Abstract
Although respiratory syncytial virus (RSV) is a significant human pathogen, no RSV vaccines are available. We have reported that a virus-like particle (VLP) RSV vaccine candidate stimulated, in mice, robust, protective anti-RSV glycoprotein TH1 biased immune responses without enhanced respiratory disease upon RSV challenge. We report here an analysis of long-term responses to these VLPs. BALB/c mice immunized, without adjuvant, with VLPs or with infectious RSV generated anti-F and anti-G protein serum antibody responses that were stable over 14 months. Neutralizing antibody titers stimulated by VLPs were robust and durable for 14 months, whereas those of RSV-immunized animals declined significantly by 3 months. F protein-specific antibody-secreting cells were detected in the bone marrows of VLP-immunized mice but not in the marrows of RSV-immunized mice. Adoptive transfer of enriched splenic B cells from VLP-immunized mice into immunodeficient rag−/− mice resulted in anti-F and anti-G protein serum IgG antibody responses, in recipient mice, that were protective upon RSV challenge. In contrast, transfer of splenic B cells from RSV-immunized mice produced no detectable serum antibody in the recipients, nor could these mice inhibit RSV replication upon virus challenge. Immunization with VLPs stimulated the formation of germinal center GL7+ B cells in normal mice. VLP immunization of TCR βδ−/− T-cell-deficient mice did not induce anti-RSV IgG antibodies, results consistent with T-cell-dependent immune responses. These results demonstrate that VLPs are effective in stimulating long-lived RSV-specific, T-cell-dependent neutralizing antibody-secreting cells and RSV-specific memory responses.
INTRODUCTION
Human respiratory syncytial virus (RSV) is a major cause of acute respiratory disease in infants and young children worldwide (15). It is estimated that there are from 34 to 65 million RSV cases and 160,000 to 199,000 deaths per year worldwide (28) (www.who.int/vaccine_research/diseases/ari/en). In the United States, 85,000 to 144,000 infants are hospitalized per year due to the virus (42). In addition, RSV infection in children has been linked to the subsequent development of asthma (reviewed in reference 32). Elderly and immunocompromised populations are also at significant risk for serious RSV disease. In the United States, the virus accounts for 10,000 deaths per year among individuals greater than 64 years of age and 14,000 to 60,000 hospitalizations per year (5, 6, 10). In immunocompromised populations, particularly stem cell transplant recipients (38) and individuals with cardiopulmonary diseases (46), RSV infections result in high mortality rates. Furthermore, RSV infections exacerbate chronic conditions such as chronic obstructive airway disease, asthma, and cystic fibrosis (reviewed in reference 8). Despite this significant morbidity and mortality, there are no licensed RSV vaccines available.
Complicating the management of RSV disease and RSV vaccine development, humans may experience RSV disease caused by the same virus serotype multiple times over several years or even within the same season (reviewed in references 8 and 35). The reasons for the frequent failure of RSV infection to protect against subsequent infection in humans are not clear but the inadequate immune response to RSV natural infection illustrates the inherent difficulty with RSV vaccine development as any RSV vaccine must stimulate immune responses that are more protective and durable than those from natural infection (36). The fact that many RSV vaccine candidates have failed to stimulate long-term protective responses in humans (discussed in references 8 and 35), illustrates our lack of understanding of the immune mechanisms necessary to generate long-term, protective anti-RSV immune responses.
Virus-like particles (VLPs) are increasingly recognized as safe, effective vaccines for viral diseases (14). VLPs are virus-sized particles composed of arrays of structures on their surfaces and in their cores, structures that mimic those of infectious viruses and that may preserve, in a noninfectious particle, the very potent immunogenicity of live viruses (14, 30). VLPs are formed by the assembly of the structural proteins and sometimes lipids without the incorporation of the viral genome, making VLPs incapable of multiple rounds of infection. Two VLP vaccines are licensed for use in humans, the papillomavirus vaccine and the hepatitis B virus vaccine, and a number of other VLP vaccine candidates are in preclinical testing and clinical trials (14).
We have recently described a novel VLP based on the Newcastle disease virus (NDV) core proteins and containing the RSV fusion (F) protein and glycoprotein (G) ectodomains (23). This VLP was formed with NDV nucleocapsid protein (NP) and matrix (M) protein and the ectodomains of the RSV F and G proteins fused to the transmembrane (TM) and cytoplasmic tail (CT) domains of the NDV fusion (F) protein or hemagglutinin-neuraminidase (HN) protein, respectively. These particles stimulated, in mice, robust protective immune responses (23). To determine the durability of these protective responses, we characterized the maintenance of neutralizing antibodies, as well as the presence of anti-RSV F protein antibody secreting bone marrow-associated cells, and RSV-specific memory B cells in mice 14 months after a single VLP immunization or a single RSV infection. We report that VLPs containing the RSV F and G glycoprotein ectodomains stimulated germinal center B cells, long-lived bone marrow associated anti-F-protein antibody-secreting cells (ASC) and memory B cell responses, whereas RSV infection did not. Furthermore, we report that the IgG response to VLPs is T cell dependent.
MATERIALS AND METHODS
Cells, virus, and plasmids.
ELL-0 (avian fibroblasts), Vero cells, Hep2 cells, and COS-7 cells were obtained form the American Type Culture Collection, whereas 293T cells were obtained from the laboratory of Frank Ennis. ELL-0 cells were maintained in Eagle minimal essential medium (Gibco) supplemented with 10% fetal calf serum. COS-7 cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with penicillin, streptomycin, and 10% fetal calf serum. Vero cells and Hep2 cells were grown in DMEM supplemented with penicillin, streptomycin, and 5% fetal calf serum. RSV, A2 strain, was obtained from R. Finberg.
The cDNAs encoding the NDV NP and M protein have been previously described (33). The genes encoding the chimera proteins F/F and H/G contain the sequences encoding the ectodomains of the RSV F and G proteins, respectively, and the sequences encoding the transmembrane and cytoplasmic domains of the NDV F and HN proteins, respectively. The construction and characterization of these chimera genes has been previously described (23, 27).
VLP preparation, purification, and characterization.
For preparations of VLPs to be used as immunogens (VLP-H/G+F/F and VLP-H/G), ELL-0 cells growing in T-150 flasks were transfected with cDNAs encoding the NDV M protein and NP and the chimeric protein H/G or the cDNAs encoding the chimera proteins H/G and F/F, as previously described (23, 27). At 24 h posttransfection, heparin was added to the cells at a final concentration of 10 μg/ml as previously described (23, 27) to inhibit rebinding of released VLPs to cells. At 48, 72, and 96 h posttransfection, cell supernatants were collected and VLPs were purified, by sequential pelleting and sucrose gradient fractionation as previously described (23, 27). Concentrations of proteins in the purified VLPs were determined by silver-stained polyacrylamide gels and by Western analysis as previously described (23).
Antibodies.
Polyclonal rabbit anti-NDV antibody was raised against UV inactivated, purified NDV as previously described (24). Polyclonal goat anti-RSV antibody (Biodesign) was used in Western blots to detect the RSV G protein. RSV F monoclonal antibody (clone 131-2A; Chemicon) was used in plaque assays and immunofluorescence of fixed cells. Anti-RSV HR2 antibody is a polyclonal antibody specific to the HR2 domain of the RSV F protein and has been previously described (23). Anti-NDV F tail is a polyclonal antibody raised against the cytoplasmic tail domain of the NDV F protein as previously described (24). Secondary antibodies against goat, mouse, and rabbit IgGs were purchased from Sigma. The antibodies used for fluorescence-activated cell sorting (FACS) analysis of murine spleen and bone marrow cells were anti-CD19 APC (Biolegend), anti-GL7 fluorescein isothiocyanate, and anti-B220 PerCp (BD Pharmingen).
Preparation of RSV, RSV plaque assays, and antibody neutralization.
RSV, grown in Hep2 cells, was prepared as previously described (23, 27). RSV plaque assays were accomplished on Vero cells as previously described (23, 27).
For antibody neutralization assays, mouse sera were complement inactivated (56°C for 30 min) and then diluted in DMEM without serum. RSV stocks were diluted to approximately 75 to 150 PFU in 100 μl. Dilutions of mouse serum in 100 μl were added to the virus, followed by incubation for 1 h at 37°C. The mixture was then added to prewashed, confluent monolayers of Vero cells growing in 24-well tissue culture dishes, and the cells were incubated at 37°C for 1 h. The antibody-virus mixture was removed, and 1 ml of methylcellulose overlay was added to each well as previously described (23, 27). Plates were incubated for 3 to 4 days, and plaques were stained as previously described (23, 27). Neutralization titer was defined as the log2 of the reciprocal of the dilution of serum that reduced virus titer by 60%.
Animals, animal immunization, and RSV challenge.
Four-week-old BALB/c mice from Jackson Laboratories or Taconic laboratories were housed (groups of five) under pathogen-free conditions in micro-isolator cages at the University of Massachusetts Medical Center animal quarters. BALB/c rag1−/− (generously provided by Ann Rothstein, UMass Medical School) and TCRβδ−/− mice (generously provided by Eva Szomolanyi-Tsuda, UMass Medical School) were generated from breeding pairs housed under pathogen free conditions and received acidified (HCl; pH 2.8–3.2) water containing trimethoprim-sulfamethoxazole (Goldline Laboratories) ad libitum for seven consecutive days every other week (39).
All protocols requiring open cages were accomplished in biosafety cabinets. BALB/c mice were immunized by intramuscular (i.m.) inoculation of 10 to 30 μg of total VLP protein in 0.05 ml of phosphate-buffered saline (PBS) containing 10% sucrose. For infection of wild-type BALB/c or rag−/− mice with RSV, the animals were lightly anesthetized with isoflurane and then infected by intranasal (i.n.) inoculation of RSV (7.5 × 105 to 22.5 × 105 PFU/mouse in 50 μl). Mice challenged with live RSV were lightly anesthetized as described above and infected i.n. with 7.5 × 105 PFU of virus.
Detection of virus in lung tissue.
At 4 days after RSV challenge (26), mice were sacrificed by CO2 asphyxiation. The lungs were removed aseptically, placed in 0.5 ml of 30% sucrose in PBS, and frozen on dry ice. The lungs were stored at −80°C. Upon thawing, lungs were weighed and then homogenized in the storage buffer using a Dounce homogenizer (Kontes). The homogenate was centrifuged at 12,000 rpm for 15 min, and the virus titer in the supernatant was determined by plaque assay as described above.
Determination of antibody titers by ELISA.
For serum collection, blood was obtained by tail vein nicks and centrifuged in BD Microtainer serum separator tubes to remove red blood cells. For enzyme-linked immunosorbent assay (ELISA), extracts containing G protein target antigen were prepared from 293T cells transfected with pCAGGS-G as previously described (23). Dilutions of transfected cell extract used as target were adjusted so that the amounts of G protein were comparable from experiment to experiment as determined by Western blotting. The F protein target used for ELISAs was purified recombinant F protein (generous gift of Novavax, Inc.). Each well contained 25 ng of F protein. All antigens were diluted in carbonate buffer (50 mM; pH 9.6). A total of 50 μl was added to each well of a microtiter plate (Costar) and incubated overnight at 4°C. Wells were washed with PBS and blocked with 50 μl of PBS containing 1% bovine serum albumin (BSA) at room temperature for 1 to 2 h, washed three times in PBS, and drained. Serial dilutions of mouse sera (50 μl in PBS-BSA) were added to the microtiter wells, followed by incubation for 1 h at room temperature. The plates were washed three times and a biotinylated anti-mouse IgG antibody (1:4,000 dilution; Sigma) in 50 μl of PBS-BSA was added for 1 h at room temperature. After washing, horseradish peroxidase (HRP)-conjugated neutravidin (1:4,000 dilution; Pierce) was added to 50 μl of PBS-BSA (1:4,000 dilution) for 1 h at room temperature prior to further washing. The HRP activity in each well was detected using TMB (3,3′,5,5′-tetramethylbenzidene) substrate (Sigma) at 50 μl/well and incubated for 15 to 20 min, at times determined in preliminary experiments to be within the linear range of the assay. The reaction was stopped with 50 μl of 1 N H2SO4, and the optical density at 450 nm (OD450) was read in a plate reader (Molecular Devices). Alternatively, alkaline phosphatase coupled to streptavidin was utilized to detect bound antibody using as a substrate 4-nitrophenyl phosphate disodium salt hexahydrate at 1 mg/ml in 1 M diethanolamine and 0.5 μM MgCl2. The OD450 was determined. As previously described, the titers of anti-G protein antibodies were defined as the reciprocal of the serum dilution that gave an OD450 of 3-fold over background (23). Titers for anti-F protein antibodies were defined as the reciprocal of the serum dilution that gave an OD450 of 0.2 since background values for this target antigen were zero (23).
Enzyme-linked immunospot (ELISpot) assay of spleen or bone marrow cells.
Splenocytes prepared from disrupted spleens were filtered through Nytex mesh (100- to 120-μm pore size) washed in balanced salt solution (BSS) containing 5% fetal calf serum or 0.3% BSA, and red blood cells were lysed in Gey's solution (Sigma). Washed cells, resuspended in BSS, were counted and resuspended at a concentration of 1 × 107 to 2 × 107 live cells/ml in complete medium (RPMI) containing 10% serum, penicillin-streptomycin, glutamine, and β-mercaptoethanol (5 × 10−5 M). Bone marrow cells were flushed from femurs using a 25-gauge needle and BSS buffer, filtered, washed, counted, and resuspended at 2 × 107/ml.
For ELISpot assays, ELISpot plates (Millipore) were coated overnight with purified F protein (25 ng/well in PBS). Wells were washed eight times in water, drained, and incubated for 1 h in compete medium (RPMI containing 10% serum, pen-strep, glutamine and BME at 5 × 10−5 M). Fourfold serial dilutions of spleen or bone marrow cells were added in triplicate to precoated wells, followed by incubation at 37°C for 6 h. The plates were washed eight times and blocked overnight in PBS containing 1% BSA or 3% fetal calf serum. The wells were incubated with biotinylated anti-mouse IgG (Southern; 1:2,000 dilution) for 1 h at room temperature, followed by eight water washes. The wells were then incubated for 1 h at room temperature with streptavidin-AP (Southern; 1:4,000 dilution) diluted in PBS-BSA, washed, and then developed with BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium; 1 tablet/10 ml of autoclaved Millipore water) until purple spots appeared. The spots were counted using a CTL immunospot S5 analyzer.
Purification of splenic B cells and adoptive transfer.
Splenocytes from disrupted spleens were washed, and red blood cells were lysed with Gey's solution, washed, resuspended at 5 × 107/ml in anti-thy1.2 (J1J10), and incubated on ice for 45 min. The cells were then washed and treated with rabbit complement (Pel-Freeze H2) for 45 min at 37°C (39). FACS analysis using anti-CD19 demonstrated that B cells were ca. 90% pure. Adoptive transfer of purified B cells to rag−/− mice (1.2 × 107 cells/mouse) was accomplished by periorbital inoculation.
Detection of germinal center B cells.
Lymph nodes (LN; deep cervical, mediastinal, brachial, axillary, inguinal, and popliteal) were harvested from control, VLP-immunized, or RSV-infected mice at 7 days posttreatment. The cell suspensions were made, the RBCs were lysed, and FACS staining was performed to assess the presence of GL7+ germinal center B cells. Analysis was performed on a BD FACSCalibur flow cytometer using FlowJo (Treestar) software.
Statistical analysis.
Statistical analyses (Student t test) of the data were accomplished using GraphPad Prism 5 software.
RESULTS
Long-term antibody responses to VLP-H/G+F/F and RSV.
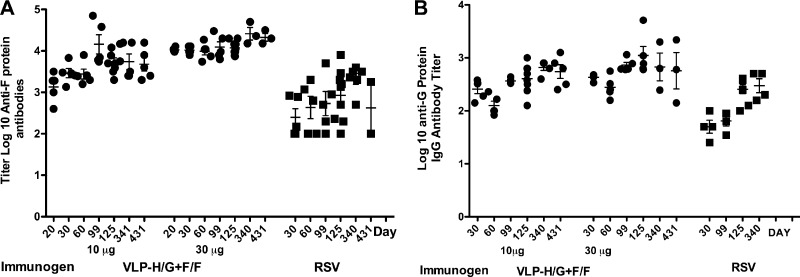
To assess the ability of VLPs containing both the RSV F protein and G protein ectodomains to induce persistent levels of serum antibodies to the RSV proteins, the anti-F and anti-G protein antibody titers were measured, by ELISA, periodically over 430 days after a single immunization and compared to mice infected intranasally (i.n.) with a single dose of RSV. VLPs containing the RSV F and G protein ectodomains (VLP-H/G+F/F) were purified and characterized as previously described (23). Groups of five mice were injected, intramuscularly (i.m.) to mimic vaccination, with either 10 or 30 μg of total VLP/mouse, whereas another group of mice was infected i.n. with RSV to mimic natural infection. Negative control groups received buffer–10% sucrose i.m. Sera were collected from these animals from 30 to 430 days, and the total anti-F and anti-G protein IgG antibody titers with time are shown in Fig. 1A and B, respectively. VLP immunization resulted in robust anti-F and anti-G protein IgG antibody titers that remained relatively constant for 430 days. Similarly, serum anti-F and anti-G protein anti-IgG antibody titers after i.n. infection with RSV remained relatively constant over the course of the experiment, although the titers were lower than those observed with VLP immunization. These results are consistent with the VLP stimulation of long-lived antibody responses in the absence of adjuvants at both doses of VLP tested.
Fig 1.
Titers of serum anti-F and anti-G protein antibodies with time after immunization or infection. Groups of five BALB/c mice were immunized i.m. with VLP-H/G+F/F. One group received 10 μg of total VLP/mouse (0.7 μg of F protein and 0.8 μg of G protein) or 30 μg of total VLP protein/mouse (2.1 μg of F protein and 2.4 μg of G protein). Antibody titers in sera, with time after immunization, were measured as described in Materials and Methods. Another group of five mice was infected with RSV i.n. (2.25 × 106 PFU/mouse). Mice immunized i.m. with PBS (50 μl) served as negative controls. (A) Anti-F protein antibody titers; (B) anti-G protein antibody titers.
Long-term neutralizing antibody responses to VLP-H/F+F/F and infectious RSV.
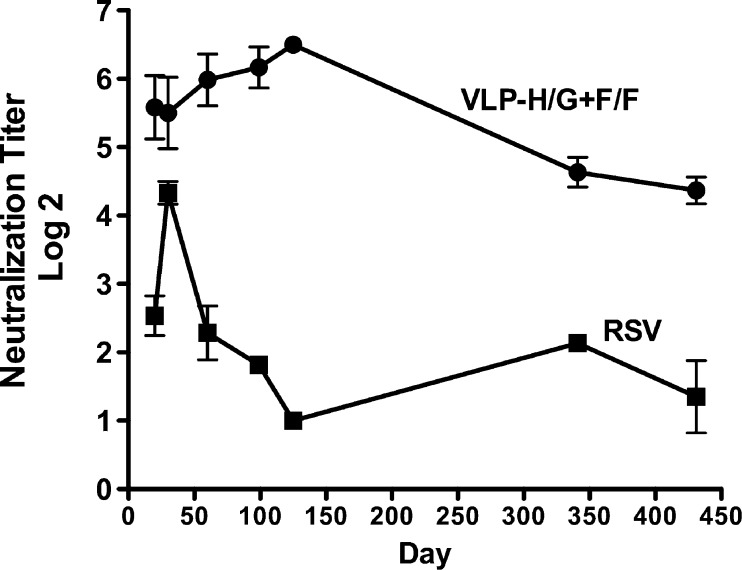
In humans, whereas RSV infection may result in detectable antibody responses that persist for some time, protective responses diminish rapidly, resulting in susceptibility to subsequent infection in many individuals (reviewed in references 8 and 35). To assess the longevity of neutralizing antibody responses in the murine system, the neutralization titers of sera obtained over time after VLP immunization or RSV infection were determined. Figure 2 shows that the neutralizing antibody titers after VLP immunization were maximal by 60 to 100 days and, while decreasing slightly between 125 and 430 days, remained high for 14 months. However, after RSV i.n. infection, serum neutralizing antibodies levels failed to reach those seen with VLP immunization and declined markedly by 100 days, despite the fact that total anti-F and G protein IgG antibody titers were relatively stable after RSV infection. These results suggest that VLP immunization produced an antibody response that is different in character than that induced by RSV infection.
Fig 2.
Serum neutralization titers with time after immunization or infection. Sera from mice immunized with 30 μg of VLP, described in the legend to Fig. 1, were pooled and neutralization titers were determined as described in Materials and Methods. Each point is the average of three separate plaque assays, each performed in duplicate. The standard deviations are shown.
Long-term protection from RSV challenge.
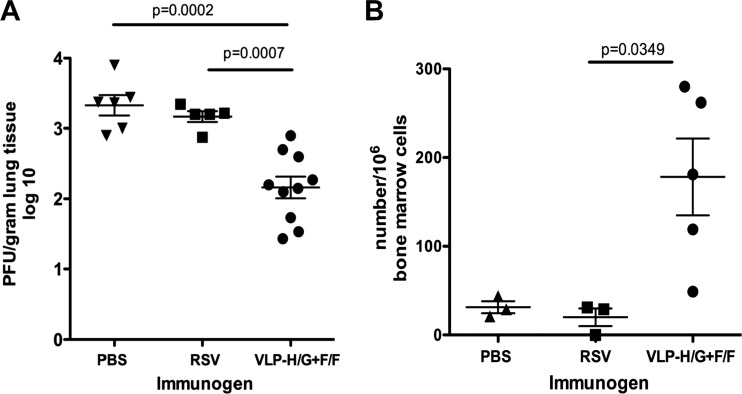
To determine the longevity of protection after VLP immunization or RSV infection, we challenged mice infected with RSV or immunized with VLPs 14 months previously. Mice immunized with a low dose of VLPs (10 μg of total VLP/mouse), mice infected i.n. with RSV, and PBS controls were infected with RSV i.n. After 4 days, the mice were sacrificed, and virus titers in the lungs are determined (Fig. 3A). Clearly, even at the low concentration of VLPs used in this experiment, there was still significant protection from RSV replication after 14 months. In contrast, mice infected with RSV 14 months previously showed no evidence of protection from RSV replication relative to the PBS controls upon virus challenge. These results are consistent with the observation, shown in Fig. 2, that sera from mice immunized with VLPs had significant neutralization titers at 14 months, whereas sera from mice infected with RSV did not. We have previously reported that mice challenged with RSV 38 days (27) or 52 days (23) after a single i.n. RSV infection were completely protected. Thus, protective responses after RSV infection are transient.
Fig 3.
Long-lived serum antibody and bone marrow antibody-secreting cells. At 430 days (approximately 14 months) postimmunization, five mice immunized with 10 μg of total VLP-H/G+F/F protein/mouse were challenged with RSV (7.5 × 105 PFU/mouse). Five mice previously infected with RSV (2.25 × 106 PFU/mouse) were similarly challenged with RSV. (A) Four days after challenge, the lungs were removed, and the virus titers were determined as described in Materials and Methods. Virus titers (per g of lung tissue) in each lobe of the lungs of VLP-immunized mice were determined separately, while titers in one randomly selected lobe of the PBS- and RSV-immunized mice were determined. Means and standard deviations are shown. Bars indicate groups compared for statistical analysis using GraphPad Prism software (t tests), with P values shown above the bar. The differences between control (PBS) and RSV-infected mice were not significant (P = 0.381). (B) Bone marrows from five VLP-immunized mice or three mice infected with RSV (2.25 × 106 PFU/mouse) or three mice sham immunized with PBS were harvested and prepared for ELISpot analysis as described in Materials and Methods. The panel shows the number of cells secreting anti-F protein antibodies per 106 bone marrow cells. The means and standard deviations are shown. The bar at the top indicates the groups compared for statistical analysis using GraphPad Prism software (t tests), with the P value shown above the bar. The differences between PBS and RSV groups were not significant.
Long-lived plasma cells in bone marrows of VLP-immunized mice.
The presence of significant RSV-specific antibody levels 14 months after immunization with VLPs was consistent with the generation of long-lived, antibody-secreting plasma cells, cells that reside primarily in the bone marrow. To determine the frequency of long-lived anti-F protein antibody-secreting bone marrow-associated cells in these mice, the bone marrows of the RSV-challenged mice characterized in Fig. 3A were harvested, and the numbers of anti-F protein antibody-secreting cells (ASC) were determined directly by ELISpot. Figure 3B shows that VLP-immunized mice had significant numbers of these cells relative to mice infected with RSV or unimmunized controls. These results suggest that VLPs containing the RSV F and G protein ectodomains stimulated long-lived anti-RSV F protein ASC, whereas a single RSV infection did not.
Assessment of memory responses to VLPs.
To evaluate the generation of memory B cells specific for RSV glycoproteins after VLP immunization, we performed an adoptive transfer experiment. Groups of mice immunized with either 30 μg of VLP/mouse i.m., infectious RSV i.n., or buffer i.m. were sacrificed after 14 months, and B-cell-enriched splenic populations (90% B cells) were injected into syngeneic immunodeficient, BALB/c rag−/− mice. After 6 days, the mice were challenged with RSV by i.n. inoculation. At 4 days after challenge, the mice were sacrificed, and sera, spleens, and lungs were harvested for ELISA, ELISpot, and virus titer analysis, respectively.
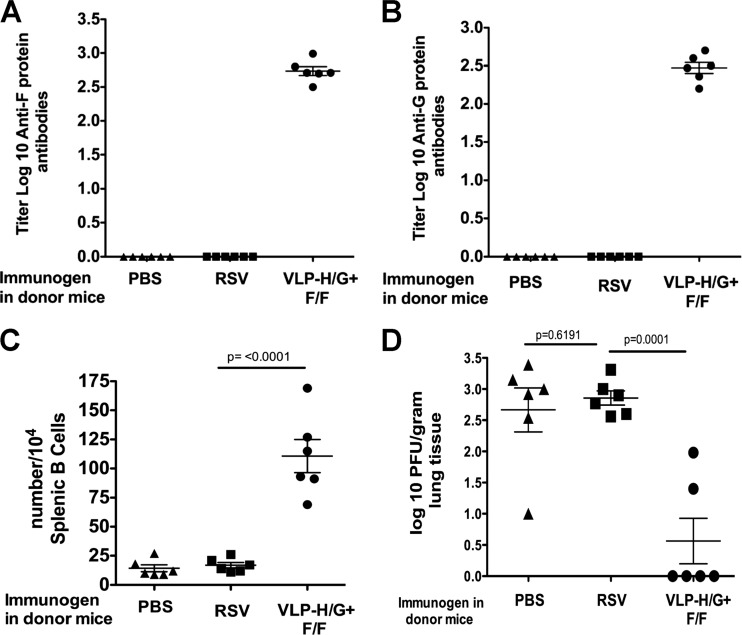
Figure 4A and B show the titers of anti-F protein and anti-G protein IgG antibody, respectively, in the sera of recipient mice. Sera from mice that received B cells from VLP-immunized mice had significant titers of anti-RSV F and G glycoprotein antibodies, whereas mice that received B cells from RSV-infected mice or sham-vaccinated mice had no detectable serum antibodies to RSV F or G proteins.
Fig 4.
Properties of B cell recipient mice. B cells from groups of five mice immunized with 30 μg of total VLP-H/G+F/F protein/mouse, infected with RSV (2.25 × 106 PFU/mouse), or sham immunized with PBS were harvested and purified 430 days after immunization as described in Materials and Methods. Purified B cells were transferred to six rag−/− mice (106 cells/mouse). After 6 days, the recipient mice were challenged with RSV (7.5 × 105 PFU/mouse). At 4 days after challenge the mice were sacrificed, and sera were harvested. (A and B) Anti-F protein and anti-G protein antibody titers, respectively, in the sera of recipient mice, were determined as in legend to Fig. 1 and in Materials and Methods with the means and standard deviations indicated. (C) Spleen cells from recipient mice were prepared for ELISpot analysis as described in Materials and Methods. The panel shows the number of anti-F protein antibody-secreting cells per 104 splenic B cells in each mouse in each group with the means and standard deviations indicated. The bar at the top of the graph indicates groups compared for statistical analysis using GraphPad Prism software (Student t test), with P values shown above the bar. Differences between the PBS and RSV groups were not significant. (D) Titers of RSV in the lungs of RSV challenged B cell recipient mice were determined as described in Materials and Methods, with means and standard deviations indicated. Bars indicate groups compared for statistical analysis using GraphPad Prism software (Student t test), with P values shown above the bar.
RSV-specific anti-F protein immunoglobulin-secreting B cells in the spleens of recipient mice were readily detected by ELISpot analysis in recipients receiving B cells from VLP-immunized mice (Fig. 4C). However, mice that received B cells from RSV-infected mice were devoid of immunoglobulin-secreting cells, as were the buffer controls.
To determine whether the transferred B cells could provide protection to recipient mice from RSV challenge, the titers of RSV in the lungs were determined at 4 days after i.n. infection of recipient mice with RSV. Figure 4D shows that RSV replication was significantly reduced in mice that received B cells from VLP-immunized donors, while mice that received B cells from RSV-infected mice were not protected. The titers of RSV in the lungs of these mice were not significantly different from levels in mice that received B cells from control vaccinated mice. These combined results are consistent with the generation in donor mice of memory B cells specific for RSV F protein by VLP immunization. In contrast, there is no evidence for spleen-associated memory B cells 430 days after RSV infection, a result that is consistent with the lack of long-term protection from challenge in these mice.
It should be noted that the titers of RSV in wild-type PBS control mice (Fig. 3A) and rag−/− PBS control mice (Fig. 4D) are very similar. This unexpected result may indicate that the well-known partial restriction of RSV replication in BALB/c mice may not be due, entirely, to the murine adaptive immune responses to RSV.
T cell dependence of anti-G and anti-F protein responses to VLPs.
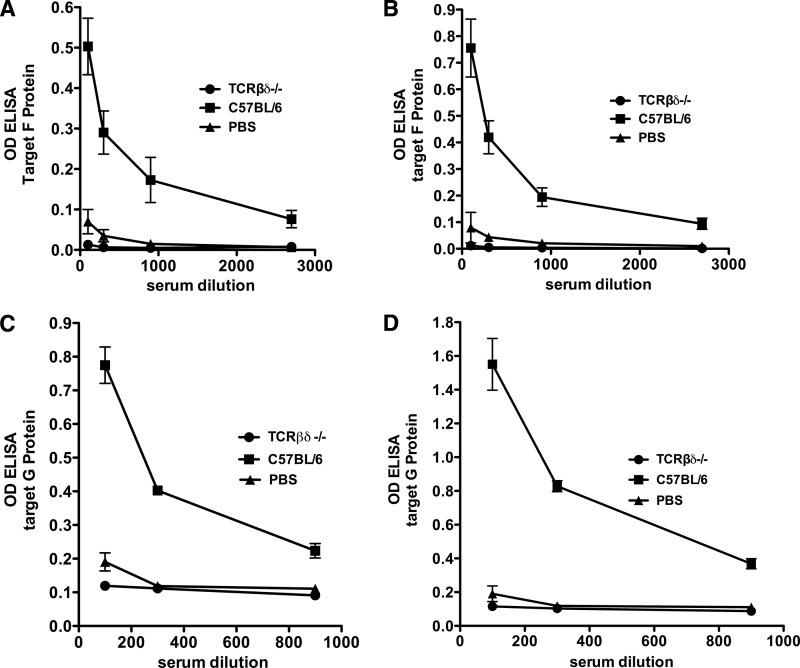
The development of long-lived, antibody-secreting plasma cells and memory B cells is classically characteristic of T-cell-dependent (TD) immune stimulation (discussed in reference 19), although there is increasing evidence that T-cell-independent (TI) responses can produce B cell memory (31; reviewed in references 2 and 43). To determine whether VLPs require T cells to produce anti-RSV F protein IgG immune responses, a group of T-cell-deficient C57BL/6-TCRβδ knockout mice and a control group of C57BL/6 wild-type mice were immunized with a single dose of VLP-H/G+F/F. Another group of mice received buffer immunization. Anti-F protein IgG antibody responses at 21 and 29 days after immunization were measured by ELISA. Figure 5A and B show that while wild-type mice developed robust anti-F protein antibody responses, no anti-F protein IgG antibody was detected in the T-cell-deficient mice, even at low serum dilutions. Similarly, to determine whether VLPs require T cells to produce anti-RSV G protein IgG antibody responses, a group of C57BL/6-TCRβδ knockout mice and a control group of C57BL/6 wild-type mice were immunized with a single dose of VLP-H/G, a VLP that contains only the ectodomain of the RSV G protein (27). Figure 5C and D show that no anti-G protein IgG antibody was detected in mutant mouse sera, whereas sera from wild-type mice contained anti-G protein IgG antibody. These results are consistent with VLP-H/G+F/F or VLP-H/G acting as TD antigens to elicit anti-F protein and anti-G protein IgG responses.
Fig 5.
T cell dependence of anti-G and anti-F protein responses to VLPs. (A and B) C57BL/6 or C57BL/6-TCRβδ−/− mice (five and eight mice/group, respectively) were immunized (i.m.) with 30 μg of total VLP-H/G+F/F protein. Five C57BL/6 mice immunized with PBS served as negative controls. At 21 (A) and 29 (B) days after immunization, serum anti-F antibodies were measured by ELISA using neutravidin-coupled HRP. The OD450 at different serum dilutions is shown. Each point is the mean of five or eight mice with standard deviations indicated. (C and D) C57BL/6 or C57BL/6-TCRβδ−/− mice (five mice/group) were immunized (i.m.) with 30 μg of total VLP-H/G protein. Five C57BL/6 mice immunized with PBS served as negative controls. At 20 (C) and 30 (D) days after immunization, sera from mice in each group were pooled, and anti-G protein antibodies in pooled sera were measured in triplicate by ELISA using alkaline phosphatase coupled to streptavidin. The figure shows the OD405 at different serum dilutions. Each point is the mean of three determinations of pooled sera with the standard deviations indicated.
Germinal center reaction.
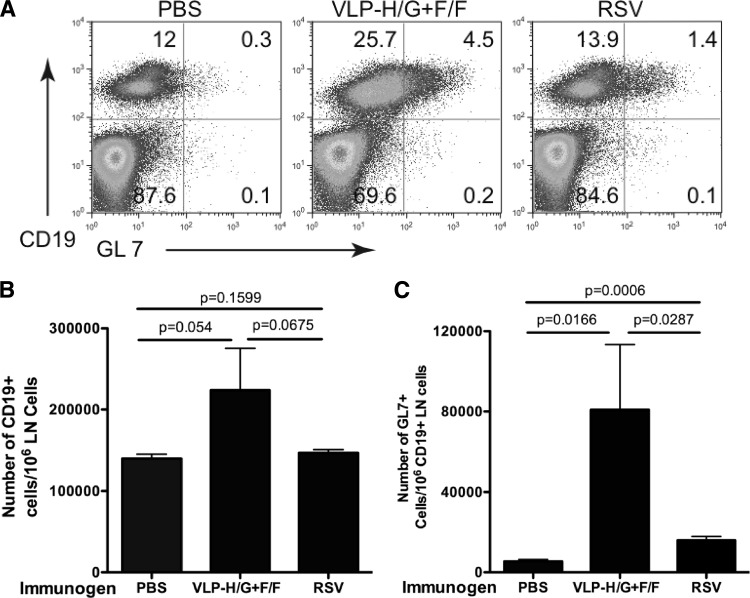
A key step in antibody affinity maturation and the production of long-lived plasma cells and memory B cells during TD responses is the induction of the germinal center reaction (GC) in spleens and lymph nodes. To assess whether VLP immunization stimulated GC formation, cells were harvested from lymph nodes of VLP-H/G+F/F-, RSV-, or buffer-immunized mice at 7 days postimmunization and analyzed by flow cytometry for the formation of germinal center B cells (CD19+, GL7+). Figure 6A shows the proportion of B cells and of germinal center B cells in representative animals. Figure 6B shows the average numbers of CD19+ cells/106 lymph node (LN) cells. Although VLP immunization resulted in slightly higher levels of CD19+ LN cells, the levels were not statistically significantly different from those observed after PBS or RSV immunization. Figure 6C shows the average numbers of GL7+ cells/106 CD19+ cells in LN of these mice. The induction of GC B cells after RSV infection has been previously reported (see, for example, reference 3). Clearly, VLP-H/G+F/F stimulated the induction of GC B cells more effectively than did RSV infection. This result supports the data, shown in Fig. 4 and 5, that VLPs stimulate a T-cell-dependent germinal center reaction necessary for the production of RSV-specific long-lived ASC and memory B cells.
Fig 6.
Stimulation of germinal center GL7+ B cells. Groups of five mice were immunized with VLP-H/G+F/F (i.m.), RSV (i.n.), or PBS. At 7 days after immunization, caudal, axillary, inguinal, deep cervical, mediastinal, and brachial lymph nodes were harvested and combined. B-cell-enriched cell suspensions, prepared as described in Materials and Methods, were incubated with anti-CD19 and anti-GL7 antibodies and analyzed by flow cytometry. (A) CD19+, GL7+ cells in representative mice from each group. (B) Average numbers of CD19+ cells per 106 lymph node cells in each group. (C) Average number of GL7+ cells per 106 CD19+ cells in each group. Means with standard deviations are shown. The bars at the top indicate groups compared for statistical analysis using GraphPad Prism software (Student t test), with P values shown above the bar.
DISCUSSION
A major problem in formulating a successful vaccine for RSV is that natural infection in humans does not result in complete protection from subsequent infection. Immune responses are weak and short-lived and ineffective against repeated infections with the same strain of virus, even in the same season (8, 9, 35). Furthermore, high titers of serum antibody can fail to protect a considerable proportion of both adults and infants. These observations led to the comment by Pulendran and Ahmed (36) that an effective RSV vaccine, in contrast to most vaccines, must stimulate better immune responses than natural infection. Development of such a vaccine has thus far been unsuccessful since many clinical trials of vaccine candidates show modest immunogenicity and short-lived protection.
Vaccine development is further complicated by the finding that RSV infection is reported to produce long-lasting protective responses in rodents, the preferred preclinical model, suggesting that studies in rodents may not directly translate to humans. However, in mice, induction of protective responses requires multiple infections and a single infection has been shown inadequate for long-lasting protective responses (see, for example, reference 44), a finding more in line with observations of the course of infection in humans.
We have chosen to analyze, in mice, the longevity of responses to a single immunization with our RSV VLP vaccine candidate and to compare its relative effectiveness to a single infection with RSV. We found a single RSV infection of BALB/c mice resulted in prolonged serum anti-F and anti-G protein antibody responses, but, as previously reported, neutralizing antibody titers were transient, and there was no evidence of long-lived bone marrow-associated anti-F protein antibody-secreting cells nor B cell memory responses, all contributing factors in making protection short-lived. In striking contrast, a single VLP immunization resulted in stable serum antibody titers and, more importantly, stable, robust neutralizing antibody titers for a period of over a year. Thus, these VLPs stimulate more effective protective immunity than does RSV infection, a critical criterion for an effective RSV vaccine.
Our results showed that these long-term responses correlated with the presence of long-lived anti-F protein antibody secreting cells in bone marrow and memory B cells. B cell memory and long-lived high-affinity antibody responses to conventional protein antigens arise when antigen-specific B cells proliferate and differentiate during the germinal center (GC) reaction (reviewed in reference 25). Within the GC, B cells with somatically mutated B cell receptors undergo affinity maturation by selection and class switch recombination, processes requiring specialized T follicular helper cells (TFH). To assess the T cell requirement for VLP immunogenicity, we sought to determine whether antibody responses could be induced in mice that were deficient in T cells (TCRβδ−/− mutant mice). Our results clearly show that VLPs did not stimulate any anti-F or G protein IgG antibodies in T-cell-deficient mice, indicating that the F and G chimera proteins presented by these VLPs behave as classic TD antigens. Further support for the T cell dependence of B cell responses to our VLPs is our finding that the VLPs stimulated a germinal center reaction, as indicated by the appearance of GL7+, CD19+ B cells in the lymph nodes 7 days after VLP immunization at levels significantly increased over those detected after buffer immunization or RSV infection.
Our results also show that protection against RSV challenge could be effected with memory B cells, induced as a result of VLP immunization and adoptively transferred into BALB/c rag−/− mice before RSV infection. Comparable numbers of B cells prepared from RSV-infected or PBS injected donors did not confer protection. The memory B cells from VLP-primed mice were activated in the presence of limited numbers of T cells. Studies with model hapten-protein conjugates have demonstrated that T cells are required for the differentiation of memory B cells into IgG secreting plasma cells (see, for example, references 4 and 45). However, it has been reported that virus-specific memory B cells generated by cytomegalovirus infection or tick-borne encephalitis virus infection can be activated in the absence of cognate or bystander T cells upon a second exposure to viral antigen (12, 17, 47). Similarly, a complex of ovalbumin and alum, which forms a particulate antigen, did not require T cell help for the activation of memory B cells (21). These results led MacLeod et al. to suggest that requirements for T cells in activation of memory B cells vary with the form of the antigen and that stimulation of B cell memory responses to particulate antigens may be T cell independent (22). Our results suggest that VLPs containing RSV F and G proteins may induce memory B cells that can be activated by virus infection in the presence of minimal levels of T cells. In contrast, infectious RSV, also a particulate antigen, did not generate memory B cells with similar properties. The reasons for differences in immune responses between virus and VLPs are unknown and the subject of future investigation. The differences may be related to differences in the routes of immunization. Alternatively, virus infection may suppress innate immune responses important for long-term immune memory development.
Stimulation of innate immunity is necessary for the induction of durable B cell and T cell responses (reviewed in references 1, 7, 20, 34, and 41). Furthermore, signal pathways induced by the stimulation of multiple Toll-like receptors (TLRs) synergize to enhance adaptive immunity (16, 29, 37), as well as determine the quality of the adaptive response (13, 37). Adjuvants, as stimulators of innate immunity are often included in vaccine formulations to enhance adaptive immune responses. Our data suggest that VLP-H/G+F/F must be self-adjuvanting since this VLP stimulated long-term immunity in the absence of added adjuvant. It is not known how VLP-H/G+F/F stimulates the innate immune responses important for the long-term adaptive responses we have observed, although it may be speculated that TLR4 and TLR7 or TLR3 are involved. RSV F protein has been reported to activate TLR4 (11, 18) and VLP-H/G+F/F may stimulate through TLR3, TLR7, or TLR9, which recognize various forms of nucleic acid. It has been reported that parainfluenza virus 5 (PIV5) VLPs assemble random host RNAs likely due to nonspecific interaction of the PIV5 NP with cellular transcripts (40). If our VLPs, which contain the NDV NP protein, contain host transcripts, they may stimulate via TLR pathways that respond to single-stranded RNA. It has been reported that removing host nucleic acid associated with Qβ VLPs significantly reduces their immunogenicity (1). Kasturi et al. have found that the best stimulation of long-term antibody responses to antigen-associated nanoparticles requires both TLR4 and TLR7 ligands (16).
In summary, our results demonstrate that VLPs based on NDV core proteins and containing the ectodomains of the RSV F and G proteins stimulate, in murine systems, robust, long-lived neutralizing antibodies, anti-RSV antibody-secreting bone marrow cells, and RSV-specific memory B cells. These responses are in contrast to those observed after a single RSV infection, which stimulated transient neutralizing antibodies, no detectable antibody-secreting bone marrow cells, and no memory B cells.
ACKNOWLEDGMENTS
This study was supported by grants from the National Institute of Allergy and Infectious diseases of the National Institutes of Health (AI093791 [T.G.M.] and AI041054 and AI084800 [R.T.W. and M.R.S.]). Core resources supported by the Diabetes Research Center grant DK32520 were also used.
We thank Rachel Gerstein with help with flow cytometry analysis and Ann Rothstein and Eva Szomolanyi-Tsuda for mutant mice.
Footnotes
Published ahead of print 15 August 2012
REFERENCES
- 1. Bessa J, Kopf M, Bachmann MF. 2010. Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J. Immunol. 184:4615–4619 [DOI] [PubMed] [Google Scholar]
- 2. Defrance T, Taillardet M, Genestier L. 2011. T cell-independent B cell memory. Curr. Opin. Immunol. 23:330–336 [DOI] [PubMed] [Google Scholar]
- 3. Delgado MF, et al. 2009. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 15:34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duffy D, Yang C-P, Heath A, Garside P, Bell EB. 2006. Naive T-cell receptor transgenic T cells help memory B cells produce antibody. Immunology 119:376–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759 [DOI] [PubMed] [Google Scholar]
- 6. Falsey AR, Walsh EE. 2000. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13:371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. 2007. MyD88 is required for the formation of long-term humoral immunity to virus infection. J. Immunol. 178:5124–5131 [DOI] [PubMed] [Google Scholar]
- 8. Hall CB. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917–1928 [DOI] [PubMed] [Google Scholar]
- 9. Hall CB, Long CE, Schnabel KD. 2001. Respiratory syncytial virus infections in previously healthy working adults. Clin. Infect. Dis. 33:792–796 [DOI] [PubMed] [Google Scholar]
- 10. Han LL, Alexander JP, Anderson LJ. 1999. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J. Infect. Dis. 179:25–30 [DOI] [PubMed] [Google Scholar]
- 11. Haynes LM, et al. 2001. Involvement of Toll-like receptor 4 in innate immunity to respiratory syncytial virus. J. Virol. 75:10730–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hebeis BJ, et al. 2004. Activation of virus-specific memory B cells in the absence of T cell help. J. Exp. Med. 199:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Iwasaki A, Medzhitov R. 2004. Toll-like receptor control of adaptive immune responses. Nat. Immunol. 5:987–995 [DOI] [PubMed] [Google Scholar]
- 14. Jennings GT, Bachmann MF. 2008. The coming of age of virus-like particle vaccines. Biol. Chem. 389:521–536 [DOI] [PubMed] [Google Scholar]
- 15. Karron RA. 2008. Respiratory syncytial virus and parainfluenza virus vaccines, 5th ed Saunders-Elsevier, Philadelphia, PA [Google Scholar]
- 16. Kasturi SP, et al. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Klenovsek K, et al. 2007. Protection from CMV infection in immunodeficient hosts by adoptive transfer of memory B cells. Blood 110:3472–3479 [DOI] [PubMed] [Google Scholar]
- 18. Kurt-Jones EA, et al. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398–401 [DOI] [PubMed] [Google Scholar]
- 19. Lanzavecchia A, Sallusto F. 2009. Human B cell memory. Curr. Opin. Immunol. 21:298–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lanzavecchia A, Sallusto F. 2007. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr. Opin. Immunol. 19:268–274 [DOI] [PubMed] [Google Scholar]
- 21. Leclerc C, et al. 1995. Stimulation of a memory B cell response does not require primed helper T cells. Eur. J. Immunol. 25:2533–2538 [DOI] [PubMed] [Google Scholar]
- 22. MacLeod MKL, Clambey ET, Kappler JW, Marrack P. 2009. CD4 memory T cells: what are they and what can they do? Semin. Immunol. 21:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGinnes LW, et al. 2011. Assembly and immunological properties of Newcastle disease virus-like particles containing the respiratory syncytial virus F and G proteins. J. Virol. 85:366–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McGinnes LW, Reitter JN, Gravel K, Morrison TG. 2003. Evidence for mixed membrane topology of the Newcastle disease virus fusion protein. J. Virol. 77:1951–1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McHeyzer-Williams M, Okitsu S, Wang N, McHeyzer-Williams L. 2011. Molecular programming of B cell memory. Nat. Rev. Immunol. 12:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Murawski MR, et al. 2009. Respiratory syncytial virus activates innate immunity through Toll-like receptor 2. J. Virol. 83:1492–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Murawski MR, et al. 2010. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice with no evidence of immunopathology. J. Virol. 84:1110–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nair H, et al. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. 2005. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6:769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noad R, Roy P. 2003. Virus-like particles as immunogens. Trends Microbiol. 11:438–444 [DOI] [PubMed] [Google Scholar]
- 31. Obukhanych TV, Nussenzweig MC. 2006. T-independent type II immune responses generate memory B cells. J. Exp. Med. 203:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Openshaw PJ, Tregoning JS. 2005. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin. Microbiol. Rev. 18:541–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pantua HD, McGinnes LW, Peeples ME, Morrison TG. 2006. Requirements for the assembly and release of Newcastle disease virus-like particles. J. Virol. 80:11062–11073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pasare C, Medzhitov R. 2005. Control of B-cell responses by Toll-like receptors. Nature 438:364–368 [DOI] [PubMed] [Google Scholar]
- 35. Power UF. 2008. Respiratory syncytial virus (RSV) vaccines: two steps back for one leap forward. J. Clin. Virol. 41:38–44 [DOI] [PubMed] [Google Scholar]
- 36. Pulendran B, Ahmed R. 2011. Immunological mechanisms of vaccination. Nat. Immunol. 12:509–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Querec T, et al. 2006. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 203:413–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raboni SM, et al. 2003. Respiratory tract viral infections in bone marrow transplant patients. Transplant 76:142–146 [DOI] [PubMed] [Google Scholar]
- 39. Schmidt MR, et al. 2008. Human BLyS facilitates engraftment of human PBL derived B cells in immunodeficient mice. PLoS One 3:e3192 doi:10.1371/journal.pone.0003192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schmitt AP, Leser GP, Waning DL, Lamb RA. 2002. Requirements for budding of paramyxovirus simian virus 5 virus-like particles. J. Virol. 76:3952–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schwarz K, et al. 2003. Role of Toll-like receptors in costimulating cytoxic T cell responses. Eur. J. Immunol. 33:1465–1470 [DOI] [PubMed] [Google Scholar]
- 42. Shay DK, et al. 1999. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 282:1440–1446 [DOI] [PubMed] [Google Scholar]
- 43. Shlomchik MJ, Weisel F. 2012. Germinal centers. Immunol. Rev. 247:5–10 [DOI] [PubMed] [Google Scholar]
- 44. Singleton R, Etchart N, Hou S, Hyland L. 2003. Inability to evoke a long-lasting protective immune response to respiratory syncytial virus infection in mice correlates with ineffective nasal antibody responses. J. Virol. 77:11303–11311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vieira P, Rajewsky K. 1990. Persistence of memory B cells in mice deprived of T cell help. Int. Immunol. 2:487–494 [DOI] [PubMed] [Google Scholar]
- 46. Walsh EE, Falsey AR, Hennessey PA. 1999. Respiratory syncytial and other virus infections in persons with chronic cardiopulmonary disease. Am. J. Respir. Crit. Care Med. 160:791–795 [DOI] [PubMed] [Google Scholar]
- 47. Weisel FJ, et al. 2010. Unique requirements for reactivation of virus-specific memory B lymphocytes. J. Immunol. 185:4011–4021 [DOI] [PubMed] [Google Scholar]