Abstract
Bovine spongiform encephalopathy (BSE) is a transmissible spongiform encephalopathy (TSE) (or prion disease) that is readily transmissible to sheep by experimental infection and has the shortest incubation period in animals with the ARQ/ARQ PRNP genotype (at codons 136, 154, and 171). Because it is possible that sheep in the United Kingdom could have been infected with BSE by being fed contaminated meat and bone meal supplements at the same time as cattle, there is considerable interest in the responses of sheep to BSE inoculation. Epidemiological evidence suggests that very young individuals are more susceptible to TSE infection; however, this has never been properly tested in sheep. In the present study, low doses of BSE were fed to lambs of a range of ages (∼24 h, 2 to 3 weeks, 3 months, and 6 months) and adult sheep. The incidence of clinical BSE disease after inoculation was high in unweaned lambs (∼24 h and 2 to 3 weeks old) but much lower in older weaned animals The incubation period was also found to be influenced by the genotype at codon 141 of the PRNP gene, as lambs that were LF heterozygotes had a longer mean incubation period than those that were homozygotes of either type. The results suggest that sheep in the United Kingdom would have been at high risk of BSE infection only if neonatal animals had inadvertently ingested contaminated supplementary foodstuffs.
INTRODUCTION
Transmissible spongiform encephalopathies (TSEs) are prion diseases that affect many mammals and are associated with the presence of the protein PrPSc, a protease-resistant form of a normal host protein, PrPC. PrPSc is believed to form all or part of the infectious agent (44). The natural sheep TSE is scrapie; however, sheep can also be experimentally infected with bovine spongiform encephalopathy (BSE) by several routes, including intracerebral, oral (17), and intravenous (28). The manifestation of clinical signs of BSE following inoculation depends upon a number of factors, the most important being the PRNP genotype. In the Neuropathogenesis Unit, Edinburgh, United Kingdom (NPU), Cheviot sheep, animals homozygous for the ARQ PRNP gene allele (codons 136, 154, and 171 indicated in order) are most susceptible (23). However, for reasons that are currently unknown, a various number of adult ARQ/ARQ sheep will survive a challenge with BSE (16). Understanding this aspect of resistance is of fundamental importance. One potential influence on disease outcome following infection with any TSE is the age at which the individual is challenged. Because of the etiology of scrapie, which suggests that in sheep the risk for infection is very high during the perinatal period, many sheep challenge studies, including the study described in reference 3, have used young lambs rather than adult sheep as infection models. In addition, epidemiological studies in cattle (15) have suggested that in general, younger individuals are more susceptible to infection with BSE, and studies in human beings have shown that more individuals at relatively young ages have been affected by variant Creutzfeldt-Jakob disease (vCJD) (caused by a zoonotic infection with BSE) than by sporadic CJD (6). No systematic time course study in sheep has previously been reported in which the age at infection has varied within a single controlled experiment to demonstrate definitively whether susceptibility of sheep to any TSE changes with age.
At the time that this study was set up (2001), there was considerable concern about whether BSE might infect (or already has infected) the United Kingdom sheep flock. In the 10 years that it took to complete our study, surveillance programs have searched in the United Kingdom and throughout Europe for evidence of BSE in small ruminants. BSE-like signs have not been found in sheep but have been reported in very few instances in goats (31). However, it was not possible to rule out BSE completely with the use of these negative and low figures, so several attempts have been made to estimate the maximum possible numbers of cases. In one study in Britain the estimated maximum proportion of sheep TSE cases that could have been BSE was 0.66% (53). In a European study the estimated maximum numbers of BSE cases entering the human food chain in 2009 (95% confidence) were 0 to 240 sheep (of >57 million slaughtered) and 0 to 381 goats (of >7 million slaughtered) (14). In the present study, if we were able to show that any particular age group of sheep was at higher risk of BSE infection, then taking into account current feeding practices we could also consider whether infection of the United Kingdom sheep flock was likely to have occurred.
The objective of this project was therefore to challenge sheep of different ages with BSE in a range of relatively low doses and to establish at which age the animals were most likely to become infected and go on to develop clinical BSE.
MATERIALS AND METHODS
Sheep.
Cheviot sheep of the ARQ/ARQ genotype were sourced from the Defra flock of New Zealand (scrapie-free) origin (27) and were housed indoors in concrete pens in the sheep unit at The Institute for Animal Health, Compton, United Kingdom. Prior to arrival at Compton, some ewes were implanted with ARQ/ARQ embryos so that the resulting lambs could be inoculated soon after birth. The study involved sheep of various age groups: two groups of suckling lambs (∼24 h and 2 to 3 weeks of age), weaned lambs (3 months of age), young adults (6 months of age), and adults (15 to 27 months of age).
Following inoculation (see below) sheep were transferred into temporary pens for around 4 weeks and then moved to different group pens for life span observation. This separation was performed in order to minimize the chances of cross-infection from any inoculum passing through intact in the feces. All animals were monitored on a daily basis and were humanely culled when TSE clinical signs developed. Intercurrent illness affected a small number of sheep, which were culled for welfare reasons, and the experiment was terminated when survivors reached more than 2,000 days (5.5 years) after challenge. All work was reviewed and approved by the local ethical review committee and performed under license from the United Kingdom Home Office.
BSE inoculation.
Sheep were orally dosed with various amounts of BSE-infected cattle brain (provided by the TSE Archive, Veterinary Laboratories Agency [VLA]) through the intrabuccal route, which is known to have been successfully used with adult sheep (16). A full-titration assay of the BSE homogenate was carried out in RIII mice, producing a titer of 103.2 infectious units per g, which is comparable with BSE sources used in our other experiments (16). Sheep (7 to 12 per age and dose group) were challenged with low doses of BSE cattle brain. Each age group had 3 dose groups in which each animal received a dose of 0.05 g, 0.5 g, or 1 g, and the lambs in the 24-h age group had an additional dose group that received 0.1 g. The aim was to deliver the same numbers of infectious units per animal in each dose group. The inocula were prepared from the same batch of BSE-infected brain macerate by homogenization and dilution in sterile phosphate-buffered saline according to the required dose. Each sheep received the dose by intrabuccal administration in a total volume of 2.5 ml or 5 ml, depending on the dilution factor required, and these volumes were tolerated by both the adult sheep and the very young lambs. Control sheep (9 to 11 per age group) were age matched to the infected sheep, and each received 0.5 g of normal cow brain (also sourced from VLA). Controls were culled throughout in order to provide samples that were approximately age matched to those of the clinical cases.
Confirmation of BSE clinical disease.
A range of central nervous system and lymphoid tissues were recovered from all sheep in the study, including from animals that died. Tissue samples were fixed in neutral buffered formalin or frozen immediately on dry ice and then transferred to storage at −70°C. Immunohistochemical testing with the BG4 anti-PrP antibody was performed on brain, spleen, and tonsil tissues and a range of lymph nodes (mesenteric, retropharyngeal, prescapular) (18) to confirm clinical BSE disease. Western blotting was carried out with the 6H4 anti-PrP antibody on a selection of brain extracts (including control tissues) to confirm the presence of PrPSc with the glycoform pattern expected with BSE (36).
PRNP gene sequencing.
PRNP genotyping was performed on PCR-amplified DNA fragments generated from genomic DNA, which was extracted from tissue samples collected during the postmortem procedure. Small pieces of tissue (≤1 mg) were digested with 7 mg/ml proteinase K (Qiagen, United Kingdom) in 600 μl buffer (0.34% SDS, 0.34 mM EDTA, 3.4 mM Tris [pH 8.0], 0.1 M Na acetate, 0.33× phosphate-buffered saline [PBS]) at 37°C for 1 to 5 h or overnight. Protein was then removed by standard phenol-chloroform extraction. PCR amplification of isolated DNA (50 to 100 ng) was performed with Sigma JumpStart REDTaq DNA polymerase and buffers, 200 μM (each) deoxynucleoside triphosphates (dNTPs) (Roche, Switzerland), and 0.4 μM each of the oligonucleotide primers PS-141d (GGAATGTGAAGAACATTTATGACCTAGAAT) and PS + 109u (CAAGAGAGAAGCAAGAAATGAGACA). PCR conditions were 3 min at 95°C followed by 40 cycles of 30 s at 95°C, 30 s at 61°C, and 1 min at 72°C and a final elongation step of 10 min at 72°C. Purified PCR fragments (in a volume of 1 to 3 μl) were sequenced using oligonucleotide PS + 50u (CCCCCAACCTGGCAAAGATTAAGA) and a BigDye Terminator v3.1 cycle-sequencing kit. Purified reactions were run on an Applied Biosystems 3130 genetic analyzer (Applied Biosystems).
Data analysis.
The data on incubation period (time between inoculation and culling due to BSE clinical signs) were analyzed by survival analyses based on Cox's proportional hazards model ignoring interval censoring. Data from animals that survived to the end of the experiment or were culled due to reasons unrelated to BSE were treated as censored data. The Efron approximation was used to handle ties when the partial likelihood was calculated. The proportions of clinical cases were analyzed with a generalized linear model framework fitted with the assumption of Bernoulli distributions for the proportion of clinical cases with a logit link function. These analyses were carried out using R software version 2.13.1. Genotype information at PrP codon 141 was analyzed using GraphPad Prism on a subset of the data.
RESULTS
Incidence of BSE in the sheep groups.
The outcome following oral challenge of sheep with low doses of BSE is presented in Table 1. The presence of TSE was confirmed, or ruled out, by immunohistochemical detection of disease-associated PrP (PrPSc) in brain and lymphoid tissue from all sheep that showed clinical signs of disease or had to be culled/died for intercurrent reasons. The experiment was terminated when survivors reached at least 2,000 days postinoculation (dpi). There were differences in the numbers of animals developing clinical signs, confirmed by immunohistochemical testing, in each age group. In the ∼24-h and the 2- to 3-week age challenge groups there were high numbers of BSE cases, and there was just one survivor to over 2,000 dpi in the 2- to 3-week 0.5-g group. The incubation periods ranged widely, with large standard deviations (SD), and there was no link with the dose of BSE.
Table 1.
Incubation periods and survival times of sheep orally challenged with BSE at a range of ages
| Age at challenge | Dose of BSE (g) | BSE cases/no. challenged | Incubation period (daysa [SD]) | Survival of non-BSE sheepb for <2,000 days (no. if >3) | Survival of non-BSE sheepb for >2,000 days (no. if >3) |
|---|---|---|---|---|---|
| ∼24 h | 0.05 | 8/9 | 865 (202) | 524 | NAc |
| 0.1 | 4/7 | 774 (212) | 317, 335, 964 | NA | |
| 0.5 | 6/11 | 1,044 (215) | 39–672 (n = 5) | NA | |
| 1.0 | 7/10 | 685 (164) | 9, 135, 150 | NA | |
| 2–3 wk | 0.05 | 11/12 | 740 (162) | 104 | NA |
| 0.5 | 11/12 | 949 (189) | NA | 2,263 | |
| 1.0 | 12/12 | 745 (207) | NA | NA | |
| 3 mo | 0.05 | 0/10 | NA | 8, 193 | 2,121–2,237 (n = 8) |
| 0.5 | 0/10 | NA | 8, 989, 1,890 | 2,172–2,179 (n = 7) | |
| 1.0 | 0/10 | NA | 217–1,381 (n = 4) | 2,143 (n = 6) | |
| 6 mo | 0.05 | 0/10 | NA | 519, 1,273 | 2,021–2,062 (n = 8) |
| 0.5 | 3/10 | 729, 742, 932 | 922 | 2,021–2,062 (n = 6) | |
| 1.0 | 4/10 | 821 (183) | 682 | 2,062 (n = 5) | |
| 15–27 mo | 0.05 | 1/10 | 2,289 | 1,214, 1,227 | 2,039–2,290 (n = 7) |
| 0.5 | 2/10 | 594, 849 | 1,723 | 2,191–2,304 (n = 7) | |
| 1.0 | 1/10 | 930 | 1,010,d 1,359 | 2,290–2,297 (n = 7) |
If fewer than four cases, individual incubation periods or survival times are presented (days after infection); the survival rates for four or more animals are given as a range.
Animals culled for intercurrent illness/welfare reasons or at the end of the experiment.
NA, not applicable.
One animal with PrPSc-positive staining in Peyer's patches with no other BSE signs.
The remaining age groups (challenged at 3 months to adult age) had much lower BSE incidences, with for example the 3-month group having no confirmed BSE-affected sheep and 60 to 80% of challenged animals having a survival period of >2,000 dpi. The 6-month age group had seven cases of BSE, four in the 1-g dose group and three in the 0.5-g dose group, but again 50 to 60% of the animals challenged at 6 months of age had a survival period of >2,000 dpi. In the adult group there was one case of BSE in the 0.05-g dose group and there were two cases in the 0.5-g dose group. The adult 1-g dose group had one animal with a clinical BSE case at 930 dpi and one additional animal that despite the absence of BSE clinical signs had slight PrPSc staining in Peyer's patches (PP). The latter sheep was culled at 1,010 dpi for welfare reasons. Overall in the adult sheep group 70 to 80% of animals survived to >2,000 dpi. The results show clearly that the two groups with the youngest lambs (∼24 h and 2 to 3 weeks old) had the highest BSE clinical disease incidence of all age groups, regardless of the dosing regimen (Fig. 3A). A number of sheep challenged orally with normal cow brain were culled at the same times as the TSE clinically affected sheep, and none showed detectable disease-associated PrP in either the central nervous system or the peripheral lymphoid tissue.
Fig 3.
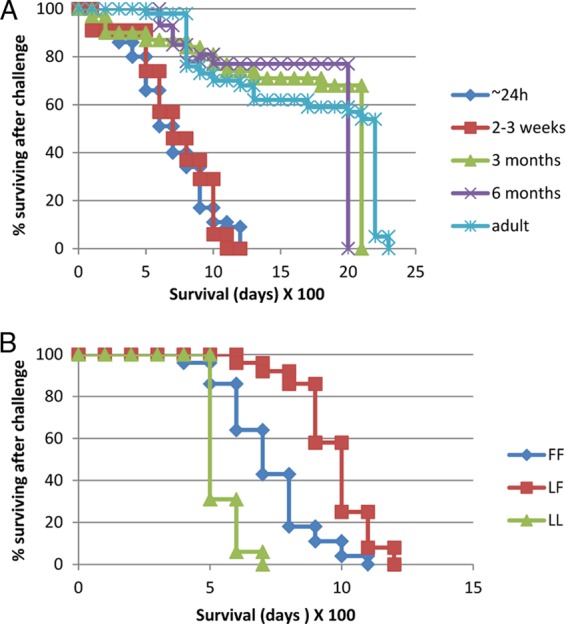
Survival times of sheep challenged with BSE. (A) Sheep of different age groups challenged with BSE; (B) sheep of different PRNP genotypes (codon 141) challenged with BSE.
Immunohistochemical testing with the antibody BG4 was carried out on sections taken from various regions of the brain, and positive cases had PrPSc distributed in brain tissues, mostly in the medulla as described previously (18, 19). Immunohistochemical testing was also carried out on a range of peripheral lymphoid tissues. In the majority of cases animals showing positive staining in brain tissue were also positive in lymph nodes (Fig. 1), which was as expected from our previous pathogenesis studies (19). All of the negative controls and survivors and all but one animal with intercurrent death had no signs of PrPSc deposition in any tissue. The exception was the animal with intercurrent death at 1,010 dpi from the 1-g adult group mentioned above. Western blotting showed typical BSE-like glycoform profiles of PrPSc (Fig. 2) in clinical BSE animals only. As expected the proteinase K-treated PrPSc from the ovine BSE brains had a lower molecular weight for the unglycosylated band than is seen with natural scrapie (Fig. 2, compare lanes 1 and 2) and a 1-in-3 dilution series showed the preponderance of the diglycosylated band (Fig. 2, lanes 2 through 4).
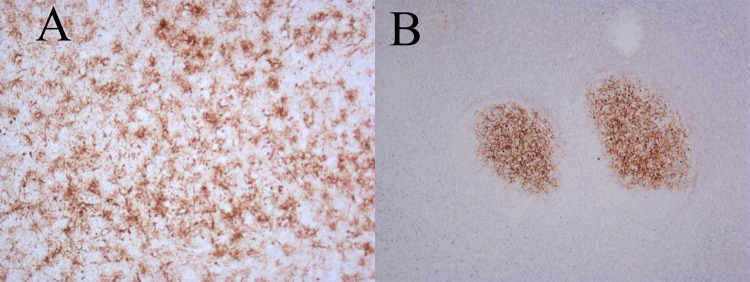
Fig 1.
Immunohistochemical staining for PrPSc in BSE-challenged sheep brain and lymph node tissue samples. Immunohistochemical testing using BG4 staining of PrPSc in tissues of one representative sheep from the ∼24-h challenge group and clinically affected by BSE. A, thalamus; B, tonsil. Magnification, ×10.
Fig 2.
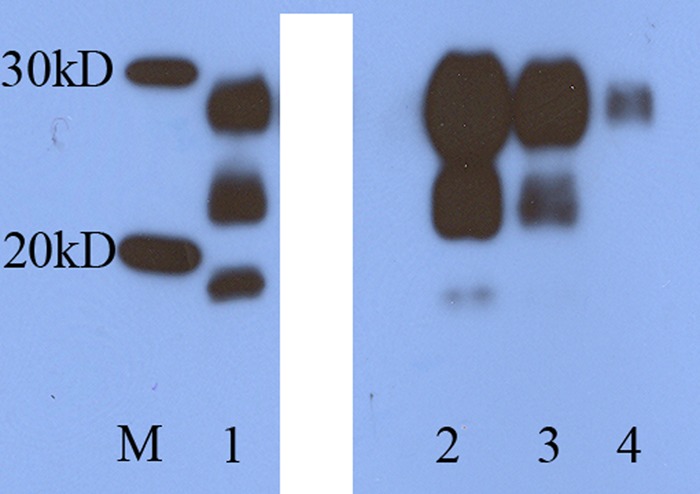
Western blot of brain tissue from BSE-challenged sheep brain. Western blot using 6H4 showing proteinase K-treated PrPSc in brain tissue from a representative clinically affected sheep challenged with BSE at 6 months of age. Size markers (M), natural scrapie control (1), sheep BSE (2), sheep BSE diluted 1/3 (3), and sheep BSE diluted 1/9 (4). This is a single gel with an irrelevant lane blanked out using Photoshop CS5. No other changes were made.
Survival analysis tested the effect of age at challenge on the development of BSE clinical signs in the sheep. With the use of a generalized linear model there was no evidence that the dose of BSE had a significant effect on the mean proportion of clinical cases in the sheep (P = 0.264), so results were pooled for each age group (Fig. 3A). The age at challenge had a statistically significant effect on whether or not the challenged animal developed BSE. Older sheep (6 months and adult) had significantly lower (p < 0.001) mean hazard ratios (0.09 and 0.04, respectively) relative to ∼24-h lambs. The 2- to 3-week lambs were also more likely to develop BSE than the 6-month and adult groups (p < 0.0001). Comparison of the ∼24-h and 2- to 3-week lamb groups showed that the mean hazard ratio was not significant between the two ages (p = 0.712). Similarly for the 6-month challenge groups relative to adult sheep the mean hazard ratio was not significant (p = 0.266). Since there were no clinical cases in the 3-month challenge groups, they were compared with the other groups using a generalized linear model, which again showed that the age group had a statistically significant effect on the mean proportion of BSE cases (P < 0.001). The main age-related difference in incidence of BSE was therefore between unweaned (∼24-h and 2- to 3-week) animals with high susceptibility and weaned (3-month, 6-month, and adult) animals with much lower susceptibility.
PRNP gene sequence.
When the challenge groups were set up in 2001, in ARQ/ARQ sheep, no account was taken of genotype at any other PRNP gene codon as we were not at that time aware of any additional associations with disease. DNA sequencing was carried out on a limited number (n = 68) of the sheep in this study in order to establish if polymorphisms at PRNP gene codons other than the standard three (136, 154, and 171) might be linked to the observed differences in susceptibility. No other codons were found to be variable apart from those corresponding to L141F and P168L. All sheep were homozygously P168 apart from one single animal that was PL168. The latter sheep, which was mentioned above and was from the adult 1-g challenge group, was culled with intercurrent illness at 1,010 dpi and found to have slight PrPSc detectable in Peyer's patch tissue. However, the L141F polymorphism was found at high frequency throughout all groups, and incubation periods of BSE in the different age groups (for a reduced data set of 68 animals) were compared with the PRNP genotype at codon 141. There was no observed effect of dose on incubation period length (not shown) so the data were pooled for each genotype, and incubation periods of clinical animals are shown in Fig. 3B. A significant lengthening of incubation period was observed in 141-LF animals compared with 141-LL and 141-FF sheep (P < 0.001). The mean incubation period for LL (n = 15) was 605 days, SD 56; for FF (n = 28) 774 days, SD 166; and for LF (n = 24) 1,013 days, SD 138. There was no link with resistance, as 12 survivors were also genotyped mostly from the 3-month challenge group and were as follows: LL (n = 1) survival time, 2,143 days; FF (n = 4) survival times, 1,214, 1,359, 1,723, and 2,143 days; and LF (n = 7) survival times, 1,010 (the animal which was also PL168 and PrPSc positive in Peyer's patches only), 1,227, and 2,143 (five animals) days.
DISCUSSION
In our study of sheep challenged orally at a range of ages with low doses of BSE, we have found clear evidence that neonatal lambs are much more susceptible than weaned lambs and adults. In our previous studies we used a dose of 5 g of cattle BSE brain per (adult) sheep and this resulted in clinical disease at an average incubation period of around 800 days (16). The 5-g dose, or even larger amounts, has become the normal oral dose when clinical disease is required for pathogenesis studies in our own and other labs (3, 47, 52, 59). In the present study, the lower doses (ranging from 0.1 g to 1 g per animal) were chosen such that the sheep in the adult group would not all become clinically affected by BSE. In order to demonstrate clearly any higher susceptibility in the younger age groups. Smaller amounts were also thought to more accurately reflect levels of dietary sources of BSE that may theoretically have affected United Kingdom sheep. Our results demonstrate clearly that the ∼24-h and 2- to 3-week-old lambs were much more likely to develop BSE after oral challenge than older sheep (3 months, 6 months, and adult). The BSE incubation period did not alter with the doses used, but there was an association of codon 141 with the length of the incubation period in that animals of the ARQ/ARQ genotype had the shortest incubation periods if they were also LL141, longer if FF141, and longer still if LF141. New Zealand Cheviot sheep have a polymorphism at codon 168 that has been associated with BSE resistance (22), and codon 112 has been shown in other sheep also to be associated with differences in BSE (47). However, in the sheep in the present study, codons 168 and 112 did not vary, except in one single animal that was PL168, and no additional codon variants were found. Codon 141 heterozygotes have been shown previously to have low susceptibility to scrapie (32, 46), but this is the first time an association with oral BSE infection of sheep has been published with detailed incubation period data, although the observation of longer survival of LF141 sheep has been presented in a conference abstract (54).
There are indications from epidemiological studies that TSEs are more infectious to younger sheep (12, 38) and cattle (5); however, there are, to our knowledge, no previously reported similar studies using direct experimental challenge of sheep to investigate the relationship of age to TSE susceptibility. Indeed, there are very few examples of challenge of neonatal ruminants in the literature at all. In a very early study, before PrP genetics was elucidated in sheep, fetal and newborn Suffolk sheep were experimentally infected with scrapie (24). The animals challenged as fetuses showed signs of infection in tissues at ∼250 days of age, and the newborns survived to 147 to 210 days of age. However, without PrP genotype information it is difficult to interpret the results. In two more recent studies, neonatal and 4-month-old ARQ/ARQ lambs were orally infected with scrapie, following which clinical disease developed in 100% of the former at 24 months of age (26) and around 50% of the latter at 32 months. However, the study was very limited and was disjointed, as it was carried out in two separate experiments with a long time gap between them, and no additional PrP genotype information was reported regarding the ARQ allele in the challenged animals. There are more published studies of neonatal infection in mice and hamsters although the information is also limited and at times contradictory. Neonatal mice have been reported to be in some instances less likely than weaned young mice to develop scrapie following challenge by intraperitoneal injection (43), and weaned young hamsters were found to be more susceptible to intracerebral challenge than adults (40).
There are undoubtedly multiple possible routes that can be used by TSE infectivity to invade and spread within the body, and different routes may predominate as others are closed. This makes it difficult to investigate which particular route has been taken in any individual experiment; however, it is clear from our results that the very early stages of life are high-risk periods for sheep to become infected if exposed to BSE in foodstuffs. The evidence that neonatal sheep are much more susceptible to BSE infection than weaned animals warrants further investigation in order to further our understanding of the epidemiology of BSE and its zoonosis, with, in the end, the aim of control and prevention of infection of all TSEs in sheep.
From our results we suggest that there are at least two strong and testable hypotheses for control of the age-related differences in initiation of infection. Our first suggestion is that the environment of the neonatal gut may be more favorable to BSE survival than that of weaned animals. The rumen in adult sheep contains billions of microorganisms that aid in the digestion of fibrous grass and hay. Regurgitation and rechewing of this material prepares the ingested fiber for the subsequent enzymatic and acid breakdown before moving on into the small intestine. Any ingested PrPSc/infectivity would have to survive this considerably destructive process prior to crossing the gut wall. There have been many attempts, with conflicting results, to establish whether PrPSc can survive ruminant digestion processes. Some studies have shown no effect on PrPSc after incubation in rumen, colon, or ileum fluids (7, 41), and work on BSE in cattle has suggested that PrPSc must survive the passage through much of the, if not the entire, digestive tract (55). However, without detailed infectivity bioassay analysis it is difficult to be sure what this means. In contrast, many other studies have indicated that PrPSc is readily degraded in gut fluids or in gut loop inoculations (11, 30, 48). Despite this rather confusing picture, experimentally infected adult sheep will develop BSE clinical signs after oral dosing, and our results clearly show that from as little as 0.5 g BSE cattle brain, sufficient infectivity can be taken up through the gut walls to establish infection even if much of the PrPSc is digested.
Ingestion and digestion of nutrients is very different in young lambs. A reflex of the esophageal groove allows the suckling lamb to ingest milk directly into the abomasum, thereby aiding the absorption of larger protein molecules. During this phase of development, the level of proteolytic activity in the digestive tract is low and is further reduced by trypsin inhibitors in colostrum (57, 62). This may mean that ingested PrPSc/infectivity is subjected to greatly reduced protease action compared with older animals. Survival of PrPSc/infectivity in the digestive fluids of neonatal lambs has not, to our knowledge, been investigated. It is possible therefore that before weaning, the lamb gut is a less harsh environment, allowing more infectivity to survive than in weaned lambs. The pH in the abomasum is variable in young ruminants and after ingestion of milk is very much higher than that in older animals (10), which may reduce protein digestion and aid absorption of colostral immunoglobulin. The effects of gut conditions can be profound. In one recent study, treatment of mice with omeprazole (a proton pump inhibitor) had the effect of increasing gastric fluid pH from 1.2 to 5.3 and more than doubled the rate of disease in intragastric scrapie infection compared with controls with normal gastric pH at infection (37).
Our second hypothesis is that in neonates there is a more efficient mechanism for absorption of macromolecules (and therefore also potentially PrPSc/infectivity) through the gut wall than in older animals. There are several different mechanisms for uptake of macromolecules from the gut, and it is possible that any one, or all, of these could be exploited to establish infection. In neonates these processes, which differ in detail between rodents and ruminants, can be broadly divided into those involved in transfer of maternal immunoglobulins to the newborn and those involved in general uptake of macromolecules through M cells and/or villous epithelium. Neonatal mice have an Fc receptor on gut enterocyte membranes that recognizes milk IgG and facilitates its selective transcytosis across the mucosa to the submucosa and then into the blood (58). The process ceases abruptly at or before weaning (21), when IgG receptors disappear. In contrast, in very young sheep, maternal immunoglobulins plus macromolecules and lymphocytes from colostrum are transferred by a nonselective process of enterocyte pinocytosis across the gut to the bloodstream and then to the lambs' lymph nodes (39, 56), a mechanism that terminates by approximately 48 h after birth. Although uptake of PrPSc/infectivity could occur via this route, it has not been tested by experimentation, nor does it explain the high susceptibility of our 2- to 3-week age group, in which it should have ceased to act.
In studies of general uptake mechanisms from the gut in older animals, experiments suggest that Peyer's patches (PP) are important routes of entry of PrPSc/infectivity. Ileal PP are among the first tissues that stain positive for PrPSc in studies of sheep affected by natural scrapie and within experimental studies of BSE and scrapie (2, 59, 60). The amount of gut-associated lymphoid tissues (GALT), and in particular PP, decreases at a variable rate as the animal matures, so that in the adult sheep it is much reduced. After around 2 to 3 months of age, ileal PP undergo progressive involution, and by ∼15 months the organized lymphoid elements of the terminal ileum have almost completely regressed (35, 45, 50, 51); however, the process is so gradual, taking place over many months, that it is unlikely to be the main factor controlling the sudden drop in disease transmission seen between the group challenged at 2 to 3 weeks of age and that challenged at 3 months.
Within PPs there is compelling evidence in mice that supports M cells as the route of entry; for example, transgenic mice in which the M cells had been depleted were found to be resistant to scrapie infection (13). We are not aware of any published detailed description of M cell development in sheep neonates, and this is clearly necessary before M cells can be reliably associated with the high susceptibility seen in this age group. However, in a study of infection of gut loops in live sheep, an additional M-cell-independent route of entry has been implicated. Villous columnar epithelial cells in the mucosa of the ileum of adult sheep were seen to incorporate PrPSc early in the infection process, well before M-cell-associated uptake (30). It is also known from work in rodents and humans that small but significant amounts of macromolecules are absorbed through gut mucosa and can remain intact in adults, as it is possible to demonstrate the presence in blood of active forms of proteins ingested from the gut, for example, proteases (33) and horseradish peroxidase (61). It is not clear whether the efficiency of this process is greater in younger sheep; however, it does seem to be in young cattle (4) and mice (42), and a recent elegant study in sheep may provide details of a mechanism. Use of in vivo gut loop inoculation of lambs of 7 to 32 days of age with a full-length recombinant PrP polypeptide molecule (rPrP) implicated macrophages in the uptake of rPrP from the gut lumen and through the villous epithelium and suggested that this may be how the nonselective uptake of macromolecules may occur (1). Of course, a demonstration of uptake does not confirm that the infectious process has been successfully initiated or that clinical disease will follow as a direct result, but it is possible that prion infection is opportunistic and able to exploit several different infection routes, some or all of which may be more active in younger animals. Further studies are needed to assess fully whether the M cell or mucosal route is the direct cause of the major change in susceptibility that we have shown for BSE in neonatal sheep and older animals.
Although our results fit well with the idea that after weaning the survival of PrPSc and/or uptake from the sheep gut is considerably reduced, there are other factors that may influence infection initiation and spread. Once PrPSc/infectivity has traveled through the gut wall there are further crucial steps in pathogenesis that may be both developmentally regulated and rate limiting for the spread of infection. TSE neuroinvasion occurs in the periphery and results in dissemination to the brain via autonomic nerves, in particular parasympathetic and sympathetic efferent nerves projecting to the gut and thought to be common pathways (59). Developmental studies of the enteric nervous system (8, 20, 49) in young lambs are rare, but in one reported study (34) the number of neurons in the myenteric plexus of the rumen of suckling lambs was found to be 140 ± 10 per cm2, whereas in young ruminating sheep (5 months of age) there were only 10 ± 3 per cm2 and in adult sheep, 7 ± 2 per cm2. If this is reflected throughout the gut, the numbers of neurons for the uptake of PrPSc/infectivity could be considerably reduced in weaned sheep compared with newborns. Alternatively, once TSE infection is transported across the gut wall, it could take a route via the lymphoreticular system (LRS) prior to invasion of the central nervous system. In the LRS the importance of follicular dendritic cells (FDCs) in the dissemination of infectivity has been elegantly demonstrated in mice (9, 29). Lambs, however, have different immune system status from neonatal mice and are well endowed with FDCs in their lymphoid tissues before birth (25), so this is unlikely to be important in our unweaned sheep.
How do our findings affect the risk analysis for the chances of BSE having infected the United Kingdom sheep flock? Although in some systems solid food (creep feed) is introduced to lambs before weaning, ingestion of large amounts of solid food (for example, protein supplements) is more likely to take place during and after weaning. Sheep should therefore have had considerable protection against BSE infection as a result of a combination of their physiological maturation and these husbandry practices. Our results, which show that risk of infection following exposure to BSE declines markedly after weaning, may therefore help explain the surveillance studies (14, 53) that suggest that despite being susceptible to BSE, United Kingdom sheep were actually at low risk of having been naturally infected with BSE.
ACKNOWLEDGMENTS
This work was funded by contract SE1844 from the United Kingdom Department for Environment, Food and Rural Affairs (Defra).
We acknowledge the contribution to this project of Hugh Simmons and the Arthur Rickwood Sheep Unit staff who provided the study sheep, including embryo derivation. We thank all staff involved in the care of the animals, both at the Institute for Animal Health, Compton (Greenfield Sheep Unit staff), and The Roslin Institute (Dryden staff). We also thank Mari Norimatsu, Richard Lysons, and Sue Halliday for assistance and Chris Bostock for constant encouragement and support during the initial stages of the project. Statistical analysis on the survival of sheep in this study was carried out by M. Nath, BIOSS.
Footnotes
Published ahead of print 22 August 2012
REFERENCES
- 1. Akesson CP, et al. 2012. Phenotypic characterization of cells participating in transport of prion protein aggregates across the intestinal mucosa of sheep. Prion 6:174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andreoletti O, et al. 2000. Early accumulation of PrPSc in gut associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115–3126 [DOI] [PubMed] [Google Scholar]
- 3. Andreoletti O, et al. 2006. Bovine spongiform encephalopathy agent in spleen from an ARR/ARR orally exposed sheep. J. Gen. Virol. 87:1043–1046 [DOI] [PubMed] [Google Scholar]
- 4. Ano Y, et al. 2008. Incorporation of beta-amyloid protein through the bovine ileal epithelium before and after weaning: model for orally transmitted amyloidosis. Microbiol. Immunol. 52:429–433 [DOI] [PubMed] [Google Scholar]
- 5. Arnold ME, Wilesmith J. 2004. Estimation of the age-dependent risk of infection to BSE of dairy cattle in Great Britain. Prev. Vet. Med. 66:35–47 [DOI] [PubMed] [Google Scholar]
- 6. Boelle PY, Cesbron JY, Valleron AJ. 2004. Epidemiological evidence of higher susceptibility to vCJD in the young. BMC Infect. Dis. 4:26–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bohnlein C, Groschup MH, Maertbauer E, Pichner R, Gareis M. 2012. Stability of bovine spongiform encephalopathy prions: absence of prion protein degradation by bovine gut microbiota. Zoonoses Public Health 59:251–255 [DOI] [PubMed] [Google Scholar]
- 8. Brookes SJH, Costa M. 2002. Cellular organization of the mammalian enteric nervous system, p 393–468 In Brookes SJH, Costa M. (ed), Innervation of the gastrointestinal tract. Taylor and Francis, New York, NY [Google Scholar]
- 9. Brown KL, Wathne GJ, Sales J, Bruce ME, Mabbott N. 2009. The effects of host age on follicular dendritic cell status dramatically impair scrapie agent neuroinvasion in ages mice. J. Immunol. 183:5199–5207 [DOI] [PubMed] [Google Scholar]
- 10. Constable PD, Grunberg W, Carstensent L. 2009. Comparative effects of two oral rehydration solutions on milk clotting, abomasal luminal pH, and abomasal emptying rate in suckling calves. J. Dairy Sci. 92:296–312 [DOI] [PubMed] [Google Scholar]
- 11. Dagleish MP, et al. 2010. Digestion and transportation of bovine spongiform encephalopathy-derived protein in the sheep intestine. J. Gen. Virol. 91:3116–3123 [DOI] [PubMed] [Google Scholar]
- 12. Diaz C, Vitezica ZG, Rupp R, Andreoletti O, Elsen J-M. 2005. Polygenic variation and transmission factors involved in the resistance/susceptibility to scrapie in a Romanov flock. J. Gen. Virol. 86:849–857 [DOI] [PubMed] [Google Scholar]
- 13. Donaldson DS, et al. 2012. M cell-depletion blocks oral prion disease. Mucosal Immunol. 5:216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. EFSA 2010. EFSA Panel on Biological Hazards (BIOHAZ): scientific opinion on BSE/TSE infectivity in small ruminant tissues. EFSA J. 8:1875 doi 10.2903/j.efsa.2010.1875 [Google Scholar]
- 15. Ferguson NM, Donelly CA, Woolhouse MEJ, Anderson RM. 1997. The epidemiology of BSE in cattle herds in Great Britain, II. Model construction and analysis of transmission dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 352:803–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Foster JD, et al. 2004. Maternal transmission studies of BSDE in sheep. J. Gen. Virol. 85:3159–3163 [DOI] [PubMed] [Google Scholar]
- 17. Foster JD, Hope J, Fraser H. 1993. Transmission of bovine spongiform encephalopathy to sheep and goats. Vet. Rec. 133:339–341 [DOI] [PubMed] [Google Scholar]
- 18. Foster JD, Parnham D, Chong A, Goldmann W, Hunter N. 2001. Clinical signs, histopathology and genetics of experimental transmission of BSE and natural scrapie to sheep and goats. Vet. Rec. 148:165–171 [DOI] [PubMed] [Google Scholar]
- 19. Foster JD, Parnham DW, Hunter H, Bruce M. 2001. Distribution of the prion protein in sheep terminally affected with BSE following experimental oral transmission. J. Gen. Virol. 82:2319–2316 [DOI] [PubMed] [Google Scholar]
- 20. Furness JB. 2006. Structure of the enteric nervous system, p 1–28 In Furness JB. (ed), The enteric nervous system. Blackwell, Malden, MA [Google Scholar]
- 21. Gardner MLG. 1988. Gastrointestinal absorption of intact proteins. Annu. Rev. Nutr. 8:329–350 [DOI] [PubMed] [Google Scholar]
- 22. Goldmann W, et al. 2006. Ovine prion protein variant A(136)R(154)L(168)Q(171) increases resistance to experimental challenge with bovine spongiform encephalopathy agent. J. Gen. Virol. 87:3741–3745 [DOI] [PubMed] [Google Scholar]
- 23. Goldmann W, Hunter N, Smith G, Foster J, Hope J. 1994. PrP genotype and agent effects in scrapie: change in allelic interaction with different isolates of agent in sheep, a natural host of scrapie. J. Gen. Virol. 75:8989–8995 [DOI] [PubMed] [Google Scholar]
- 24. Hadlow WJ. 1999. Reflections on the transmissible spongiform encephalopathies. Vet. Pathol. 36:523–529 [DOI] [PubMed] [Google Scholar]
- 25. Halleraker M, Press CM, Landsverk T. 1994. Development and cell phenotypes in primary follicles of foetal sheep lymph nodes. Cell Tissue Res. 275:51–62 [DOI] [PubMed] [Google Scholar]
- 26. Hamir AN, Kunkle RA, Greenlee JJ, Richt JA. 2009. Experimental oral transmission of United States origin scrapie to neonatal sheep. J. Vet. Diagn. Invest. 21:64–68 [DOI] [PubMed] [Google Scholar]
- 27. Houston EF, Halliday SI, Jeffrey M, Goldmann W, Hunter N. 2002. New Zealand sheep with scrapie-susceptible PrP genotypes succumb to experimental challenge with a sheep-passaged scrapie isolate (SSBP/1). J. Gen. Virol. 83:1247–1250 [DOI] [PubMed] [Google Scholar]
- 28. Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. 2000. Transmission of BSE by blood transfusion in sheep. Lancet 356:999–1000 [DOI] [PubMed] [Google Scholar]
- 29. Ierna M, Farquhar CF, Outram GW, Bruce ME. 2006. Resistance of neonatal mice to scrapie is associated with inefficient infection of the immature spleen. J. Virol. 80:474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jeffrey M, et al. 2006. Transportation of prion protein across he intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J. Pathol. 209:4–14 [DOI] [PubMed] [Google Scholar]
- 31. Jeffrey M, et al. 2006. Immunochemical features of PrPd accumulation in natural and experimental goat transmissible spongiform encephalopathies. J. Comp. Pathol. 134:171–181 [DOI] [PubMed] [Google Scholar]
- 32. Laegreid WW, et al. 2008. Scrapie resistance in ARQ sheep. J. Virol. 82:10318–10320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lorkowski G. 2012. Gastrointestinal absorption and biological activities of serine and cysteine proteases of animal and plant origin: review on absorption of serine and cysteine proteases. Int. J. Physiol. Pathophysiol. Pharmacol. 4:10–27 [PMC free article] [PubMed] [Google Scholar]
- 34. Marruchella G, et al. 2009. Ileal tract and Peyer's patch innervations in scrapie-free versus scrapie-affected ovines. Arch. Virol. 154:709–714 [DOI] [PubMed] [Google Scholar]
- 35. Marruchella G, Ligios C, Di Guardo G. 2012. Age, scrapie status, PrP genotype and follicular dendritic cells in ovine Peyer's patches. Res. Vet. Sci. 93:853–856 [DOI] [PubMed] [Google Scholar]
- 36. Martin S, et al. 2005. Immunohistochemical characteristics of disease-associated PrP are not altered by host genotype or route of inoculation following infection of sheep with bovine spongiform encephalopathy. J. Gen. Virol. 86:839–848 [DOI] [PubMed] [Google Scholar]
- 37. Martinsen TC, Benestad SL, Moldal T, Waldum HL. 2011. Inhibitors of gastric acid secretion increase the risk of prion infection in mice. Scand. J. Gastroenterol. 46:1418–1422 [DOI] [PubMed] [Google Scholar]
- 38. Matthews L, Coen PG, Foster JD, Hunter N, Woolhouse MEJ. 2001. Population dynamics of a scrapie outbreak. Arch. Virol. 146:1173–1186 [DOI] [PubMed] [Google Scholar]
- 39. Mayer B, et al. 2002. Redistribution of the sheep neonatal Fc receptor in the mammary gland around the time of parturition in ewes and its localization in the small intestine of neonatal lambs. Immunology 107:288–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McKinley MP, DeArmond SJ, Torchia M, Mobley WC, Prusiner SB. 1989. Acceleration of scrapie in neonatal Syrian hamsters. Neurology 39:1319–1324 [DOI] [PubMed] [Google Scholar]
- 41. Nicholson EM, et al. 2007. Exposure of sheep scrapie brain homogenate to rumen stimulating conditions does not result in a reduction of PrPSc levels. Lett. Appl. Microbiol. 44:631–636 [DOI] [PubMed] [Google Scholar]
- 42. Okamoto M, et al. 2003. Experimental transmission of abnormal prion protein (PrPSc) in the small intestinal epithelial cells of neonatal mice. Vet. Pathol. 40:723–727 [DOI] [PubMed] [Google Scholar]
- 43. Outram GW, Dickinson AG, Fraser H. 1973. Developmental maturation of susceptibility to scrapie in mice. Nature 241:536–537 [DOI] [PubMed] [Google Scholar]
- 44. Prusiner SB. 1998. Prions. Proc. Natl. Acad. Sci. U. S. A. 95:13363–13383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reynolds JD, Morris B. 1983. The evolution and involution of Peyer's patches in fetal and post natal sheep. Eur. J. Immunol. 13:626–635 [DOI] [PubMed] [Google Scholar]
- 46. Santucciu C, et al. 2010. Association of N176K and L141F dimorphisms of the PRNP gene with lack of pathological prion protein deposition in placentas of naturally and experimentally scrapie-affected ARQ/ARQ sheep. J. Gen. Virol. 91:2402–2407 [DOI] [PubMed] [Google Scholar]
- 47. Saunders GC, et al. 2009. Protective effect of the T112PrP variant in sheep challenged with bovine spongiform encephalopathy. J. Gen. Virol. 90:2569–2574 [DOI] [PubMed] [Google Scholar]
- 48. Scherbel C, et al. 2006. Degradation of scrapie associated prion protein (PrPSc) by the gastrointestinal microbiota of cattle. Vet. Res. 37:695–703 [DOI] [PubMed] [Google Scholar]
- 49. Schneider DA, et al. 2008. Myenteric neurons of the ileum that express somatostatin are a target of prion neuroinvasion in an alimental model of sheep scrapie. Acta Neuropathol. 115:651–661 [DOI] [PubMed] [Google Scholar]
- 50. St Rose SG, et al. 2007. Quantification of Peyer's patches in Cheviot sheep for future scrapie pathogenesis experiments Vet. Immunol. Immunopathol. 116:163–171 [DOI] [PubMed] [Google Scholar]
- 51. St Rose SG, et al. 2006. Comparative evidence for a link between Peyer's patch development and susceptibility to transmissible spongiform encephalopathies, BMC Infect. Dis. 6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stack M, et al. 2009. Three serial passages of bovine spongiform encephalopathy in sheep do not significantly affect discriminatory test results. J. Gen. Virol. 90:764–768 [DOI] [PubMed] [Google Scholar]
- 53. Stack M, et al. 2006. Monitoring for bovine spongiform encephalopathy in sheep in Great Britain, 1998–2004. J. Gen. Virol. 87:2099–2107 [DOI] [PubMed] [Google Scholar]
- 54. Tan BC, et al. 2010. Codon 141 in ovine PRNP gene modulates incubation time in sheep orally infected with BSE. Prion 4:195 [Google Scholar]
- 55. Terry LA, et al. 2003. Detection of disease-specific PrP in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy. Vet. Rec. 152:387–392 [DOI] [PubMed] [Google Scholar]
- 56. Tizard IR. 1992. Veterinary immunology: an introduction, 4th ed W. B. Saunders Co., Philadelphia, PA [Google Scholar]
- 57. Tizard IR. 2000. Veterinary immunology. 6th ed W. B. Saunders Co., Philadelphia, PA [Google Scholar]
- 58. Van de Perre P. 2003. Transfer of antibody in mother's milk. Vaccine 21:3374–3376 [DOI] [PubMed] [Google Scholar]
- 59. Van Keulen LJM, Vromans ME, Dolstra CH, Bossers A, Van Zijderveld FG. 2008. Pathogenesis of bovine spongiform encephalopathy in sheep. Arch. Virol. 153:445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Van Keulen LJM, Vromans MEW, Van Zijderveld FG. 2002. Early and late pathogenesis of natural scrapie infection in sheep. APMIS 110:23–32 [DOI] [PubMed] [Google Scholar]
- 61. Warshaw AL, Walker WA, Cornell R, Isselbacher KJ. 1971. Small intestinal permeability to macromolecules. Transmission of horseradish peroxidase in to mesenteric lymph and portal blood. Lab. Invest. 25:675–596 [PubMed] [Google Scholar]
- 62. Yvon M, Levieux D, Valluy MC, Pelisser JP, Mirand PP. 1992. Colostrum protein digestion in newborn lambs. J. Nutr. 123:586–596 [DOI] [PubMed] [Google Scholar]