Abstract
Evidence was obtained on the occurrence of protein threonine, serine, and tyrosine (Tyr) kinases in developing coconut (Cocos nucifera L.) zygotic embryos, based on in vitro phosphorylation of proteins in the presence of [γ-32P]ATP, alkaline treatment, and thin-layer chromatography analysis, which showed the presence of [32P]phosphoserine, [32P]phosphothreonine, and [32P]phosphotyrosine in [32P]-labeled protein hydrolyzates. Tyr kinase activity was further confirmed in extracts of embryos at different stages of development using antiphosphotyrosine monoclonal antibodies and the synthetic peptide derived from the amino acid sequence surrounding the phosphorylation site in pp60src (RR-SRC), which is specific for Tyr kinases. Anti-phosphotyrosine western blotting revealed a changing profile of Tyr-phosphorylated proteins during embryo development. Tyr kinase activity, as assayed using RR-SRC, also changed during embryo development, showing two peaks of activity, one during early and another during late embryo development. In addition, the use of genistein, a Tyr kinase inhibitor, diminished the ability of extracts to phosphorylate RR-SRC. Results presented here show the occurrence of threonine, serine, and Tyr kinases in developing coconut zygotic embryos, and suggest that protein phosphorylation, and the possible inference of Tyr phosphorylation in particular, may play a role in the coordination of the development of embryos in this species.
Substantial progress in the understanding of the events that govern embryo formation has been achieved in a variety of animal and nonplant systems; however, much less is known of this subject in plants (Brownlee and Berger, 1995). During embryogenesis a precise control of events such as cell division, differentiation, and growth is required (Turner, 1991). In animal systems these events appear to be regulated by protein phosphorylation through a concerted action of kinases (Simon et al., 1993; Turner, 1994; Hou et al., 1995) and phosphatases (Cyert and Thorner, 1989; Sun and Tonks, 1994). Most of the protein phosphorylation in cells occurs in residues of Ser and Thr, and in minor proportion in residues of Tyr. In the case of Tyr phosphorylation, a role in embryogenesis in animal cells has been suggested, since changes in the levels of protein Tyr phosphorylation have been shown to accompany embryo development in vertebrates (Maher and Pasquale, 1988; Turner, 1991, 1994; Gilardi-Hebenstreit et al., 1992; Nieto et al., 1992; Snider, 1994) and invertebrates (Shilo, 1992; Perrimon, 1993).
The occurrence of protein phosphorylation and kinase activity in plants has been reported in several species (for review, see Stone and Walker, 1995). According to these reports, it seems that protein phosphorylation occurs more abundantly in Ser and Thr residues than in Tyr residues (Reddy et al., 1987; Saluja et al., 1987; Stone and Walker, 1995). Nevertheless, Tyr protein phosphorylation and Tyr kinase activity have already been reported in a few plant species, such as pea (Torruela et al., 1986; Håkansson and Allen, 1995), alfalfa (Duerr et al., 1993), tobacco (Suzuki and Shinshi, 1995; Zhang et al., 1996), and maize (Trojanek et al., 1996).
The present study reports the occurrence of protein kinase activities in developing coconut zygotic embryos, which can phosphorylate proteins in Thr, Ser, and Tyr residues. Particular changes in the patterns of phosphorylated proteins and Tyr kinase activity during coconut embryo development are also described.
MATERIALS AND METHODS
Plant Material
Seeds were collected from coconut (Cocos nucifera L.) groves in the Yucatán Península, México, and transported to the laboratory. The embryo developmental stages were arbitrarily defined, taking the first pollinated inflorescence as stage 0; stages 1 to 16 correspond to inflorescences of increasing maturity. Embryos were excised from the seeds in the laboratory and immediately processed.
Tissue Homogenization
Embryos were excised from seeds and immediately homogenized in buffer containing: 50 mm Tris-HCl, pH 7.4, 10 mm sodium pyrophosphate, 50 mm NaCl, 250 mm Suc, 10% glycerol, 1 mm EGTA, 0.2 mm orthovanadate, 1 mm PMSF, 1 mm β-mercaptoethanol, and 1 μg/mL leupeptin and aprotinin (extraction buffer). Homogenates were centrifuged at 14,000g for 30 min. Supernatants were further centrifuged at 100,000g for 45 min at 4°C. The supernatants (5–7 mg protein/mL), referred as the soluble fraction, were snap-frozen and stored in liquid nitrogen prior to analysis.
Protein Quantification
Protein concentration of samples was measured according to the method of Smith et al. (1985), using the bicinchorinic acid protein assay reagent and BSA as the standard (Pierce).
In Vitro Phosphorylation Assay
Aliquots of soluble fractions (100 μg of protein) were incubated at 30°C with 20 mm Hepes, pH 7.0, 10 mm MgCl2, 0.1 mm orthovanadate, and 50 μm ATP plus 2 μCi/nmol [γ-32P]ATP (NEN-Dupont). After 15 min, the reaction was stopped with Laemmli buffer (Laemmli, 1970) and heated for 5 min at 85°C. The samples were analyzed by 10% SDS/PAGE (40 V, overnight) and electroblotted onto Nitroplus transfer membrane (Micron Separations, Westborough, MA) for 5 h at 50 V. Phosphoprotein bands were visualized by autoradiography.
Immunoblot Analysis
Proteins in the soluble fractions were phosphorylated (or not) in vitro as described above, except that [γ-32P]ATP was not added. After 15 min, the reaction was stopped with Laemmli buffer and heated for 5 min at 85°C. The samples were analyzed by 10% SDS/PAGE (40 V, overnight) and either stained with Coomassie blue or electroblotted onto Nitroplus transfer membrane for 5 h at 50 V. Filters were blocked in 5% defatted milk in TBS-T buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, and 0.1% Tween 20) for 60 min. Membranes were washed in TBS-T buffer twice for 15 min and then probed in a 1:1000 dilution of recombinant anti-PY coupled to horseradish peroxidase RC20H (Transduction Laboratories, Lexington, KY) for 1 h at room temperature. The membranes were washed twice for 15 min in 100 mL (each time) of TBS-T buffer. Immunodetection was revelated in a dark room by exposing membranes for 1 min in enhanced chemiluminescence reagent (Amersham).
Protein Tyr Kinase Assay
This assay was done as described for in vitro phosphorylation in the presence of 10 μg of RR-SRC (GIBCO-BRL), and the reaction was stopped by precipitation with a cold solution of 10% TCA. The mixture was centrifuged at 14,000g for 10 min at 4°C. The supernatant was loaded onto phosphocellulose filters (Life Technologies). Filters were washed twice with 1% acetic acid and water and dried, and the radioactivity was determined by Cerenkov counting. Two controls were used in this assay: one with the extract alone in the absence of RR-SRC and another in the presence of RR-SRC but in the absence of protein extract. In both cases the activity was less than 1 pmol and was subtracted.
Alkaline Treatment
Proteins in the soluble fraction (100 μg) were phosphorylated in vitro in the presence of 10 μCi/nmol of [γ-32P]ATP (NEN-Dupont) for 15 min, and the reaction was stopped with Laemmli buffer and heated for 5 min at 60°C. Samples were analyzed by 10% SDS/PAGE (40 V, overnight). Proteins were blotted onto a Hybond-PVDF membrane (Amersham) at 50 V for 5 h. Membranes were divided into two identical fractions that contained the same samples. One membrane was incubated in 1 m KOH at 56°C for 90 min, and the control membrane was maintained in water in the same conditions as the KOH-treated membrane. Membranes were independently washed twice (5 min each time) in 100 mL (each time) of TN buffer (10 mm Tris-HCl, pH 7.4, and 150 mm NaCl) followed by two washes in 1 m Tris-HCl, pH 7.0, and distilled water. Membranes were dried and exposed to autoradiography for 48 h at −80°C.
Protein Hydrolysis
[32P]-labeled proteins were excised from PVDF membranes as strips (0.2 cm wide). The strips were rewet in methanol for 1 min and then rewet in 0.5 mL of water and placed into a glass ampule containing 300 μL of 6 n HCl (Sigma). The ampules were sealed and incubated at 110°C for 2 h in an oven (Duo-Vac, Lab-Line, Melrose Park, IL). At the end of the hydrolysis, the ampules were cooled at room temperature and centrifuged at 14,000g for 1 min. Then seals were broken and the hydrolysates were transferred to 13- × 100-mm glass tubes (Pyrex) and dried for 30 min in a Speedvac (Savant, Fullerton, CA). The dried hydrolysates were resuspended in 50 μL of distilled and deionized water and dried in the Speedvac as above. This step was repeated three times, and the hydrolysates were resuspended in 10 μL of distilled and deionized water.
Phosphoamino Acid Analyses
Protein of hydrolysate aliquots (7 μL) was loaded onto 20- × 20-cm cellulose TLC plates (Aldrich precoated TLC plates: cellulose on glass with 254-nm fluorecent indicator, 250-μm layer thickness, and fiber length of 2–20 μm). In each sample, 10 μg of internal phosphoamino acid standards (phosphoserine, phosphothreonine, and phosphotyrosine; Sigma) were included, and the phosphoamino acid standards were also loaded onto parallel lanes singly and as a mixture. The plates were developed first with n-propanol:0.5 n HCl (2:1, v/v) followed by n-butanol:acetic acid:water (100:22:50, v/v) in the same direction. The phosphoamino acid standards were located by staining with ninhydrin spray reagent (Sigma) and pencil marked. The plates were dried before autoradiography at −80°C with an intensifier.
Extracts of Cell Line A431
Extracts of the human epidermoid carcinoma cell line A431 were used as a postive control, since it is well documented (Sorkin et al., 1991; Rodríguez-Zapata and Hernández-Sotomayor, 1998) that this line contains kinase activity for Ser, Thr, and Tyr residues and substrates for these kinases. One of these substrates, the EGF receptor, has Ser, Thr, and Tyr residues that can be phosphorylated, and it also has Tyr kinase activity. A431 cells were treated with EGF and extracted with Triton X-100 as described previously (Hernández-Sotomayor et al., 1991). A431 cells were kindly provided by Dr. Graham Carpenter (Vanderbilt University, Nashville, TN).
RESULTS
Embryo Development
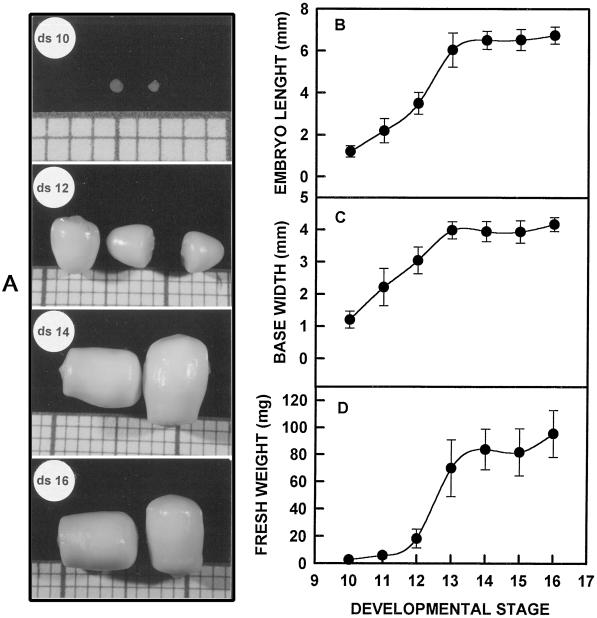
Changes in size and form were observed during embryo development. Form changed from round (stages 10–12) to cylindrical (stages 14–16). Length, base width, and fresh weight increased as the embryo developed, reaching maximum values, 6.6 mm, 3.7 mm, and 90 mg respectively, at stages 13 to 14 (Fig. 1).
Figure 1.
A, Developing zygotic embryos excised from coconuts at different developmental stages. Grid square side = 1 mm. B through D, Evaluation of embryo growth: B, length, C, base width, and D, fresh weight. Bars denote sd; n = 50.
Alkaline Treatment
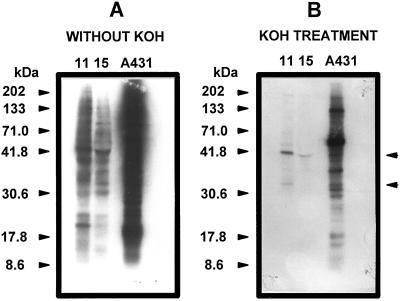
[γ-32P]ATP-phosphorylated proteins from the soluble fraction of developmental stages 11 and 15 were transferred to Hybond-PVDF membranes, treated with KOH, and autoradiographed (48 h at −80°C). Both the control membrane that was not treated with KOH and cell line A431 showed a complex pattern of bands from the developing embryo samples, some showing high-intensity signals (Fig. 2A). After alkaline treatment a high content of 32P was removed, as shown by the decrease in signal intensity of bands (Fig. 2B). Several of the bands disappeared, but some remained, although with a weaker signal, such as those at 175, 156, 125, 62, 44, 37, and 33 kD (stage 11), and 37 kD (stage 15). In contrast, some bands remained with a strong signal, such as those at 41 kD (stages 11 and 15). In line A431 the signal of several bands remained after alkaline treatment (Fig. 2B). The signal of the EGF receptor (170 kD) was not detected before or after treatment.
Figure 2.
Effect of alkaline treatment on in vitro [γ-32P]ATP-labeled proteins of soluble fractions from coconut zygotic embryos of developmental stages 11 and 15. [32P]-Labeled proteins were separated by 10% SDS-PAGE and electroblotted onto nylon membranes. Membranes were left without treatment (A) or treated with 1 m KOH (B), and bands were visualized by autoradiography. Figures with arrowheads on the left show the molecular mass markers. The arrowheads on the right show the protein bands that remained phosphorylated after the KOH treatment. A431 denotes a carcinoma cell line that was included as a positive control. The amount of protein per lane was 100 μg. The results shown are representative of four separate experiments.
Phosphoamino Acid Analysis of [32P]-Labeled Protein Hydrolysates
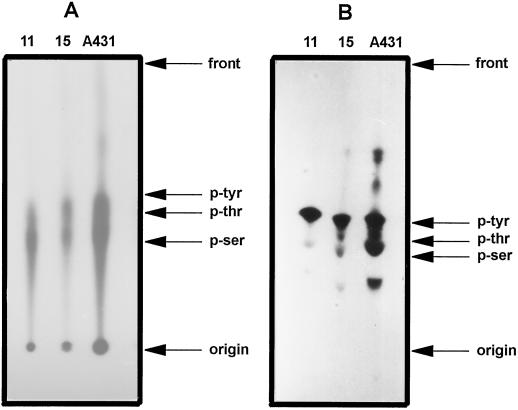
TLC of 32P-labeled protein from coconut hydrolysates of the full complement proteins (developmental stages 11 and 15) showed two signals that comigrated with authentic phosphothreonine and phosphoserine, but phosphotyrosine was not detectable (Fig. 3A). In cell line A431 the three phosphoamino acids were detectable. However, phosphoamino acid analysis of blotted proteins that remained [32P] labeled after treatment with KOH not only revealed the occurrence of phosphothreonine and phosphoserine, but also of phosphotyrosine, which was the most abundant phosphoamino acid (Fig. 3B). In hydrolysates of proteins from A431 cells the three phosphoamino acids were detected, as well as three additional bands that were not identified.
Figure 3.
TLC analysis of phosphoamino acids of in vitro [γ-32P]ATP-labeled proteins of the soluble fraction from embryos at developmental stages 11 and 15. Proteins were hydrolyzed and 7-μL aliquots of the hydrolysates were loaded onto phosphocellulose plates and developed. A, Results of analysis of all of the [32P]-labeled proteins. B, Results of the 41-kD protein only. Plates were autoradiographed after development, and the phosphoamino acids were identified by comparison with authentic, unlabeled phosphoamino acid standards. The A431 cell line hydrolysate was included as a positive control. The results are representative of three separate experiments.
Tyr Kinase Assay
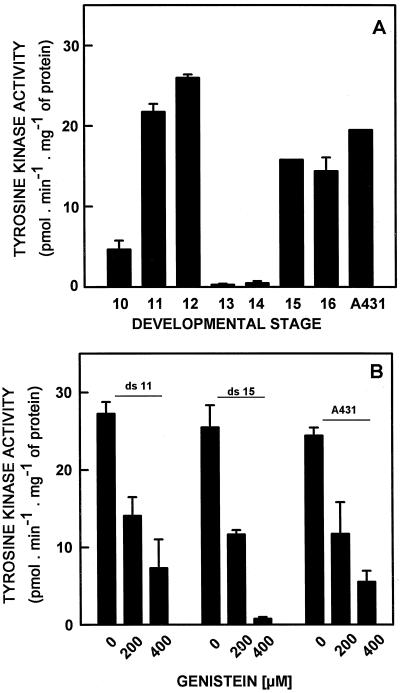
Soluble fractions from embryos of stages 10 to 16 were able to phosphorylate RR-SRC (Pike et al., 1982). This peptide is specific for Tyr kinases, because in its sequence it has a single phosphorylation site, a Tyr residue (RRLIEEDAE[Y]AARG), and it is extensively used to characterize Tyr kinase activity (Pike et al., 1982). The results showed two peaks of activity, one during early development (stages 11–12) and another during late development (stages 15–16) (Fig. 4A). Extracts of A431 cells were able to phosphorylate RR-SRC as well. The ability of embryo-soluble fractions (stages 11 and 15) to phosphorylate RR-SRC was reduced with the addition of genistein (extensively used in animal cells as a specific Tyr kinase inhibitor: O'Dell et al., 1991; Fry and Bridges, 1995) to about 50% (stages 11 and 15) when used at 200 μm, and to 25% (stage 11) and 5% (stage 15) when used at 400 μm (Fig. 4B). Similarly, the addition of genistein to extracts of A431 cells also reduced their ability to phosphorylate RR-SRC (Fig. 4B).
Figure 4.
Tyr kinase activity in the soluble fractions of coconut zygotic embryos at developmental stages 10 to 16 (A) and the effect of (200 and 400 μm) genistein on this activity in soluble fractions of embryos at stages 11 and 15 (B). Tyr kinase activity was assayed in vitro with the synthetic substrate RR-RSC. The specific activity was calculated as picomoles of 32P incorporated into RR-SRC min−1 mg−1 protein. The A431 carcinoma cell line was included as a positive control. The data shown are the averages of from three separate experiments. Bars denote sd.
Immunoblot Analysis
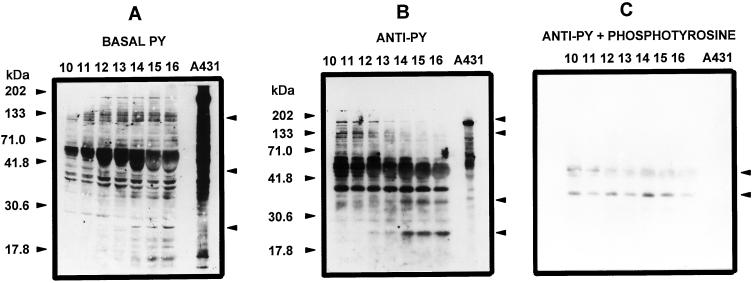
Soluble fractions of developing embryos (stages 10–16) were incubated (or not for controls) for in vitro phosphorylation prior to SDS-PAGE and protein blotting. The occurrence of basal phosphorylation in Tyr residues, which was detected as anti-PY, revealed the presence of several bands in the untreated controls (Fig. 5A). The intensity of some of the bands changed during development. For bands at 51, 53, and 41 kD, it was greater during early development (stages 10–12) and decreased during late development (stages 13–16). In contrast, the bands at 37, 35, and 24 kD showed low intensity during the early stages, which increased notably later (at stage 14). In samples subjected to in vitro phosphorylation, the intensity of those bands observed in the controls increased and additional bands were detectable (Fig. 5B). Both basal and enhanced Tyr phosphorylation showed a differential pattern, depending on the developmental stage. The intensity of the bands at 175, 151, 125, 108, 75, 67, 62, 51, 44, and 41 kD was greater during early development (stages 10–13) than in later stages of development. In contrast, two additional major phosphotyrosine-containing bands of approximately 31 and 24 kD were observed only during late development (stages 14–16). When 1 mm phosphotyrosine was added to the antibody preparation, with the exception of two bands at 47.5 and 37 kD, the band patterns were not revealed (Fig. 5C). These bands did not appear when the concentration of phosphotyrosine was increased to 2 mm (not shown). When extracts from tissue surrounding the embryo and tissues of vegetative parts (root, stem, and leaf) were assayed, no signal was observed in the immunoblots (not shown).
Figure 5.
Patterns of Tyr-phosphorylated proteins from extracts of coconut zygotic embryos at developmental stages 10 to 16. Proteins (100 μg) were separated by 10% SDS-PAGE, electroblotted onto nitrocellulose membranes, and immunodetected using the anti-PY RC20 H. Proteins were assayed directly (A) or subjected before electrophoresis to in vitro phosphorylation without (B) or with (A) 1 mm phosphotyrosine. Figures and arrowheads on the left denote molecular mass markers. Arrowheads on the right denote bands that change their intensity with development (A and B) or to those that remained after treatment with phosphotyrosine (C). The results shown are representative of five separate experiments.
Immunodetection of the extract of A431 cells revealed bands at 107, 86, and 37 kD, and a major one at 170 kD corresponds to the EGF receptor (Fig. 5, A and B). Surprisingly, the EFG receptor Tyr phosphorylation was detected by immunoblot and not after alkaline treatment (Fig. 2). A possible explanation for this discrepancy is the fact that to have signal after the alkali treatment, the proteins had to incorporate 32P-PO4 during the in vitro phosphorylation. If those proteins, like the EGF-receptor band, were already Tyr phosphorylated in vivo, the incorporation of 32P expected in vitro would not be the same as for a protein that was not totally Tyr phosphorylated in vivo. It is important to remember that in this case, A431 cells were incubated in vivo with EGF to activate Tyr phosphorylation. With the addition of phosphotyrosine the pattern was not revealed (Fig. 5C).
DISCUSSION
During early development (developmental stages 10–12) of coconut zygotic embryos, the growth rate was fast, whereas during late development (stages 13–16) the growth rate was very slow. These differences in growth were accompanied by differentiation of the embryo organs. In early developing embryos, the haustorium either was not present or was in formation and the plumule was not well defined, whereas in mature embryos, the haustorium was already present and the plumule was clearly differentiated. SDS-PAGE protein patterns evolved as embryos differentiated. These proteins included protein kinase activity, as evidenced by the ability of extracts from developing embryos to generate [32P]-labeled proteins in the presence of [γ-32P]ATP.
Phosphotyrosine residues are stable at alkaline pH, but the O-linked phosphoamino acids phosphoserine and phosphothreonine are not (Bourassa et al., 1988; Noiman and Shaul, 1995). In the present study alkaline treatment displaced most of the radioactivity from the [32P]-labeled proteins, suggesting abundant phosphorylation in Thr and/or Ser residues, and in a much smaller proportion in Tyr residues, in those proteins that remained labeled (33, 41, and 62 kD). [32P]-labeled proteins resistant to alkaline treatment have also been reported in other plants species: in soluble fractions from transformed roots of Catharanthus roseus a protein at 63 kD (Rodríguez-Zapata and Hernández-Sotomayor 1998), and in maize seedlings a group of proteins ranging from 45 to 60 kD (Trojanek et al., 1996). In both reports these [32P]-labeled proteins were able to bind monoclonal anti-PY.
The occurrence of [32P]Thr, [32P]Ser, and [32P]Tyr in coconut embryos was confirmed by phosphoamino acid analysis of [32P]-labeled proteins, an approach previously used to show the occurrence of these three phosphoamino acids in [32P]-labeled proteins in wheat embryos (Saluja et al., 1987) and maize seedlings (Trojanek et al., 1996). Therefore, these results show the occurrence of kinase activity in developing coconut embryos that includes Thr, Ser, and Tyr kinases.
Evaluation by SDS-PAGE of the pattern of phosphorylated proteins showed changes as embryos developed; some bands were more intense or only present during early development and some bands were more intense or only present during late development (data not shown). This suggests that kinase activity may play a role during coconut embryo development, as has already been proposed for kinase activity in animal embryo development, particularly for Tyr kinases (Maher and Pasquale, 1988; Turner, 1991; Clark et al., 1992; Simon et al., 1993; Hou et al., 1995). We sought further insight into Tyr kinase activity and Tyr phosphorylation of proteins, taking advantage of the availability of highly specific tests such as synthetic substrates that can only be phosphorylated in Tyr (RR-SRC), specific Tyr kinase inhibitors, and anti-PY.
Embryo extracts were able to phosphorylate the synthetic substrate RR-SRC, and this phosphorylation was strongly reduced with the addition of genistein, a specific Tyr kinase inhibitor used in studies with animal systems (O'Dell et al., 1991; Fry and Bridges 1995), and was recently reported as an inhibitor of Tyr kinase in plant systems (Håkansson and Allen, 1995; Bernasconi, 1996; Rodríguez-Zapata and Hernández-Sotomayor, 1998). These results confirm the presence of Tyr kinase activity in developing coconut embryos. The level of this kinase activity in coconut embryos was several times higher than those reported in transformed roots of C. roseus using the same substrate (Rodríguez-Zapata and Hernandez-Sotomayor, 1998), and in maize seedlings using the substrates poly(Glu-80, Tyr-20) and [Val-5]ang II (Trojanek et al., 1996). It is important to point out that the synthetic peptide RR-SRC is not phosphorylated by all Tyr kinases and it is posssible that other protein Tyr kinases are active during other stages of embryogenesis.
As in the case of general phosphorylation assays, the expression of Tyr kinase activity in the developing embryos was dynamic. It showed two peaks, one during early and another during late embryo development. Similar temporal changes of Tyr kinase activity and substrates during tissue development have been reported in animal systems (Maher and Pasquale, 1988; Turner, 1991, 1994). In these studies high levels of phosphotyrosine-containing proteins were detected during early development, whereas barely noticeable levels were found in more differentiated tissues. In the present study Tyr kinase activity was only found associated with embryo tissues. When extracts of tissue surrounding the embryo and tissues of vegetative parts were assayed, no Tyr kinase activity was detected.
The possible inference of Tyr phosphorylation of embryo proteins was further supported by the following observations: (a) protein bands gave a positive signal with anti-PY during immunoblot analysis of embryo extracts, signals that increased in intensity in some bands after phosphorylation in vitro; and (b) when (2 mm) phosphotyrosine was added to the antibody preparation, the band patterns were not revealed. The pattern of phosphotyrosine-containing proteins changed as embryos developed, some bands increased or decreased in intensity, and some bands appeared or disappeared as development progressed. Immunodetection of phosphotyrosine-containing proteins was only found associated with embryo tissues. No detection was associated with extracts of tissue surrounding the embryo and tissues of vegetative parts.
Collectively, the results presented here show the occurrence of protein Thr, Ser, and Tyr kinases in developing coconut zygotic embryos. The temporal changes that occur in protein phosphorylation suggest that kinase activity, and Tyr kinase activity in particular, may play a role in the coordination of the development of zygotic embryos in this species. Further investigations will focus on identifying the genes for these kinases, as well as their physiological role.
ACKNOWLEDGMENTS
We thank Dr. Graham Carpenter for generous assistance with reagents and advice, Dr. Roger Ashburner for revision of the manuscript, and Sue Carpenter for administrative support with the Fogarty International Research Collaboration Award grant.
Abbreviations:
- A431
human carcinoma cell line
- anti-PY
antiphosphotyrosine antibodies
- EGF
epidermal growth-factor receptor
- RR-SRC
synthetic peptide derived from the amino acid sequence surrounding the phosphorylation site in pp60src
Footnotes
This work was supported by Fogarty International Research Collaboration Award (grant no. RO3TW00263), the Consejo Nacional de Ciencia y Tecnología (CONACYT) (grant no. 0014P-N9506), the Commission of the European Communities EC-STD (grant no. ERBTS3*CT940298), and a CONACYT Fellowship to I.I.-F. (no. 89535).
LITERATURE CITED
- Bernasconi P. Effect of synthetic and natural protein tyrosine kinase inhibitors on auxin efflux in zucchini (Cucurbita pepo) hypocotyls. Physiol Plant. 1996;96:205–210. [Google Scholar]
- Bourassa C, Chapdelaine A, Roberts KD, Chevalier S. Enhancement of the detection of alkali-resistant phosphoproteins in polyacrylamide gels. Anal Biochem. 1988;169:356–362. doi: 10.1016/0003-2697(88)90295-3. [DOI] [PubMed] [Google Scholar]
- Brownlee C, Berger F. Extracellular matrix and pattern in plant embryos: on the lookout for developmental information. Trends Genet. 1995;11:344–348. doi: 10.1016/s0168-9525(00)89104-0. [DOI] [PubMed] [Google Scholar]
- Clark SG, Stern MJ, Horvitz HR. C. elegans cell-signalling gene seems encodes a protein with SH2 and SH3 domains. Nature. 1992;356:340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Cyert MS, Thorner J. Putting it on and taking it off: phosphoprotein phosphatase involvement in cell cycle regulation. Cell. 1989;57:891–893. doi: 10.1016/0092-8674(89)90325-5. [DOI] [PubMed] [Google Scholar]
- Duerr B, Gawienowski M, Ropp T, Jacobs T. MsERK1: a mitogen-activated protein kinase from a flowering plant. Plant Cell. 1993;5:87–96. doi: 10.1105/tpc.5.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry DW, Bridges AJ. Inhibitors of protein tyrosine kinases. Curr Opin Biotechnol. 1995;6:662–667. doi: 10.1016/0958-1669(95)80109-x. [DOI] [PubMed] [Google Scholar]
- Gilardi-Hebenstreit P, Nieto MA, Frain M, Mattei MG, Chestier A, Wilkinson DG, Charnay P. An Eph-related receptor protein tyrosine kinase gene segmentally expressed in the developing mouse hindbrain. Oncogene. 1992;7:2499–2506. [PubMed] [Google Scholar]
- Håkansson G, Allen JF. Histidine and tyrosine phosphorylation in pea mitochondria: evidence for protein phosphorylation in respiratory redox signalling. FEBS Lett. 1995;372:238–242. doi: 10.1016/0014-5793(95)00990-q. [DOI] [PubMed] [Google Scholar]
- Hernández-Sotomayor SMT, Mumby M, Carpenter G. Okadaic acid-induced hyperphosphorylation of the epidermal growth factor receptor. J Biol Chem. 1991;266:21281–21286. [PubMed] [Google Scholar]
- Hou XS, Chou TB, Melnick MB, Perrimon N. The torso receptor tyrosine kinase can activate raf in ras-independent pathway. Cell. 1995;81:63–71. doi: 10.1016/0092-8674(95)90371-2. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maher PA, Pasquale E. Tyrosine phosphorylated proteins in different tissues during chick embryo development. J Cell Biol. 1988;106:1747–1755. doi: 10.1083/jcb.106.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto MA, Gilardi-Hebenstreit P, Charnay P, Wilkinson DG. A receptor protein tyrosine kinase implicated in the segmental patterning of the hindbrain and mesoderm. Development. 1992;116:1137–1150. doi: 10.1242/dev.116.4.1137. [DOI] [PubMed] [Google Scholar]
- Noiman S, Shaul Y. Detection of histidine-phosphoproteins in animal tissues. FEBS Lett. 1995;364:63–66. doi: 10.1016/0014-5793(95)00358-g. [DOI] [PubMed] [Google Scholar]
- O'Dell TJ, Kandell ER, Grant SGN. Long-term potentiation in the hippocampus is blocked by tyrosine kinase inhibitors. Nature. 1991;353:558–560. doi: 10.1038/353558a0. [DOI] [PubMed] [Google Scholar]
- Perrimon N. The torso receptor protein-tyrosine kinase signalling pathway: an endless story. Cell. 1993;74:219–222. doi: 10.1016/0092-8674(93)90412-j. [DOI] [PubMed] [Google Scholar]
- Pike LA, Gallis B, Casnellie JE, Bornstein P, Krebs EG. Epidermal growth factor stimulates the phosphorylation of synthetic tyrosine-containing peptides A431 cell membranes. Proc Natl Acad Sci. 1982;79:1443–1447. doi: 10.1073/pnas.79.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AS, Raina A, Gunnery S, Datta A. Regulation of protein synthesis in plant embryo by protein phosphorylation. Plant Physiol. 1987;83:988–993. doi: 10.1104/pp.83.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Zapata LC, Hernández-Sotomayor SMT. Evidence of protein tyrosine kinase activity in Catharanthus roseus roots transformed by Agrobacterium rhizogenes. Planta. 1998;204:70–77. doi: 10.1007/s004250050231. [DOI] [PubMed] [Google Scholar]
- Saluja D, Bansal A, Sachar RC. Regulation of protein kinase through de novo enzyme synthesis in germinating embryos of wheat: enzyme purification and its autophosphorylation. Plant Sci. 1987;50:37–48. [Google Scholar]
- Shilo BZ. Roles of receptor tyrosine kinases in Drosophila development. FASEB J. 1992;6:2915–2922. doi: 10.1096/fasebj.6.11.1322852. [DOI] [PubMed] [Google Scholar]
- Simon MA, Dodson GS, Rubin GM. An SH3-SH2-SH3 protein is required for p21ras activation and binds to sevenless and sos proteins in vitro. Cell. 1993;73:169–177. doi: 10.1016/0092-8674(93)90169-q. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Waters C, Overholser KA, Carpenter G. Multiple autophosphorylation site mutations of epidermal growth factor receptor. J Biol Chem. 1991;266:8355–8362. [PubMed] [Google Scholar]
- Stone JM, Walker CJ. Plant protein kinase families and signal transduction. Plant Physiol. 1995;108:451–457. doi: 10.1104/pp.108.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Tonks NK. The coordinated action of protein tyrosine phosphatases and kinases in cell signaling. Trends Biochem Sci. 1994;19:480–485. doi: 10.1016/0968-0004(94)90134-1. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Shinshi H. Transient activation and tyrosine phosphorylation of a protein kinase in tobacco cells treated with a fungal elicitor. Plant Cell. 1995;7:639–647. doi: 10.1105/tpc.7.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torruella M, Casano LM, Vallejos RH. Evidence of the activity of tyrosine kinase(s) and of the presence of phosphotyrosine proteins in pea platelets. J Biol Chem. 1986;261:6651–6653. [PubMed] [Google Scholar]
- Trojanek J, Ek P. Scoble, Muszyñska G, Engström L (1996) Tyrosine phosphorylation in proteins of maize seedlings. Eur J Biochem 235: 338–344 [DOI] [PubMed]
- Turner CE. Paxillin is a major phosphotyrosine-containing protein during embryonic development. J Cell Biol. 1991;115:201–207. doi: 10.1083/jcb.115.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE. Paxillin: a cytoskeletal target for tyrosine kinases. BioEsssays. 1994;16:47–52. doi: 10.1002/bies.950160107. [DOI] [PubMed] [Google Scholar]
- Zhang K, Letham D, John P. Cytokinins control the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone kinase. Planta. 1996;200:2–12. doi: 10.1007/BF00196642. [DOI] [PubMed] [Google Scholar]