Abstract
Breast feeding reduces the risk of developing severe respiratory syncytial virus (RSV) infections in infants. In addition to maternal antibodies, other immune-modulating factors in human milk contribute to this protection. Specific dietary prebiotic oligosaccharides, similar to oligosaccharides present in human milk, were evaluated in a C57BL/6 mouse RSV infection model. During primary RSV infection, increased numbers of RSV-specific CD4+ T cells producing gamma interferon (IFN-γ) were found in the lungs at days 8 to 10 postinfection in mice receiving diet containing short-chain galactooligosacharides, long-chain fructooligosaccharides, and pectin-derived acidic oligosaccharides (termed scGOS/lcFOS/pAOS). In a Th2-skewed formalin-inactivated (FI)-RSV vaccination model, the prebiotic diet reduced RSV-specific Th2 cytokine (interleukin-4 [IL-4], IL-5, and IL-13)-producing CD4+ T cells in the lung and the magnitude of airway eosinophilia at day 4 and 6 after infection. This was accompanied by a decreased influx of inflammatory dendritic cells (CD11b+/CD11c+) and increased numbers of IFN-γ-producing CD4+ and CD8+ T cells at day 8 after viral challenge. These findings suggest that specific dietary oligosaccharides can influence trafficking and/or effector functions of innate immune, CD4+, and CD8+ T cell subsets in the lungs of RSV-infected mice. In our models, scGOS/lcFOS/pAOS had no effect on weight but increased viral clearance in FI-RSV-vaccinated mice 8 days after infection. The increased systemic Th1 responses potentiated by scGOS/lcFOS/pAOS might contribute to an accelerated Th1/Th2 shift of the neonatal immune system, which might favor protective immunity against viral infections with a high attack rate in early infancy, such as RSV.
INTRODUCTION
Respiratory syncytial virus (RSV), a pneumovirus in the family Paramyxoviridae, infects nearly all children within the first 3 years of life (15). Primary RSV infections can cause severe bronchiolitis and pneumonia, which are associated with significantly increased risk of developing wheeze during childhood that lasts until teenage years (31, 45, 46). Symptomatic reinfections occur in every age group, but the frequency and severity of symptoms are highest in children below 5 years of age. The mechanism behind the onset of severe RSV infections is still not completely clear. Severe RSV infections that require hospitalization are most frequent in infants 2 to 4 months of age (44). Therefore, it has been proposed that inadequate innate or adaptive responses of the immature immune system contribute to disease severity; in particular, Th2 bias of the immature immune system has been suggested to be an important factor contributing to RSV disease (5, 36).
Formation of the intestinal microbiota population, starting directly after birth, is shaped during infancy and is unique for each individual throughout life (23, 37). The intestinal microbiota composition is important for establishment of gut homeostasis and affects local mucosal immunity (20). Although it has been documented that the host genetic background facilitates a core microbiome (52), factors like caesarian section, diet, and reduced microbial pressure in western countries shape host microbial populations (9, 32). There is increasing evidence that environmental factors and diet correlate with host immune function and disease susceptibility. The best-known examples are correlations found in allergy-related diseases like atopy and asthma, but a relatively new disease, like obesity, also appears to be linked to a specific composition of the intestinal microbiota (30, 47). This suggests that the microbial community and the host immune system continuously cross-communicate and reorganize, leading to a delicate balance. It is, however, largely unknown how exactly the intestinal bacterial community interferes with systemic immune processes. Some insight has come from studies of conventional specific-pathogen-free (SPF) animals, in which a microbiota depletion approach showed that the enhanced killing of Streptococcus pneumoniae and Staphylococcus aureus by bone marrow-derived neutrophils was regulated by bacterial peptidoglycans derived from the gut (8). This suggests that manipulation of microbiota can induce systemic priming of the innate immune system by systemic shedding of bacterial components that act as ligands on pattern recognition receptors.
Breast feeding reduces the risk of severe RSV bronchiolitis (6). In addition to maternal antibodies, human milk contains immune-modulating components, including oligosaccharides. Some nondigestible oligosaccharides are called prebiotics, because they stimulate the growth of commensal bacteria known to be beneficial to the host (38). It has been shown that infant formula including specific nondigestible carbohydrates, like short-chain galactooligosacharides (scGOS) and long-chain fructooligosaccharides (lcFOS), affects the incidence of upper respiratory tract infections and severity of asthma and lowers IgE antibody titers in atopic disease (1, 53, 54). In addition, in infants receiving scGOS, lcFOS, and pectin-derived oligosaccharides (pAOS), a preventive effect was found for development of atopic dermatitis (17).
In this study, the effect of a specific dietary intervention (termed scGOS/lcFOS/pAOS) with proven gut microbiota-modulating capacities in humans and mice (1, 16, 33, 56) is evaluated on virus-specific lung T cell responses in a C57BL/6 mouse model of primary RSV infection. Because earlier work in an influenza virus vaccination model has shown that prebiotic treatment induced a Th2→Th1 shift, we also tested the prebiotic diet in a formalin-inactivated (FI)-RSV vaccine model. This FI-RSV mouse model is an enhanced disease model whereby increased Th2 responses and lung eosinophilia are critical features of enhanced disease. Modulation of systemic immunity potentiated by these oligosaccharides might contribute to an accelerated Th1/Th2 shift of the neonatal immune system and thereby favors protective immunity against viral infections with a high attack rate in early infancy, such as RSV.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free 3- to 4-week-old female C57BL/6 mice (Charles River Nederland, Maastricht, The Netherlands) were housed under standard housing conditions. All animals had ad libitum access to tap water and diet. All study protocols were approved by the Animal Ethics Committee of the Medical Faculty of Utrecht University.
Diet.
AIN-93G-based diets were mixed with a specific oligosaccharide mixture (Research Diet Services, Wijk Bij, Duurstede, The Netherlands) containing 45% short-chain galactooligosacharide (scGOS; Borculo, Domo, Zwolle, The Netherlands), 100% long-chain fructooligosaccharide (lcFOS; Orafti, Wijchen, The Netherlands), and pectin-derived acidic oligosaccharides (pAOS; 5% galacturonic acid; Sudsucker, Mannheim, Germany) in a 9:1:10 ratio based on carbohydrate purity. This specific ratio is based on immune-modulating capacities in humans and mice described earlier (16, 55). The specific oligosaccharides were exchanged against 2% (wt/wt) total carbohydrates present in the control diet.
Virus and infection.
RSV strain A2 (VR-1302; ATCC) was grown on HEp-2 cells (CCL-23; ATCC) purified by polyethylene glycol 6000 precipitation and stored in phosphate-buffered saline (PBS) with 10% sucrose in liquid nitrogen until further use. Mice were anesthetized with isoflurane and intranasally (i.n.) infected with 4 × 106 PFU RSV in a volume of 50 μl diluted in PBS.
FI-RSV vaccine and vaccination.
FI-RSV was prepared by the method used for the original vaccine as tested in the 1960s in infants (39). The RSV A2 strain was grown for 48 h in HEp-2 cells. Culture medium was cleared from cell debris by low-speed centrifugation (1,000 × g, 10 min, 4°C). Formalin (F8775; Sigma-Aldrich) was added to 3 × 106 PFU RSV/ml containing supernatant at a final dilution of 1:4,000 and incubated at 37°C for 3 days with stirring. After ultracentrifugation (50,000 × g, 1 h, 4°C) of the FI-RSV preparation, resulting pellets were resuspended to 1/25 of the original volume in Iscove's modified Dulbecco's medium (IMDM; Gibco, Invitrogen) without supplements. FI-RSV was adsorbed to 4 mg/ml aluminum hydroxide (A1577; Sigma-Aldrich) overnight at room temperature while stirring. Finally, FI-RSV was pelleted by centrifugation (1,000 × g, 30 min) and resuspended to 1/4 volume in phosphate-buffered saline (PBS). This procedure resulted in a final dosage that was concentrated 100-fold and contained 16 mg/ml aluminum hydroxide. At day 0, mice were intramuscularly (i.m.) injected with 50 μl FI-RSV vaccine preparation.
Tissue isolation and preparation.
Mice were sacrificed at different time points, as indicated in the figure legends, by i.p. injection of 300 μl pentobarbital. Cells from the airways were obtained by bronchoalveolar lavage (BAL) with 3 washes with 1 ml of 0.15 M NaCl. Prior to removal, the lungs were perfused with PBS containing 100 U/ml heparin. Lungs were cut to 1- by 1-mm pieces and incubated with collagenase (2.4 mg/ml; 10103586001; Roche Applied Science) and DNase (1 mg/ml; 10104159001; Roche Applied Science) for 20 min at 37°C. Single-cell suspensions were prepared by processing the tissue through 70-μm cell strainers (BD Falcon, BD Biosciences).
RSV-specific real-time PCR.
For preparation of lung tissue homogenates, isolated snap-frozen whole lungs were crushed on dry ice, dissolved in PBS, and stored at −80°C until further use. Viral replication in lung homogenates was determined, as earlier described, via plaque assay (24). To measure total virus copy numbers by PCR, total RNA from lung homogenates was extracted using MagnaPure LC equipment, cDNA was synthesized, and viral loads were determined by real-time PCR as recently described (19). In short, viral genomic RNA was isolated using a MagnaPure LC total nucleic acid kit (Roche Diagnostics, Mannheim, Germany). The isolated viral RNA was reverse transcribed using a MultiScribe reverse transcriptase kit and random hexamers (Applied Biosystems, Foster City, CA) according to the manufacturer's guidelines. Primers and probes designed on the basis of highly conserved genomic regions of the N gene for both RSV subgroup A (RSV-A) and B (RSV-B) were used for subtyping of the RSV patient strains. The following primers and probes were used: RSA-1, 5′-AGATCAACTTCTGTCATCCAGCAA-3′; RSA-2, 5′-TTCTGCACATCATAATTAGGAGTATCAAT-3′; RSB-1, 5′-AAGATGCAAATCATAAATTCACAGGA-3′; RSB-2, 5′-TGATATCCAGCATCTTTAAGTATCTTTATAGTG-3′; RSA probe, 5′-CACCATCCAACGGAGCACAGGAGAT-3′; and RSB probe, 5′-TTCCCTTCCTAACCTGGACATAGCATATAACATACCT-3′.
Murine encephalomyocarditis virus (RNA) was used as an internal control. Samples were assayed in a 25-μl reaction mixture containing 10 μl of cDNA, TaqMan universal PCR master mix (Applied Biosystems, ABI), primers (900 nM RSV-A primers and 300 nM RSV-B primers), and fluorogenic probes (58.3 nM RSV-A probe and 66.7 nM RSV-B probe) labeled with the 5′ reporter dye 6-carboxyfluorescein (FAM) and the 3′ quencher dye 6-carboxy-tetramethyl-rhodamine (TAMRA). Amplification and detection were performed with an ABI 7900 HT system for 2 min at 50°C, 10 min at 95°C, and 45 cycles of 15 s at 95°C and 1 min at 60°C. Samples were controlled for the presence of possible inhibitors of the amplification reaction by the indicated internal control, signals of which had to range within a clear-cut interval. Sample cycle threshold values (CT) were compared to a standard curve of RSV-A2.
In vitro restimulation.
Isolated lung cells were restimulated with a synthetic peptide representing a dominant H-2-restricted RSV epitope or a dendritic cell (DC) line infected with RSV. For peptide stimulation, single-cell suspensions of lung cells (1 × 106 cells) were incubated with the H-2Db-restricted peptide (1 μg/ml) from the RSV M protein (M187-195; NAITNAKII) (28). Restimulation with RSV was accomplished by coculturing lung cells (1 × 106 cells) with RSV-infected D1 cells (2 × 105 cells). D1 is a nontransformed, growth factor-dependent, myeloid dendritic cell line derived from C57BL/6 mice (60). D1 cells were maintained in IMDM, 5% Hyclone fetal calf serum (FCS) (SH30080.03; Perbio), 1% penicillin-streptomycin, and 50 μM β-mercaptoethanol and supplemented with 30% conditioned medium from granulocyte-macrophage colony-stimulating factor (GM-CSF) producing R1 cells (mouse fibroblast NIH 3T3 cells, transfected with the GM-CSF gene) (12). D1 cells were infected for a period of 48 h with RSV (multiplicity of infection [MOI], 2) before addition to the lung cell suspension. Cell suspensions were stimulated for 6 h at 37°C, 5% CO2 in 200 μl IMDM supplemented with 2 mM l-glutamine, 25 mM HEPES buffer, 5% FCS, penicillin-streptomycin, 50 μM β-mercaptoethanol, and 50 U/ml recombinant human interleukin-2 (IL-2) (11147528001; Roche). Brefeldin A (10 μg/ml; B7651; Sigma-Aldrich) was added for the duration of the stimulation to facilitate intracellular accumulation of cytokines.
Cell surface and intracellular cytokine staining.
Lung single-cell suspensions were first preincubated with 2.4.G2, an Fc receptor-specific antibody (Ab) (anti-CD16/32), to reduce nonspecific binding and then stained for 4- or 5-color flow cytometry with the following antibodies (from BD Biosciences unless otherwise stated). To identify myeloid DC (mDC) populations, cells were stained with anti-CD11c (clone HL3), anti-major histocompatibility complex class II (MHC-II) (I-Ab/I-Eb; clone AF6-120.1), anti-CD103 (clone M290), and anti-CD11b (clone M1/70). For plasmacytoid DC (pDC) populations, cells were incubated with anti-Ly6C (clone RB6-8C5), anti-CD45R (clone RA3-6B2), anti-CD11b (clone M1/70), anti-CD11c (clone HL3), and anti-mPDCA-1 (clone JF05-1C2.4.1; Miltenyi Biotec). BAL eosinophils were identified with anti-CD45R (clone RA3-6B2), anti-CD11c (clone HL3), and anti-Siglec-F (clone E50-2440). RSV-specific CD8+ T cells were visualized with the MHC-I M187-195 tetramer, which was manufactured as previously described (18). Cytokine production by CD4+ and CD8+ T cells was measured by flow cytometry. Cells were washed with fluorescence-activated cell sorting (FACS) buffer and stained for surface markers with anti-NKG2A (clone 20d5), anti-CD8 (clone 53-6.7), and anti-CD4 (clone RM4-5). For intracellular staining, cells were fixed and permeabilized with CytoFix/CytoPerm (554722; BD) solution and Perm/Wash buffer (554723; BD). Intracellular cytokines were detected with anti-gamma interferon (IFN-γ) (clone XMG1.2), anti-IL-5 (clone TRFK5), anti-IL-4 (clone 11B11), and anti-IL-13 (clone eBio13a; eBioscience). Stained samples were measured on a FACSCanto II flow cytometer (BD, San Diego, CA) and analyzed using FacsDiva software (BD, San Diego, CA).
BAL fluid leukocyte composition.
Lung cells were spun onto glass slides (Shandon cytospin, Pittsburgh, PA) and fixed with 100% methanol for 5 min. Subsequently, cell nuclei were stained in a 1:1 dilution of May-Grünwald (3855; Mallinckrodt Baker) with buffered water (pH 6.8) for 5 min. After washing with buffered water at pH 6.8, cells were stained with a 1:8 dilution of Giemsa (1.09204.500; Merck) with buffered water (pH 6.8) for 15 min and finally washed with water. For determination of cell composition, 100 cells per sample were counted.
Statistical analysis.
For all experiments, the difference between groups was calculated using a two-way analysis of variance (ANOVA) followed by the Bonferroni test (Graphpad Prism version 4; Graphpad, San Diego, CA). Data are expressed as the means ± standard errors of the means (SEM). P < 0.05 was deemed significant and is indicated in the figures.
RESULTS
Dietary intervention with prebiotic scGOS/lcFOS/pAOS increases RSV-specific CD4+ T cell-mediated IFN-γ production during primary RSV infection.
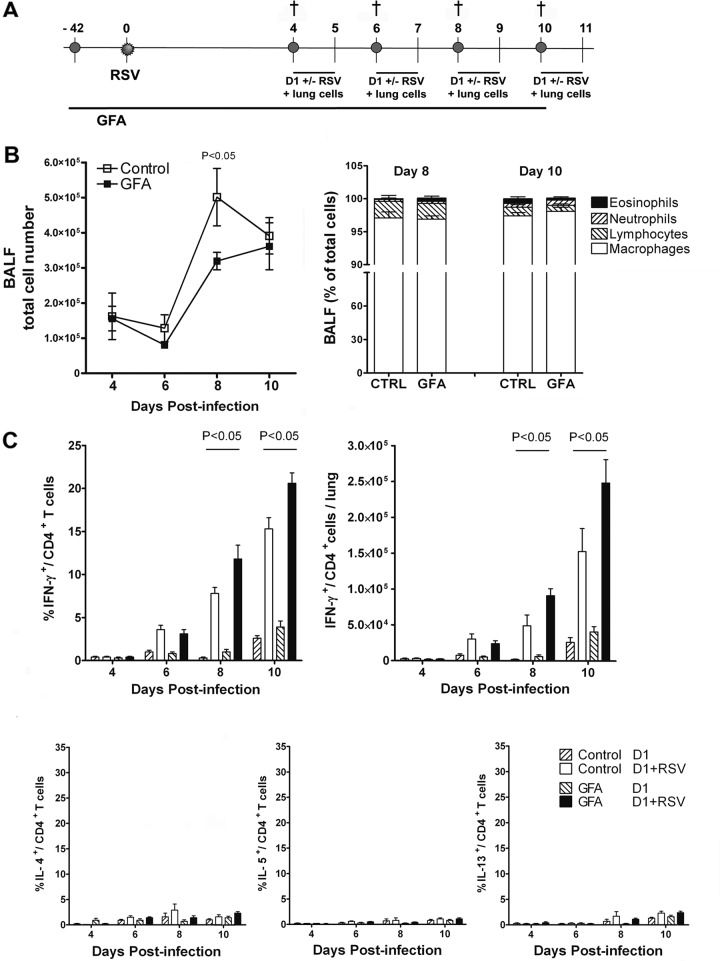
The effect of prebiotic dietary intervention on primary immune responses initiated after respiratory virus infection was investigated. We used an intranasal RSV infection model in female C57BL/6 mice. In this model, development of the RSV-specific CD4+ and CD8+ T cell responses in the lungs peak around days 8 to 10 after viral exposure (28). Dietary intervention was started in 3-week-old mice and continued until the end of the experiment. Six weeks after the start of dietary intervention, mice were i.n. infected with RSV. At 4 different time points after infection, mice were sacrificed and bronchoalveolar lavage (BAL) samples and lung single-cell suspensions were analyzed for inflammatory cell influx and T cell responses (Fig. 1A). During the course of infection, a significantly (P < 0.05) lower cellular influx in the BAL fluid was observed in mice receiving scGOS/lcFOS/pAOS compared to control mice (3.2 × 105 ± 0.2 × 105 versus 5.0 × 105 ± 0.8 × 105 cells) at day 8 postinfection. The cellular composition of BAL samples at all time points was similar to the control (Fig. 1B).
Fig 1.
Dietary intervention with scGOS/lcFOS/pAOS (GFA) increases RSV-specific CD4+ T cell-mediated IFN-γ production during primary RSV infection. (A) Mice received prebiotic or control diet starting 6 weeks before intranasal infection with RSV and were sacrificed at the indicated time points. (B) Cell composition of BAL fluid (BALF); the graph on the left shows total cell numbers, and that on the right indicates cell type as a percentage of total BAL cells. (C) Percentage of cytokine-producing CD4+ T cells (upper left, IFN-γ-producing CD4+ T cells; bottom, IL-4+, IL-5+, and IL-13+ CD4+ T cells as a fraction of total CD4+ T cells) and absolute numbers of IFN-γ+ CD4+ T cell numbers (upper right) measured after in vitro restimulation of lung cell suspensions with unloaded (D1) or RSV-loaded (D1+RSV) myeloid dendritic cells. Data shown represent the means ± SEM from 2 individual experiments performed with similar results, n = 8/group.
The effect of scGOS/lcFOS/pAOS on developing CD4+ T cell responses was measured by intracellular staining of IFN-γ (Th1) and IL-4, IL-5, and IL-13 (Th2) cytokine production after in vitro restimulation of lung cells with RSV-infected or uninfected D1 cells. The RSV-specific CD4+ T cell response developed from day 4 postinfection onward as has been shown before (28). Mainly IFN-γ was produced by the responding virus-specific cells. A significantly increased percentage of virus-specific, IFN-γ-producing CD4+ T cells was detected in the mice receiving scGOS/lcFOS/pAOS compared to control mice at day 8 after RSV infection (11.8 ± 1.6 versus 7.8 ± 0.7) and at day 10 (20.6 ± 1.2 versus 15.3 ± 1.3 CD4+ IFN-γ+ of total CD4+ T cells) (Fig. 1C). Expressed in absolute numbers/lung, this resulted in increased numbers of CD4+ IFN-γ+-producing cells (9.1 × 104 ± 1.0 × 104 versus 4.9 × 104 ± 1.5 × 104 cells) at day 8 and (2.5 × 105 ± 0.3 × 105 versus 1.5 × 105 ± 0.3 × 105 cells/lung) at day 10 postinfection. Th17 cells were barely detectable in the lungs of infected mice, and no effect of the intervention with scGOS/lcFOS/pAOS was found on IL-17-producing CD4+ T cells (data not shown).
The total number of recently activated CD8+ T cells present in the lung was visualized using activation marker NKG2A. Although increased NKG2A+ CD8+ T cell numbers could be detected in the lungs after RSV infection, no difference was found between the two dietary intervention groups (Table 1). Virus-specific CD8+ T cell responses, visualized by staining with the H-2Db/M187-195 tetrameric complex (containing the dominant RSV epitope derived from the viral matrix protein M187-195; NAITNAKII) increased with similar kinetics in both groups. The absolute number of tetramer-positive cells that produced IFN-γ was slightly increased in the scGOS/lcFOS/pAOS-receiving group (9.1 × 105 ± 1.7 × 105 versus 6.9 × 105 ± 0.9 × 105 cells/lung) at day 10 postinfection but didn't reach statistical significance. These data show that dietary intervention with scGOS/lcFOS/pAOS selectively increased RSV-specific CD4+ T cell-mediated IFN-γ production in the lungs of RSV-infected mice.
Table 1.
Dietary intervention with scGOS/lcFOS/pAOS does not affect RSV-specific lung CD8+ T cell response during primary RSV infectiona
| Day | T cell responseb |
|||||||
|---|---|---|---|---|---|---|---|---|
| % NKG2A+/CD8+ |
% IFN-γ+/CD8+ (D1-RSV) |
%TET+/CD8+ |
% IFN-γ+/CD8+ (NAITNAKII) |
|||||
| Ctrl | GFA | Ctrl | GFA | Ctrl | GFA | Ctrl | GFA | |
| 4 | 9.9 ± 1.3 | 8.7 ± 1.3 | 1.7 ± 0.2 | 1.6 ± 0.3 | 0.3 ± 0.1 | 0.5 ± 0.1 | 1.4 ± 0.1 | 1.9 ± 0.2 |
| 6 | 19.9 ± 2.0 | 20.4 ± 1.6 | 8.7 ± 0.7 | 8.6 ± 1.0 | 7.9 ± 1.0 | 10.0 ± 1.3 | 10.3 ± 1.8 | 10.8 ± 1.3 |
| 8 | 45.1 ± 2.4 | 47.2 ± 1.8 | 24.0 ± 1.8 | 26.2 ± 1.3 | 17.5 ± 1.9 | 17.7 ± 1.2 | 17.9 ± 1.2 | 20.9 ± 2.4 |
| 10 | 59.5 ± 1.9 | 59.8 ± 2.9 | 26.4 ± 2.1 | 29.2 ± 2.1 | 25.5 ± 1.7 | 28.8 ± 2.1 | 19.6 ± 1.1 | 20.8 ± 1.1 |
C57BL/6 mice received scGOS/lcFOS/pAOS (GFA) or control diet starting 6 weeks before i.n. infection with 2.0 × 106 PFU RSV. At the indicated time points after infection, lymphocytes were isolated from the lung parenchyma and stained for CD8 in combination with NKG2A or H-2Db/M187-195 tetramer. For in vitro T cell restimulation experiments, lung cells were stimulated with RSV-infected D1 cells or the RSV epitope NAITNAKII and intracellularly stained for IFN-γ.
The values depicted represent the number of NKG2A+/CD8+, IFN-γ+/CD8+, or TET+/CD8+ double-positive cells as percentages of total CD8+ T cells. Data are means ± SEM of control or GFA-treated mice from 2 individual experiments, n = 8/group.
Dietary intervention with scGOS/lcFOS/pAOS lowers the Th2 type immune response and lung eosinophilia in FI-RSV-vaccinated mice.
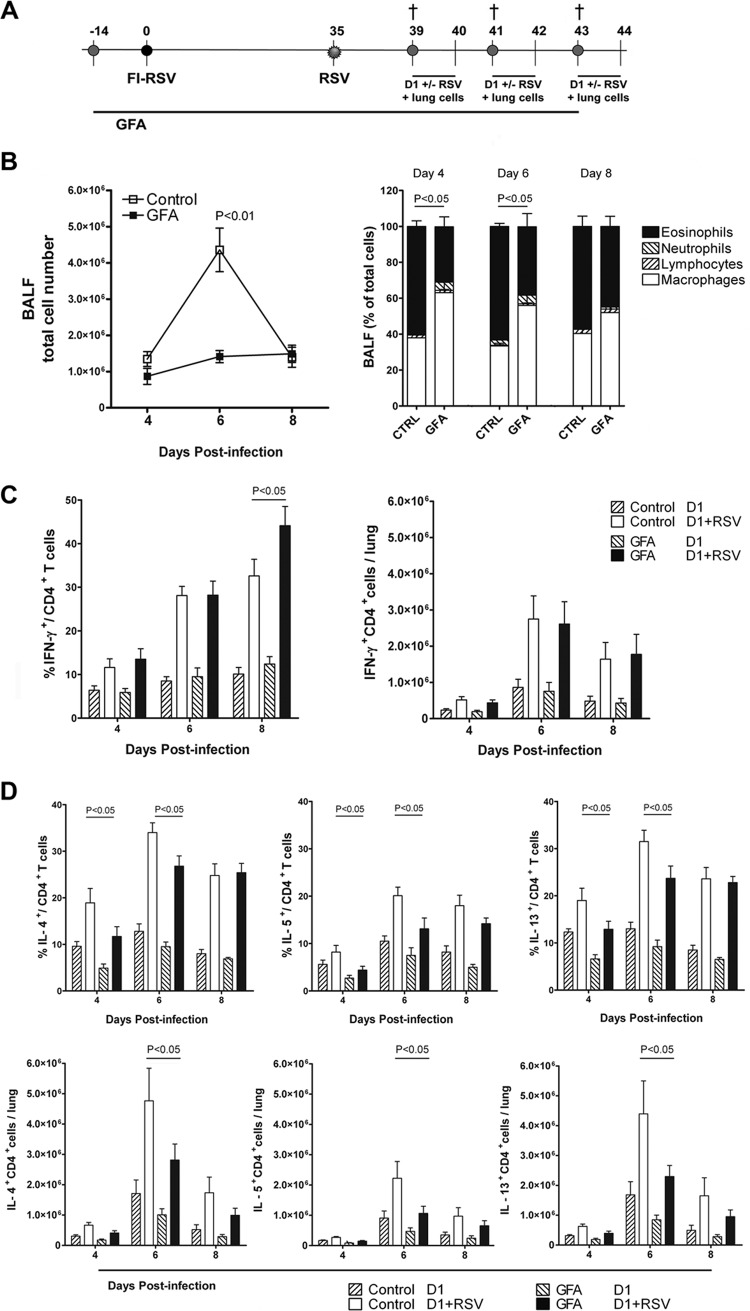
Because the prebiotic intervention showed an increased Th1 response during primary RSV infection, it was further tested in a Th2 disease model to evaluate whether dietary intervention could alter the Th1/Th2 balance. We used a murine formalin-inactivated (FI)-RSV vaccination model based on the original vaccine as tested in the 1960s in infants (39). From this model, it is known that Th2 cells play an important role in the development of enhanced disease (7). Dietary intervention started 2 weeks prior to i.m. FI-RSV vaccination and was administered until the end of the experiment. Thirty-five days after vaccination, mice were i.n. challenged with RSV. At 3 different time points after infection, mice were sacrificed and bronchoalveolar lavage (BAL) samples and lung cell suspensions were analyzed for inflammatory cell influx and T cell responses (Fig. 2A). Cellular infiltration in the lung airways peaked at day 6 postinfection and was significantly (P < 0.05) decreased in the mice receiving scGOS/lcFOS/pAOS (1.4 × 106 ± 0.2 × 106 versus 4.3 × 106 ± 0.6 × 106 cells) compared to control mice. Furthermore, analysis of the BAL fluid cell composition showed that eosinophil influx, characteristic for this model, was significantly (P < 0.05) decreased in mice receiving prebiotic diet compared to control diet at day 4 (30.8% ± 5.5% versus 59.9% ± 3.1%) and day 6 (38.1% ± 7.3% versus 63.3% ± 1.7%) after infection (Fig. 2B).
Fig 2.
Dietary intervention with scGOS/lcFOS/pAOS (GFA) decreases the Th2 type immune response and lung eosinophilia upon challenge in FI-RSV-vaccinated mice. (A) Mice received prebiotic or control diet starting 2 weeks before i.m. vaccination with 50 μl of FI-RSV. After RSV challenge at day 35 after vaccination, mice were sacrificed at the indicated time points. (B) Cell composition of BAL fluid (left, total cell numbers; right, indicated cell type as a percentage of total BAL cells). Percentages of eosinophils were significantly decreased in mice treated with the GFA diet (P < 0.05). (C and D) Percentages of IFN-γ-, IL-4-, IL-5-, and IL-13-producing cells of total CD4+ T cells and total IFN-γ-, IL-4-, IL-5-, and IL-13-producing CD4+ T cell numbers after in vitro restimulation of lung CD4+ T cells with unloaded (D1) or RSV-loaded (D1+RSV) myeloid dendritic cells. Data shown represent the means ± SEM from 2 individual experiments performed with similar results, n = 8/group.
In this model, the specific T cell response against RSV, represented as the absolute numbers of IFN-γ-, IL-4-, IL-5-, and IL-13-producing CD4+ T cells, peaked at day 6 after infection. Dietary intervention with the specific prebiotic diet significantly (P < 0.05) decreased the absolute numbers of IL-4 (2.8 × 106 ± 0.5 × 106 versus 4.8 × 106 ± 1.1 × 106 cells), IL-5 (1.1 × 106 ± 0.2 × 106 versus 2.2 × 106 ± 0.5 × 106 cells), and IL-13 (2.3 × 106 ± 0.4 × 106 versus 4.4 × 106 ± 1.1 × 106 cells) producing CD4+ T cells/lung at day 6 after challenge. Moreover, the percentages of CD4+ T cells that produced these Th2 type cytokines were significantly lower in mice receiving scGOS/lcFOS/pAOS at both day 4 and day 6 postinfection (Fig. 2D). In this model, the absolute numbers of CD4+ T cells that produced RSV-specific IFN-γ remained unaffected, while the percentage followed a pattern similar to that of the primary infection, i.e., they were significantly (P < 0.05) increased in mice receiving the specific prebiotic diet compared to the control (44.1 ± 4.4 versus 32.6 ± 3.8 CD4+ IFN-γ+ cells) at day 8 postinfection (Fig. 2C).
The development in the total number of activated (NKG2A+) CD8+ T cells (Table 2) was similar between the two diet groups. However, virus-specific CD8+ T cell response, measured by M187-195 tetramer staining, showed a significant (P < 0.05) increase in virus-specific CD8+ T cell numbers in the mice receiving scGOS/lcFOS/pAOS compared to the control (18.3% ± 2.6% versus 12.8% ± 2.6% CD8+ IFN-γ+ cells) 8 days postinfection. In vitro restimulation of lung cells with M187-195 peptide showed that these CD8+ T cells were functional, since similar fractions of IFN-γ-producing CD8+ T cells (13.8% ± 2.5% for the control versus 19.7% ± 2.8% CD8+ IFN-γ+ cells in prebiotic-receiving mice) were measured at this time point. However, expressed in absolute numbers of tetramer-positive cells that produced IFN-γ, this increase (9.1 × 105 ± 1.7 × 105 versus 6.9 × 105 ± 0.9 × 105 cells/lung) in the scGOS/lcFOS/pAOS-receiving group did not reach statistical significance. Reduced airway eosinophilia at days 4 and 6 after challenge (Fig. 2B) correlated with significantly decreased numbers of GATA-3-expressing CD4+ T cells (data not shown) and percentages of RSV-specific IL-4-, IL-5-, IL-13-producing CD4+ T cells (Fig. 2D) in the lungs of mice receiving the prebiotics. These data show that dietary intervention with scGOS/lcFOS/pAOS modulates FI-RSV-induced RSV-specific CD4+ and CD8+ T cell responses to a more Th1 type of response in the lungs of RSV-infected mice.
Table 2.
Dietary intervention with scGOS/lcFOS/pAOS increases RSV-specific lung CD8+ T cell response in FI-RSV vaccinated micea
| Day | T cell responseb |
|||||||
|---|---|---|---|---|---|---|---|---|
| % NKG2A+/CD8+ |
% IFNγ+/CD8+ (D1-RSV) |
%TET+/CD8+ |
% IFNγ+/CD8+ (NAITNAKII) |
|||||
| Ctrl | GFA | Ctrl | GFA | Ctrl | GFA | Ctrl | GFA | |
| 4 | 19.1 ± 1.5 | 17.4 ± 0.8 | 6.2 ± 0.6 | 5.2 ± 0.2 | 1.5 ± 0.3 | 1.3 ± 0.2 | 4.7 ± 0.5 | 3.4 ± 0.2 |
| 6 | 22.7 ± 2.1 | 25.0 ± 1.9 | 13.0 ± 1.2 | 14.1 ± 1.3 | 5.1 ± 0.5 | 6.8 ± 0.9 | 6.3 ± 0.8 | 8.3 ± 1.4 |
| 8 | 47.6 ± 4.7 | 52.4 ± 4.7 | 26.1 ± 2.0 | 29.6 ± 3.0 | 12.8 ± 2.6 | 18.3 ± 2.6* | 13.8 ± 2.5 | 19.7 ± 2.8* |
C57BL/6 mice received scGOS/lcFOS/pAOS (GFA) or control diet starting 2 weeks before i.m. vaccination with 50 μl of FI-RSV. After 35 days, mice were i.n. challenged with 2.0 × 106 PFU RSV. At the indicated time points, lymphocytes were isolated from the lung parenchyma and stained for CD8 in combination with NKG2A or H-2Db/M187-195 tetramer. For in vitro T cell restimulation experiments, lung cells were stimulated with RSV-infected D1 cells or the RSV epitope NAITNAKII and intracellularly stained for IFN-γ.
The values depicted represent the number of NKG2A+/CD8+, IFN-γ+/CD8+, or TET+/CD8+ double positive cells as percentages of total CD8+ T cells. The table shows the means ± SEM of control or GFA-treated mice from 2 individual experiments, n = 8/group. Values denoted with an asterisk represent a significant (P < 0.05) increase in NAITNAKII-specific (IFN-γ-producing) CD8+ T cells.
Dietary intervention with scGOS/lcFOS/pAOS lowers absolute numbers of CD11c+ CD11b+ DCs in lung tissue of FI-RSV-vaccinated mice 6 days after RSV infection.
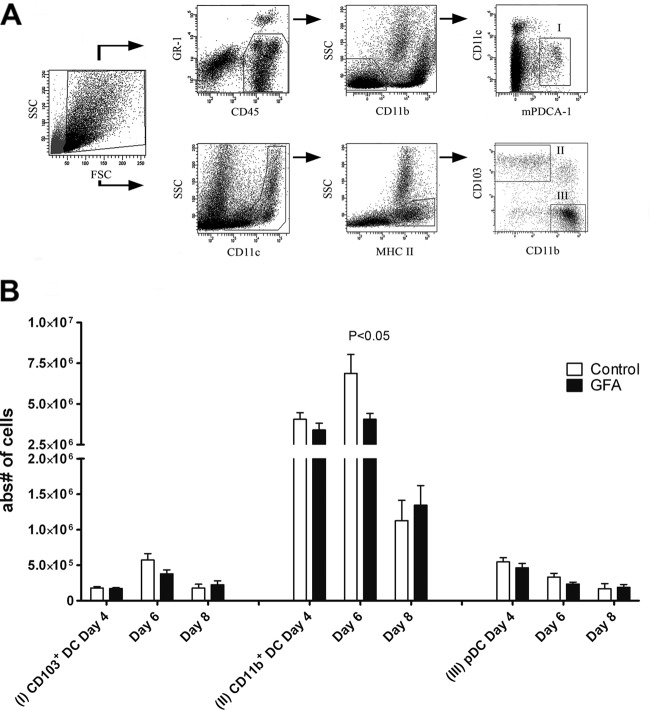
In the mouse lung, two major subsets of DC have been described, CD11clow/mPDCA-1+ plasmacytoid DC (pDC) and myeloid, or conventional, CD11c+ DC (cDC). These CD11c+ cDCs can be further divided into CD11c+ MHC-II+ CD103− CD11bhigh (CD11b+ DC), a subset located in the lung parenchyma that is important in leukocyte recruitment, and CD11c+ MHC-II+ CD103+ CD11blow (CD103+ DC), located underneath the epithelium, that can sample antigens from airways (3, 50). Different DC populations might locally be involved in polarization of Th cell subsets (29, 48). In this study, the kinetics of lung DC populations present during the acute RSV response as well as the FI-RSV-induced disease was investigated. During the acute RSV infection, no differences between the two dietary interventions on DC populations in the lungs were detected (data not shown). Within the FI-RSV vaccination model, the pDC population, known for its function in antiviral immunity and rapid production of IFN-α, decreased from day 4 to 8 after intranasal challenge with RSV-A2, but no differences were observed between the mice receiving prebiotic and control diet (Fig. 3B). No differences were found in CD103+ DC between the two intervention groups. CD11b+ DCs were the most abundant DC population on days 4 to 8 after viral challenge. This population was significantly larger (P < 0.05) at day 6 in animals receiving control diet than that of mice receiving specific prebiotic diet (Fig. 3B). However, at day 8, CD11b+ DC numbers in the lungs decreased in both groups and reached similar levels. These data indicate that dietary intervention with scGOS/lcFOS/pAOS affected the lung CD11b+ DC population, a subset known to be important in leukocyte recruitment, at day 6 after RSV challenge in the FI-RSV vaccination model.
Fig 3.
Dietary intervention with scGOS/lcFOS/pAOS (GFA) decreases CD11c+ CD11b+ DC influx in lung tissue of FI-RSV-vaccinated mice at day 6 after RSV infection. (A) Lungs of naïve mice were used for gating strategies of lung DC populations, designated I (pDC), II (CD103+ DC), and III (CD11b+ DC). (B) Days 4, 6, and 8 after intranasal challenge with RSV, absolute cell numbers of pDC, CD103+ DC, and CD11b+ DC were determined. Data shown represent the means ± SEM from 2 individual experiments performed with similar results, n = 8/group.
Dietary intervention with scGOS/lcFOS/pAOS in the C57BL/6 FI-RSV vaccination model does not affect body weight but improves viral clearance.
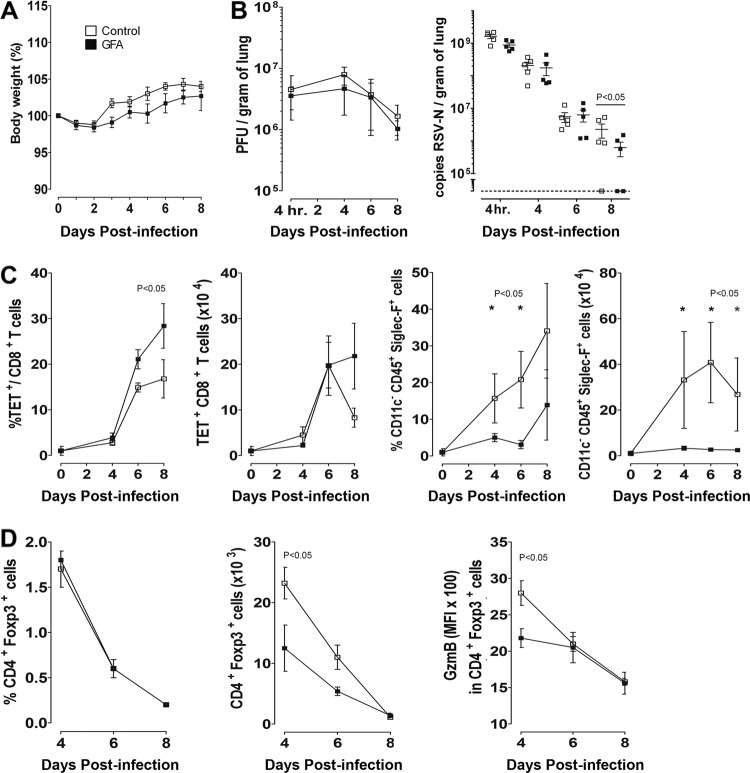
Weight loss can be used as a correlate of illness severity in mouse models of respiratory infection. Therefore, we performed daily weight measurements in individual mice to monitor the effect of dietary intervention. During the 7-week period prior to infection, the diet with scGOS/lcFOS/pAOS did not change the growth pattern of the mice compared to the control diet (data not shown). Compared to the BALB/c mouse model, C57BL/6 mice are relatively resistant to viral growth and weight loss (22, 40, 49). In accordance with these earlier studies, we did not observe significant weight loss during primary RSV infection, and no differences were observed between the diet groups (data not shown). In addition, no significant weight loss was observed in the FI-RSV vaccination model, and again weight changes between the two diet groups were not significantly different (Fig. 4A).
Fig 4.
Dietary intervention with scGOS/lcFOS/pAOS (GFA) does not affect body weight but has a significant effect on viral clearance in FI-RSV-vaccinated mice. (A) Daily weight measurements were performed for 8 days after RSV infection. The percentage of original body weight is shown. (B) Viral replication in lungs of mice was measured by plaque assay and shown as PFU/gram of lung (left). Amounts of RSV A2 present in lung of FI-RSV-vaccinated mice were measured by RT-PCR. Concentrations of RSV-N RNA were determined by comparing sample threshold values to a standard curve of RSV A2 and are shown as copies of RSV-N/gram of lung (right). (C) Development of the RSV-specific CD8+ T cell response measured as H-2Db/M187-195 tetramer-positive cells as a percentage of total CD8+ T cells (left) and absolute numbers (right) in BAL fluid. Kinetics of eosinophil (CD45+ CD11c− Siglec-F+) influx in BAL fluid as a percentage of total BAL cells are shown (left) and are depicted as absolute numbers (right). (D) Kinetics of CD4+ Foxp3+ regulatory T cells in BAL fluid of FI-RSV-vaccinated mice at days 4, 6, and 8 after infection (left) and GzmB expression in CD4+ Foxp3+ cells (right) at these time points. Data shown represent the means ± SEM, n = 5/group. Significant differences are indicated with an asterisk (P < 0.05).
We next addressed the question of whether changes observed in immune responses caused by the dietary intervention affected the lung viral load and virus elimination kinetics. Because changes in immune parameters were most pronounced in the FI-RSV vaccination model, lung viral load was measured in total lung tissue in the FI-RSV vaccination model after bronchoalveolar lavage. Virus titration was performed on HEp2 cells to determine the amount of infectious virus in the lung parenchyma, and total viral particle counts based on PCR were also determined. Eight days after i.n. infection with RSV, significantly (P < 0.05) lower viral copy counts were recovered from lungs of mice receiving scGOS/lcFOS/pAOS diet compared to mice receiving control diet in the FI-RSV-vaccinated mice (Fig. 4B). During this experimental setup, immunological parameters were monitored in the BAL fluid. As observed before in the FI-RSV vaccination model (Fig. 2), a substantial influx of eosinophils was observed in the BAL fluid in the control diet group, which was strongly inhibited in mice receiving the scGOS/lcFOS/pAOS diet. In addition, as observed in lung tissue (Table 2), we found a significantly (P < 0.05) increased percentage of virus-specific CD8+ T cells in the BAL fluid at day 8 after RSV challenge in FI-RSV-vaccinated mice (Fig. 4C).
Previously, changes in CD25+ regulatory CD4+ T cells (Tregs) were observed in an influenza vaccination model when the scGOS/lcFOS/pAOS diet was administered (55). In the experiments described in Fig. 2, we observed a systemic decrease in Treg numbers in lung, spleen, and mesenteric lymph nodes at day 4 after infection in FI-RSV-vaccinated mice receiving the scGOS/lcFOS/pAOS diet (data not shown). Tregs have previously been found to regulate RSV-specific primary immune responses after in vivo Treg depletion with antibodies (14, 26, 41) or after diphtheria toxin-induced depletion in mice expressing diphtheria toxin receptor under the control of the foxp3 gene locus (27). In the latter study, the expression of granzyme B (GzmB) in Treg locally in the lung was shown to be involved in the immune-regulatory function of Tregs in the primary RSV infection model. Although the percentage of Tregs did not differ, a significantly decreased absolute influx of Tregs was detected in the BAL fluid at day 4 after challenge in FI-RSV-vaccinated mice receiving the scGOS/lcFOS/pAOS diet. At this time point, GzmB expression was also significantly lower in Tregs of mice receiving the specific prebiotic diet (Fig. 4D). This indicates that the dietary intervention with scGOS/lcFOS/pAOS has an effect on the function of regulatory immune cells which correlates with altered RSV-specific immune responses.
DISCUSSION
In recent years, research in the fields of immunology and microbiology has revealed that commensal bacteria in the mammalian host play a role that is not limited to digestive help alone. The composition and products of gut microbiota can influence host immune and inflammatory responses (32). Its effect on systemic immunity, however, is still largely unknown and is a field of growing interest. In this study, we demonstrate that a specific mixture of orally applied, nondigestible oligosaccharides, scGOS/lcFOS/pAOS, with known prebiotic properties (16, 56), can regulate CD4+ and CD8+ T cell-mediated immune responses in the lungs of RSV-infected mice.
During primary infection, dietary intervention with scGOS/lcFOS/pAOS resulted in a lower cellular infiltrate in BAL fluid and an increased virus-specific CD4+ IFN-γ response in the lungs of RSV-infected mice. In an FI-RSV vaccination model, typical asthmatic parameters, like airway hypersensitivity, airway eosinophilia, RSV-specific IgE, and a Th2-skewed cytokine profile, are present (4, 10, 25). In this FI-RSV model, dietary intervention with scGOS/lcFOS/pAOS reduced lung cell infiltration and airway eosinophilia at days 4 and 6 after challenge and correlated with significantly decreased numbers of GATA-3-expressing CD4+ T cells (data not shown) and numbers of RSV-specific IL-4-, IL-5-, and IL-13-producing CD4+ T cells in the lungs of scGOS/lcFOS/pAOS-receiving mice. Although a slightly different ratio of oligosaccharides was used in previous studies (9:1:2), these findings extend earlier observations that a specific prebiotic mixture decreases lower airway hyperreactivity, IgE serum levels, and lung inflammatory cell influx in an ovalbumin-induced murine asthma model (57). Furthermore, it has been reported that administration of scGOS/lcFOS in combination with Bifidobacterium breve M-16V for a period of 4 weeks significantly reduced systemic production of Th2 cytokines after allergen challenge in patients with asthma and house dust mite allergy (53). These studies all underscore the immune-modulating and, more specifically, Th1-supporting effect of these specific oligosaccharide mixtures.
In the FI-RSV-induced, Th2-dominated model, we observed that decreased Th2 responsiveness at days 4 and 6 was accompanied by the development of an increased IFN-γ response in CD4+ as well as CD8+ T cells at day 8 after infection in the mice receiving scGOS/lcFOS/pAOS. Interestingly, the increase in IFN-γ production at day 8 postinfection was also seen during primary RSV infection when Th2 responses do not play a significant role. The impact detected on viral load and physical condition of the mice was marginal in this model. How the Th1 and Th2 arms of the virus-specific immune response reciprocally interact in these models is unclear. Suppression of Th2-induced disease in the FI-RSV model can be accomplished when CD8+ T cell responses are boosted early during challenge, i.e., in mice preimmunized with the dominant CD8+ T cell epitope (34, 35). However, the virus-specific CD8+ T cell response and CD4+ T cell-mediated IFN-γ responses were not different early after viral challenge in mice receiving the scGOS/lcFOS/pAOS diet. Furthermore, dietary-induced differences in the magnitude of the Th2 response (day 4 and 6 in the FI-RSV model) preceded the difference in Th1 response by CD4+ T cells and virus-specific CD8+ T cells (day 8/10) as well as viral clearance differences between the diets. Recently, a regulatory function of CD4+ Foxp3+ T cells has been described during primary RSV infection. Several approaches, including in vivo depletion with CD25-specific antibody, conditional depletion with diphtheria toxin during primary RSV infection in DEREG mice that express the diphtheria toxin receptor under the control of the Foxp3 locus, and in vivo Treg activation by IL-2 immune complexes, have shown that Tregs can regulate the early inflammatory response and the magnitude and quality of adaptive immune responses (14, 26, 27). In the present study, we found a correlation between diminished CD4+ Foxp3+ T cells in the lungs and increased virus-specific CD8+ T cell numbers at day 8 postchallenge in the FI-RSV vaccination model. In addition, we also found decreased GzmB expression in the CD4+ Foxp3+ cells in the BAL fluid of scGOS/lcFOS/pAOS-receiving mice, another indication that regulatory T cell function in these mice is influenced.
In the FI-RSV experiment, mice received the scGOS/lcFOS/pAOS diet from 2 weeks before vaccination through the entire experiment until they were sacrificed 8 days after viral challenge. Therefore, it is possible that dietary effects on Tregs (or other immune cells) affected immune responses during priming as well as during the recall response. It has been shown in a murine model of experimental allergic airway inflammation performed with DEREG mice that depletion of Treg during the priming phase of the response led to an exacerbation of allergic airway inflammation affecting serum IgE levels and lung eosinophilia (2). Because of the similarities between ovalbumin (OVA)-induced allergy models and the FI-RSV model (25), a direct effect of altered Treg function on the Th2 component in the immune response might be envisioned in the FI-RSV model without a role of Th1-mediated suppression of Th2 responses and eosinophilia. More in-depth studies looking into the role of Tregs during dietary intervention are needed to address their specific role and function in time.
The exact mechanism(s) involved in systemic immune modulation by scGOS/lcFOS/pAOS is currently unknown. Because of the prebiotic capacities of the oligosaccharides tested in this study, it is tempting to speculate that microbial products or composition influences gut and systemic immunity. Studies with germfree, restricted flora or antibiotic-treated mice have shown that commensal microbiota can influence systemic immunity by targeting specific cell types, like plasmacytoid dendritic cells, invariant NKT cells, virus-specific CD8+ memory cells, and marginal-zone B cells (13, 51, 58, 59). Products from gut bacteria provide signals for pattern recognition receptors like NOD or Toll-like receptors. Systemic immune response alterations appear to be caused by interaction of gut microbial components and such innate immune receptors. Ichinohe et al. showed that immune responses against respiratory tract influenza A virus infections could be influenced by gut commensal bacteria. Administration of broad-spectrum antibiotics in mice resulted in incompetent virus-specific CD4+ and CD8+ T cell responses, a defect that could be completely restored by intrarectal injection of the TLR4 ligand lipopolysaccharide (LPS) (21). Another recent study of mice showed that gut microbiota-derived peptidoglycan is translocated from the gut into the systemic circulation. Systemic availability of peptidoglycan is sensed by Nod1 receptors and results in enhanced neutrophil-mediated innate immunity (8). In addition to effects of bacterial composition or bacterial components, the observed immune modulation might be a result of direct interactions between oligosaccharides provided in the diet and host immune cells. Eiwegger et al. showed that small amounts of scGOS and lcFOS can cross the gut epithelial barrier, therefore these components may become systemically available (11). Lectins are known to bind carbohydrate structures and modulate immune responses. Galectins have been shown to control immune homeostasis and inflammation. Galectin-9–Tim-3 interactions regulate virus-specific primary and memory CD8+ T cell responses in herpes simplex virus infections and promote the induction of regulatory T cells (42, 43).
In summary, in our study we show that dietary intervention with a specific prebiotic oligosaccharide mixture can influence host innate and T cell responses during a respiratory virus infection by modulation of the Th1/Th2 responses in the lungs. Therefore, prophylactic dietary supplementation of scGOS/lcFOS/pAOS in infant formula could be beneficial by accelerating postnatal maturation of the infant immune system and potentiating protective immunity against respiratory virus infections with a high attack rate in early infancy, such as RSV.
ACKNOWLEDGMENTS
This study was financially supported and performed within the framework of the Dutch Top Institute Pharma project (D1-101-0).
M. A. Schijf, J. Bastiaans, J. Garssen, and B. van't Land are employees of Department of Immunology, Danone Research–Center for Specialized Nutrition, Wageningen, The Netherlands.
Footnotes
Published ahead of print 15 August 2012
REFERENCES
- 1. Arslanoglu S, et al. 2008. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J. Nutr. 138:1091–1095 [DOI] [PubMed] [Google Scholar]
- 2. Baru AM, et al. 2010. Selective depletion of Foxp3+ Treg during sensitization phase aggravates experimental allergic airway inflammation. Eur. J. Immunol. 40:2259–2266 [DOI] [PubMed] [Google Scholar]
- 3. Beaty SR, Rose CE, Jr, Sung SS. 2007. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J. Immunol. 178:1882–1895 [DOI] [PubMed] [Google Scholar]
- 4. Becker Y. 2006. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy–a review. Virus Genes 33:235–252 [DOI] [PubMed] [Google Scholar]
- 5. Belderbos ME, et al. 2009. Skewed pattern of Toll-like receptor 4-mediated cytokine production in human neonatal blood: low LPS-induced IL-12p70 and high IL-10 persist throughout the first month of life. Clin. Immunol. 133:228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bryan DL, Hart PH, Forsyth KD, Gibson RA. 2007. Immunomodulatory constituents of human milk change in response to infant bronchiolitis. Pediatr. Allergy Immunol. 18:495–502 [DOI] [PubMed] [Google Scholar]
- 7. Castilow EM, Olson MR, Varga SM. 2007. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol. Res. 39:225–239 [DOI] [PubMed] [Google Scholar]
- 8. Clarke TB, et al. 2010. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 16:228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Filippo C, et al. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U. S. A. 107:14691–14696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Swart RL, et al. 2002. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J. Virol. 76:11561–11569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eiwegger T, et al. 2010. Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr. Allergy Immunol. 21:1179–1188 [DOI] [PubMed] [Google Scholar]
- 12. Foti M, et al. 1999. Upon dendritic cell (DC) activation chemokines and chemokine receptor expression are rapidly regulated for recruitment and maintenance of DC at the inflammatory site. Int. Immunol. 11:979–986 [DOI] [PubMed] [Google Scholar]
- 13. Fujiwara D, et al. 2008. Systemic control of plasmacytoid dendritic cells by CD8+ T cells and commensal microbiota. J. Immunol. 180:5843–5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fulton RB, Meyerholz DK, Varga SM. 2010. Foxp3+ CD4 regulatory T cells limit pulmonary immunopathology by modulating the CD8 T cell response during respiratory syncytial virus infection. J. Immunol. 185:2382–2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Glezen WP, Taber LH, Frank AL, Kasel JA. 1986. Risk of primary infection and reinfection with respiratory syncytial virus. Am. J. Dis. Child. 140:543–546 [DOI] [PubMed] [Google Scholar]
- 16. Gori A, et al. 2011. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal Immunol. 5:544–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gruber C, et al. 2010. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J. Allergy Clin. Immunol. 126:791–797 [DOI] [PubMed] [Google Scholar]
- 18. Haanen JB, et al. 1999. Systemic T cell expansion during localized viral infection. Eur. J. Immunol. 29:1168–1174 [DOI] [PubMed] [Google Scholar]
- 19. Houben ML, et al. 2010. Disease severity and viral load are correlated in infants with primary respiratory syncytial virus infection in the community. J. Med. Virol. 82:1266–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hummelen R, Vos AP, van't Land B, van Norren K, Reid G. 2010. Altered host-microbe interaction in HIV: a target for intervention with pro- and prebiotics. Int. Rev. Immunol. 29:485–513 [DOI] [PubMed] [Google Scholar]
- 21. Ichinohe T, et al. 2011. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. U. S. A. 108:5354–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jessen B, Faller S, Krempl CD, Ehl S. 2011. Major histocompatibility complex-dependent cytotoxic T lymphocyte repertoire and functional avidity contribute to strain-specific disease susceptibility after murine respiratory syncytial virus infection. J. Virol. 85:10135–10143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koenig JE, et al. 2011. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. U. S. A. 108(Suppl. 1):4578–4585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kruijsen D, et al. 2010. Serum antibodies critically affect virus-specific CD4+/CD8+ T cell balance during respiratory syncytial virus infections. J. Immunol. 185:6489–6498 [DOI] [PubMed] [Google Scholar]
- 25. Kruijsen D, et al. 2011. Local innate and adaptive immune responses regulate inflammatory cell influx into the lungs after vaccination with formalin inactivated RSV. Vaccine 29:2730–2741 [DOI] [PubMed] [Google Scholar]
- 26. Lee DC, et al. 2010. CD25+ natural regulatory T cells are critical in limiting innate and adaptive immunity and resolving disease following respiratory syncytial virus infection. J. Virol. 84:8790–8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Loebbermann J, et al. 2012. Regulatory T cells expressing granzyme B play a critical role in controlling lung inflammation during acute viral infection. Mucosal Immunol. 5:161–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lukens MV, et al. 2006. Characterization of the CD8+ T cell responses directed against respiratory syncytial virus during primary and secondary infection in C57BL/6 mice. Virology 352:157–168 [DOI] [PubMed] [Google Scholar]
- 29. Lukens MV, Kruijsen D, Coenjaerts FE, Kimpen JL, van Bleek GM. 2009. Respiratory syncytial virus-induced activation and migration of respiratory dendritic cells and subsequent antigen presentation in the lung-draining lymph node. J. Virol. 83:7235–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ly NP, Litonjua A, Gold DR, Celedon JC. 2011. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? J. Allergy Clin. Immunol. 127:1087–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martinez FD. 2003. Respiratory syncytial virus bronchiolitis and the pathogenesis of childhood asthma. Pediatr. Infect. Dis. J. 22:S76–S82 [DOI] [PubMed] [Google Scholar]
- 32. Maslowski KM, Mackay CR. 2011. Diet, gut microbiota and immune responses. Nat. Immunol. 12:5–9 [DOI] [PubMed] [Google Scholar]
- 33. Moro G, et al. 2002. Dosage-related bifidogenic effects of galacto- and fructooligosaccharides in formula-fed term infants. J. Pediatr. Gastroenterol. Nutr. 34:291–295 [DOI] [PubMed] [Google Scholar]
- 34. Olson MR, Hartwig SM, Varga SM. 2008. The number of respiratory syncytial virus (RSV)-specific memory CD8 T cells in the lung is critical for their ability to inhibit RSV vaccine-enhanced pulmonary eosinophilia. J. Immunol. 181:7958–7968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Olson MR, Varga SM. 2007. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 179:5415–5424 [DOI] [PubMed] [Google Scholar]
- 36. Olson MR, Varga SM. 2008. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev. Vaccines 7:1239–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. 2007. Development of the human infant intestinal microbiota. PLoS Biol. 5:e177 doi:10.1371/journal.pbio.0050177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pineiro M, et al. 2008. FAO technical meeting on prebiotics. J. Clin. Gastroenterol. 42(Suppl 3, Pt 2):S156–S159 [DOI] [PubMed] [Google Scholar]
- 39. Prince GA, Curtis SJ, Yim KC, Porter DD. 2001. Vaccine-enhanced respiratory syncytial virus disease in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J. Gen. Virol. 82:2881–2888 [DOI] [PubMed] [Google Scholar]
- 40. Prince GA, Horswood RL, Berndt J, Suffin SC, Chanock RM. 1979. Respiratory syncytial virus infection in inbred mice. Infect. Immun. 26:764–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ruckwardt TJ, Bonaparte KL, Nason MC, Graham BS. 2009. Regulatory T cells promote early influx of CD8+ T cells in the lungs of respiratory syncytial virus-infected mice and diminish immunodominance disparities. J. Virol. 83:3019–3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sehrawat S, et al. 2010. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 6:e1000882 doi:10.1371/journal.ppat.1000882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sehrawat S, Suryawanshi A, Hirashima M, Rouse BT. 2009. Role of Tim-3/galectin-9 inhibitory interaction in viral-induced immunopathology: shifting the balance toward regulators. J. Immunol. 182:3191–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shay DK, et al. 1999. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282:1440–1446 [DOI] [PubMed] [Google Scholar]
- 45. Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B. 2000. Respiratory syncytial virus bronchiolitis in infancy is an important risk factor for asthma and allergy at age 7. Am. J. Respir. Crit. Care Med. 161:1501–1507 [DOI] [PubMed] [Google Scholar]
- 46. Simoes EA, et al. 2010. The effect of respiratory syncytial virus on subsequent recurrent wheezing in atopic and nonatopic children. J. Allergy Clin. Immunol. 126:256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sin DD, Sutherland ER. 2008. Obesity and the lung: 4. Obesity and asthma. Thorax 63:1018–1023 [DOI] [PubMed] [Google Scholar]
- 48. Smit JJ, et al. 2008. The balance between plasmacytoid DC versus conventional DC determines pulmonary immunity to virus infections. PLoS One 3:e1720 doi:10.1371/journal.pone.0001720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stark JM, et al. 2002. Genetic susceptibility to respiratory syncytial virus infection in inbred mice. J. Med. Virol. 67:92–100 [DOI] [PubMed] [Google Scholar]
- 50. Sung SS, et al. 2006. A major lung CD103 (alphaE)-beta7 integrin-positive epithelial dendritic cell population expressing Langerin and tight junction proteins. J. Immunol. 176:2161–2172 [DOI] [PubMed] [Google Scholar]
- 51. Tanaka K, Sawamura S, Satoh T, Kobayashi K, Noda S. 2007. Role of the indigenous microbiota in maintaining the virus-specific CD8 memory T cells in the lung of mice infected with murine cytomegalovirus. J. Immunol. 178:5209–5216 [DOI] [PubMed] [Google Scholar]
- 52. Turnbaugh PJ, et al. 2009. A core gut microbiome in obese and lean twins. Nature 457:480–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van de Pol MA, Lutter R, Smids BS, Weersink EJ, van der Zee JS. 2011. Synbiotics reduce allergen-induced T-helper 2 response and improve peak expiratory flow in allergic asthmatics. Allergy 66:39–47 [DOI] [PubMed] [Google Scholar]
- 54. van Hoffen E, et al. 2009. A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy 64:484–487 [DOI] [PubMed] [Google Scholar]
- 55. van't Land B, et al. 2010. Regulatory T-cells have a prominent role in the immune modulated vaccine response by specific oligosaccharides. Vaccine 28:5711–5717 [DOI] [PubMed] [Google Scholar]
- 56. Vos AP, Knol J, Stahl B, M'rabet L, Garssen J. 2010. Specific prebiotic oligosaccharides modulate the early phase of a murine vaccination response. Int. Immunopharmacol. 10:619–625 [DOI] [PubMed] [Google Scholar]
- 57. Vos AP, et al. 2007. Dietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in mice. Int. Immunopharmacol. 7:1582–1587 [DOI] [PubMed] [Google Scholar]
- 58. Wei B, et al. 2008. Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells. Eur. J. Immunol. 38:3411–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wei B, et al. 2010. Commensal microbiota and CD8+ T cells shape the formation of invariant NKT cells. J. Immunol. 184:1218–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Winzler C, et al. 1997. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185:317–328 [DOI] [PMC free article] [PubMed] [Google Scholar]