus bearing only single N-linked GlcNAc side chains showed higher IAV neutralization titers than antisera elicited against HA containing complex glycans. In agreement with these results, we also observed higher HI titers in sera of mice immunized with sH53N1 proteins bearing only single N-linked GlcNAc side chains than with those carrying complex glycans. However, the HI titers observed for the sH53N7 preparations were not similarly affected. As the HA proteins in the latter preparations carry 5 rather than 6 N-glycan side chains, these results may indicate that the positive effect of the EndoH treatment is larger for HA proteins carrying a higher number of N-linked side chains. Alternatively, the position of the additional N-glycan side chain in sH53N1 at the head of the HA protein (Fig. 2), close to the receptor binding site, may be of particular importance.
As the HA protein sequences around the glycosylation sites generally show less variation (6, 34), removal of (the larger part of) the glycan side chain may be expected to result in immunogens that induce broader immunoreactivity than their fully glycosylated counterparts. The glycosylation state of the HA proteins, however, did not affect the breadth of the antibody response as measured with an HA1 antigen microarray. It will be of interest to study whether trimming of the glycan side chains results in a broader immune response directed against the HA2 domain, which is more conserved than HA1. Previously, Wang and coworkers (34) reported that immunization with HA proteins caring single GlcNAc residues induced higher reactivity against HA proteins of another subtype than HA protein with complex glycans. However, as these HA preparations also induced higher reactivity against the homologous protein, the possibility that the higher reactivity against the nonhomologous HA proteins resulted from intrinsically higher immunogenicity of their HA preparations with single GlcNAc residues cannot be excluded.
Our results show that the immunogenicity of recombinant HAs is affected by their glycosylation state and thus by the expression system used to generate these antigens. Importantly, HAs carrying terminal mannose moieties, such as those produced in insect cells, were shown to induce lower HI antibody titers than other HA preparations. Although our data do not indicate that antisera raised against HA preparations carrying single GlcNAc side chains are more broadly reactive, at least against the HA1 domain, we conclude that the glycosylation state of recombinant antigens is a factor of significant importance when developing glycoprotein-based vaccines, such as recombinant HA proteins.
ACKNOWLEDGMENTS
We thank Willem Bartelink and Berend Jan Bosch for technical assistance; Matthijs Raaben, Sabine Versteeg, and Helma Avezaat for their help with the animal experiments; and Marius Dwars and Mieke Matthijs for providing chicken erythrocytes.
This work has been financially supported by the Economic Structure Enhancing Fund “Impulse Veterinary Avian Influenza Research in The Netherlands.” The research was supported in part by NIH grants AI36082 and AI45463 to J.P.M. R.P.D.V. is the recipient of a Rubicon grant from the Netherlands Organization for Scientific Research (NWO). R.W.S. is the recipient of a Vidi grant from the NWO and a Starting Investigator Grant from the European Research Council (ERC-StG-2011-280829-SHEV). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 22 August 2012
REFERENCES
- 1. Banerjee K, et al. 2009. Enzymatic removal of mannose moieties can increase the immune response to HIV-1 gp120 in vivo. Virology 389:108–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Banerjee K, et al. 2012. Occluding the mannose moieties on human immunodeficiency virus type 1 gp120 with griffithsin improves the antibody responses to both proteins in mice. AIDS Res. Hum. Retroviruses 28:206–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bohne-Lang A, von der Lieth CW. 2005. GlyProt: in silico glycosylation of proteins. Nucleic Acids Res. 33:W214–W219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bosch BJ, et al. 2010. Recombinant soluble, multimeric HA and NA exhibit distinctive types of protection against pandemic swine-origin 2009 A(H1N1) influenza virus infection in ferrets. J. Virol. 84:10366–10374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cornelissen LA, et al. 2010. A single immunization with soluble recombinant trimeric hemagglutinin protects chickens against highly pathogenic avian influenza virus H5N1. PLoS One 5:e10645 doi:10.1371/journal.pone.0010645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Das SR, et al. 2011. Fitness costs limit influenza A virus hemagglutinin glycosylation as an immune evasion strategy. Proc. Natl. Acad. Sci. U. S. A. 108:E1417–E1422 doi:10.1073/pnas.1108754108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Das SR, et al. 2010. Glycosylation focuses sequence variation in the influenza A virus H1 hemagglutinin globular domain. PLoS Pathog. 6:e1001211 doi:10.1371/journal.ppat.1001211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Deshpande KL, Fried VA, Ando M, Webster RG. 1987. Glycosylation affects cleavage of an H5N2 influenza virus hemagglutinin and regulates virulence. Proc. Natl. Acad. Sci. U. S. A. 84:36–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Vries RP, et al. 2010. The influenza A virus hemagglutinin glycosylation state affects receptor-binding specificity. Virology 403:17–25 [DOI] [PubMed] [Google Scholar]
- 10. Durocher Y, Butler M. 2009. Expression systems for therapeutic glycoprotein production. Curr. Opin. Biotechnol. 20:700–707 [DOI] [PubMed] [Google Scholar]
- 11. Elbein AD, Tropea JE, Mitchell M, Kaushal GP. 1990. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J. Biol. Chem. 265:15599–15605 [PubMed] [Google Scholar]
- 12. Ellebedy AH, Webby RJ. 2009. Influenza vaccines. Vaccine 27(Suppl. 4):D65–D68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hanson SR, et al. 2009. The core trisaccharide of an N-linked glycoprotein intrinsically accelerates folding and enhances stability. Proc. Natl. Acad. Sci. U. S. A. 106:3131–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim YK, et al. 2005. Production and N-glycan analysis of secreted human erythropoietin glycoprotein in stably transfected Drosophila S2 cells. Biotechnol. Bioeng. 92:452–461 [DOI] [PubMed] [Google Scholar]
- 15. Koopmans M, et al. 2012. Profiling of humoral immune responses to influenza viruses by using protein microarray. Clin. Microbiol. Infect. 18:797–807 [DOI] [PubMed] [Google Scholar]
- 16. Lee SJ, et al. 2002. Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 295:1898–1901 [DOI] [PubMed] [Google Scholar]
- 17. Loeffen WL, et al. 2011. Vaccination with a soluble recombinant hemagglutinin trimer protects pigs against a challenge with pandemic (H1N1) 2009 influenza virus. Vaccine 29:1545–1550 [DOI] [PubMed] [Google Scholar]
- 18. Maley F, Trimble RB, Tarentino AL, Plummer TH., Jr 1989. Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal. Biochem. 180:195–204 [DOI] [PubMed] [Google Scholar]
- 19. Martinelli E, et al. 2007. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-{alpha} secretion in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 104:3396–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Munk K, et al. 1992. Carbohydrate masking of an antigenic epitope of influenza virus haemagglutinin independent of oligosaccharide size. Glycobiology 2:233–240 [DOI] [PubMed] [Google Scholar]
- 21. Ohuchi M, Ohuchi R, Feldmann A, Klenk HD. 1997. Regulation of receptor binding affinity of influenza virus hemagglutinin by its carbohydrate moiety. J. Virol. 71:8377–8384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohuchi M, Ohuchi R, Matsumoto A. 1999. Control of biological activities of influenza virus hemagglutinin by its carbohydrate moiety. Microbiol. Immunol. 43:1071–1076 [DOI] [PubMed] [Google Scholar]
- 23. Osterhaus A, Fouchier R, Rimmelzwaan G. 2011. Towards universal influenza vaccines? Philos. Trans. R. Soc. Lond. B Biol. Sci. 366:2766–2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pejchal R, et al. 2011. A potent and broad neutralizing antibody recognizes and penetrates the HIV glycan shield. Science 334:1097–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reading PC, Tate MD, Pickett DL, Brooks AG. 2007. Glycosylation as a target for recognition of influenza viruses by the innate immune system. Adv. Exp. Med. Biol. 598:279–292 [DOI] [PubMed] [Google Scholar]
- 26. Reeves PJ, Callewaert N, Contreras R, Khorana HG. 2002. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc. Natl. Acad. Sci. U. S. A. 99:13419–13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ruhaak LR, Steenvoorden E, Koeleman CA, Deelder AM, Wuhrer M. 2010. 2-Picoline-borane: a non-toxic reducing agent for oligosaccharide labeling by reductive amination. Proteomics 10:2330–2336 [DOI] [PubMed] [Google Scholar]
- 28. Safdar A, Cox MM. 2007. Baculovirus-expressed influenza vaccine. A novel technology for safe and expeditious vaccine production for human use. Expert Opin. Investig. Drugs 16:927–934 [DOI] [PubMed] [Google Scholar]
- 29. Schmidt TG, Skerra A. 2007. The Strep-tag system for one-step purification and high-affinity detection or capturing of proteins. Nat. Protoc. 2:1528–1535 [DOI] [PubMed] [Google Scholar]
- 30. Shan M, et al. 2007. HIV-1 gp120 mannoses induce immunosuppressive responses from dendritic cells. PLoS Pathog. 3:e169 doi:10.1371/journal.ppat.0030169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skehel JJ, et al. 1984. A carbohydrate side chain on hemagglutinins of Hong Kong influenza viruses inhibits recognition by a monoclonal antibody. Proc. Natl. Acad. Sci. U. S. A. 81:1779–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. van Liempt E, et al. 2006. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 580:6123–6131 [DOI] [PubMed] [Google Scholar]
- 33. Vigerust DJ, et al. 2007. N-linked glycosylation attenuates H3N2 influenza viruses. J. Virol. 81:8593–8600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang CC, et al. 2009. Glycans on influenza hemagglutinin affect receptor binding and immune response. Proc. Natl. Acad. Sci. U. S. A. 106:18137–18142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang SF, et al. 2008. DC-SIGN mediates avian H5N1 influenza virus infection in cis and in trans. Biochem. Biophys. Res. Commun. 373:561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wei CJ, et al. 2008. Comparative efficacy of neutralizing antibodies elicited by recombinant hemagglutinin proteins from avian H5N1 influenza virus. J. Virol. 82:6200–6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Weldon WC, et al. 2010. Enhanced immunogenicity of stabilized trimeric soluble influenza hemagglutinin. PLoS One 5:e12466 doi:10.1371/journal.pone.0012466 [DOI] [PMC free article] [PubMed] [Google Scholar]