Abstract
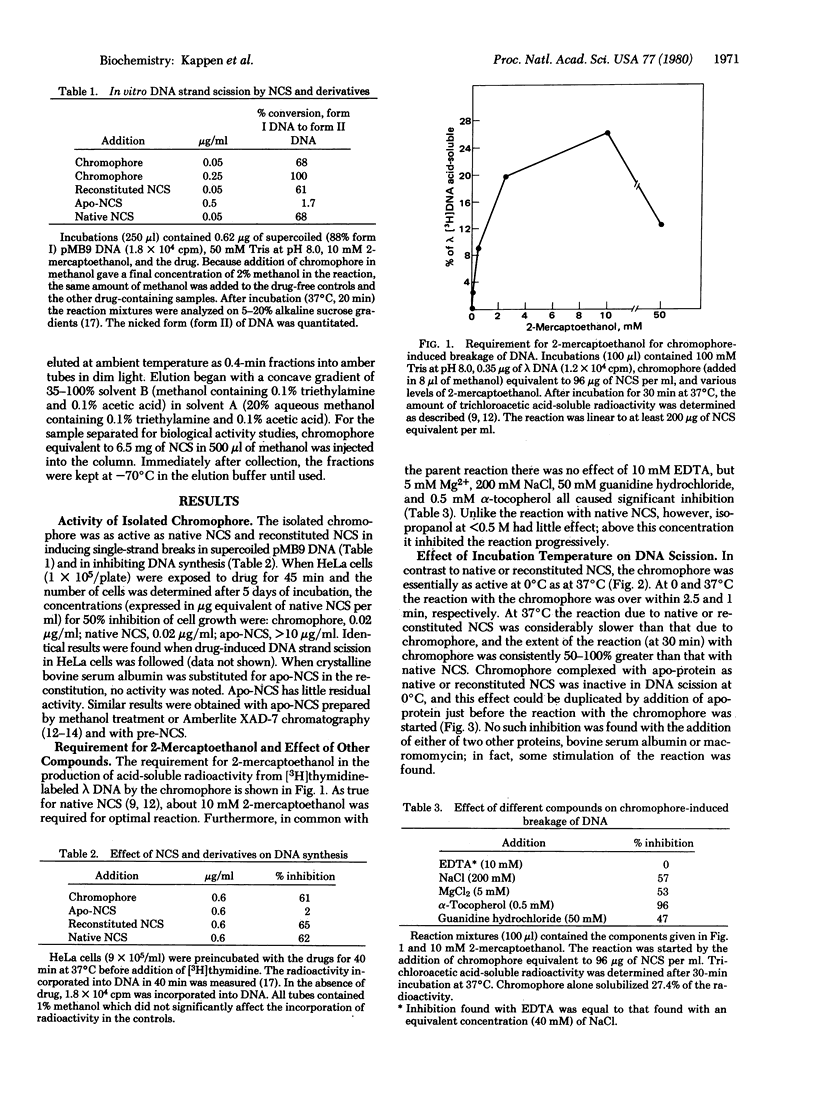
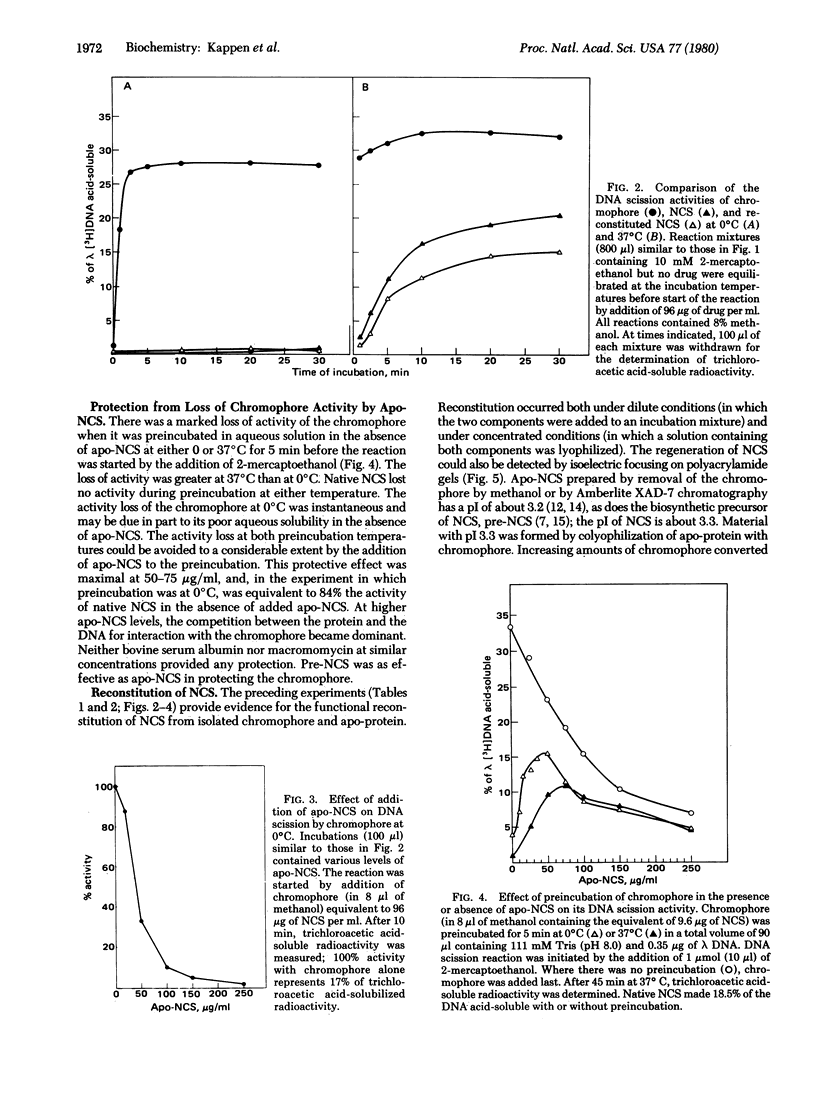
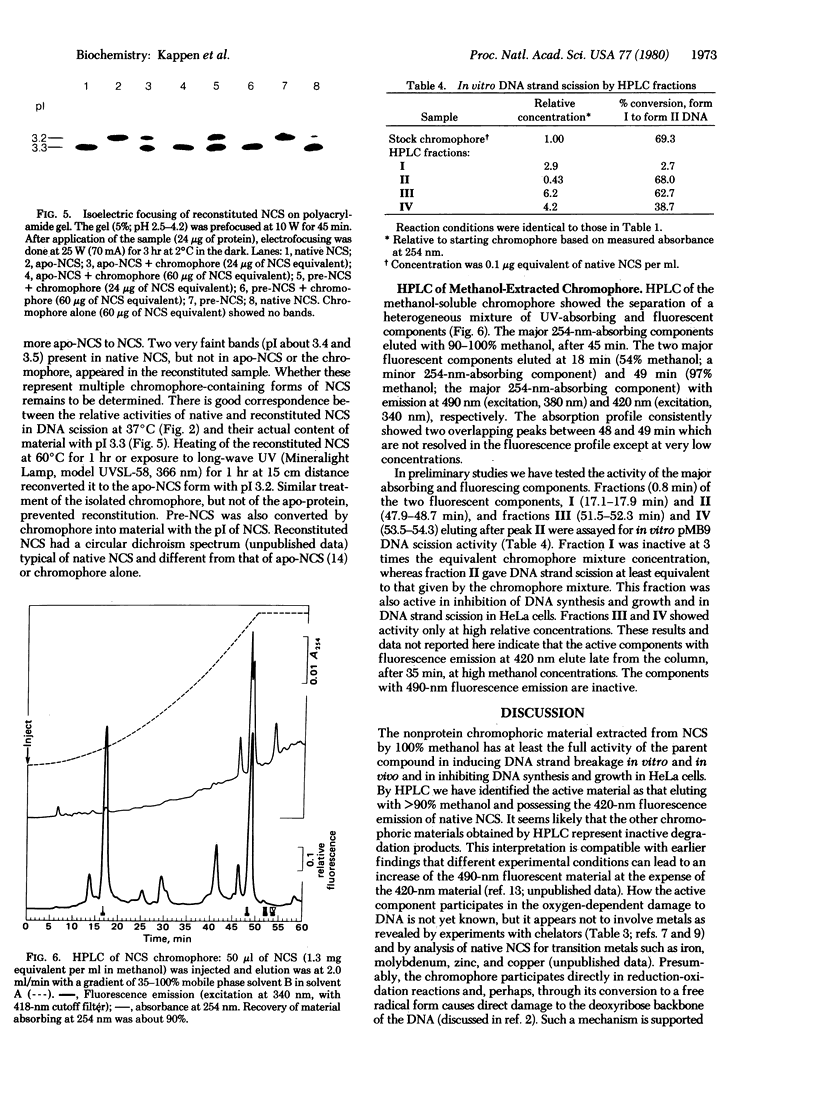
The methanol-extractable, nonprotein chromophore of the antitumor, protein antibiotic neocarzinostatin (NCS) has at least the full activity of the parent compound in inhibiting DNA synthesis and growth of HeLa cells and in causing DNA strand breaks in vivo and in vitro. In vitro DNA strand scission by the chromophore is markedly stimulated by 2-mercaptoethanol and is inhibited by guanidine hydrochloride and alpha-tocopherol. By high-pressure liquid chromatography, this activity has been localized to fractions eluting at greater than 90% methanol and having fluorescence emission at 420 nm (excitation at 340 nm). The apo-protein of NCS is inactive by itself but complexes with the chromophore so as to regulate its availability during the in vitro reaction. In DNA strand scission the chromophore acts rapidly at both 0 and 37 degrees C, whereas native and reconstituted NCS are inactive at 0 degrees C and slowly active at 37 degrees C. Complex formation with apo-NCS stabilizes the chromophore. Reconstitution of NCS (pI 3.3) from chromophore and apo-protein (pI 3.2) was shown by both activity studies and isoelectric focusing on polyacrylamide gels. "Pre-NCS," the biosynthetic precursor of NCS, is identical to apo-NCS in amino acid composition, spectral properties, isoelectric focusing on polyacryl-amide gels, and ability to complex with isolated chromophore to form material with all the properties of native NCS.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beerman T. A., Goldberg I. H. DNA strand scission by the antitumor protein neocarzinostatin. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1254–1261. doi: 10.1016/0006-291x(74)90449-5. [DOI] [PubMed] [Google Scholar]
- Beerman T. A., Poon R., Goldberg I. H. Single-strand nicking of DNA in vitro by neocarzinostatin and its possible relationship to the mechanism of drug action. Biochim Biophys Acta. 1977 Mar 18;475(2):294–306. doi: 10.1016/0005-2787(77)90020-x. [DOI] [PubMed] [Google Scholar]
- Burger R. M., Peisach J., Horwitz S. B. Effect of light and oxygen on neocarzinostatin stability and DNA-cleaving activity. J Biol Chem. 1978 Jul 25;253(14):4830–4832. [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Sequence specific cleavage of DNA by the antitumor antibiotics neocarzinostatin and bleomycin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3608–3612. doi: 10.1073/pnas.75.8.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatayama T., Goldberg I. H., Takeshita M., Grollman A. P. Nucleotide specificity in DNA scission by neocarzinostatin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3603–3607. doi: 10.1073/pnas.75.8.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida R., Takahashi T. Role of mercaptoethanol in vitro DNA degradation by neocarzinostatin. Cancer Res. 1978 Aug;38(8):2617–2620. [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Activation and inactivation of neocarzinostatin-induced cleavage of DNA. Nucleic Acids Res. 1978 Aug;5(8):2959–2967. doi: 10.1093/nar/5.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Effect of neocarzinostatin-induced strand scission on the template activity of DNA for DNA polymerase I. Biochemistry. 1977 Feb 8;16(3):479–485. doi: 10.1021/bi00622a022. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H. Mechanism of the effect of organic solvents and other protein denaturants of neocarzinostatin activity. Biochemistry. 1979 Dec 11;18(25):5647–5653. doi: 10.1021/bi00592a020. [DOI] [PubMed] [Google Scholar]
- Kappen L. S., Goldberg I. H., Samy T. S. Contrasts in the actions of protein antibiotics on deoxyribonucleic acid structure and function. Biochemistry. 1979 Nov 13;18(23):5123–5127. doi: 10.1021/bi00590a015. [DOI] [PubMed] [Google Scholar]
- Kikuchi M., Shoji M., Ishida N. Pre-neocarzinostatin, a specific antagonist of neocarzinostatin. J Antibiot (Tokyo) 1974 Oct;27(10):766–774. doi: 10.7164/antibiotics.27.766. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarus H., Raso V., Samy T. S. In vitro inhibition of human leukemic cells (CCRF-CEM) by agarose-immobilized neocarzinostatin. Cancer Res. 1977 Oct;37(10):3731–3736. [PubMed] [Google Scholar]
- Maeda H., Kuromizu K. Spontaneous deamidation of a protein antibiotic, neocarzinostatin, at weakly acidic pH. Conversion to a homologous inactive preneocarzinostatin due to change of asparagine 83 to aspartic acid 83 accompanied by conformational and biological alterations. J Biochem. 1977 Jan;81(1):25–35. doi: 10.1093/oxfordjournals.jbchem.a131443. [DOI] [PubMed] [Google Scholar]
- Meienhofer J., Maeda H., Glaser C. B., Czombos J., Kuromizu K. Primary structure of neocarzinostatin, an antitumor protein. Science. 1972 Nov 24;178(4063):875–876. doi: 10.1126/science.178.4063.875. [DOI] [PubMed] [Google Scholar]
- Napier M. A., Holmquist B., Strydom D. J., Goldberg I. H. Neocarzinostatin: spectral characterization and separation of a non-protein chromophore. Biochem Biophys Res Commun. 1979 Jul 27;89(2):635–642. doi: 10.1016/0006-291x(79)90677-6. [DOI] [PubMed] [Google Scholar]
- Poon R., Beerman T. A., Goldberg I. H. Characterization of DNA strand breakage in vitro by the antitumor protein neocarzinostatin. Biochemistry. 1977 Feb 8;16(3):486–493. doi: 10.1021/bi00622a023. [DOI] [PubMed] [Google Scholar]
- Sausville E. A., Stein R. W., Peisach J., Horwitz S. B. Properties and products of the degradation of DNA by bleomycin and iron(II). Biochemistry. 1978 Jul 11;17(14):2746–2754. doi: 10.1021/bi00607a008. [DOI] [PubMed] [Google Scholar]
- Sim S. K., Lown J. W. The mechanism of the neocarzinostatin-induced cleavage of DNA. Biochem Biophys Res Commun. 1978 Mar 15;81(1):99–105. doi: 10.1016/0006-291x(78)91635-2. [DOI] [PubMed] [Google Scholar]



