Abstract
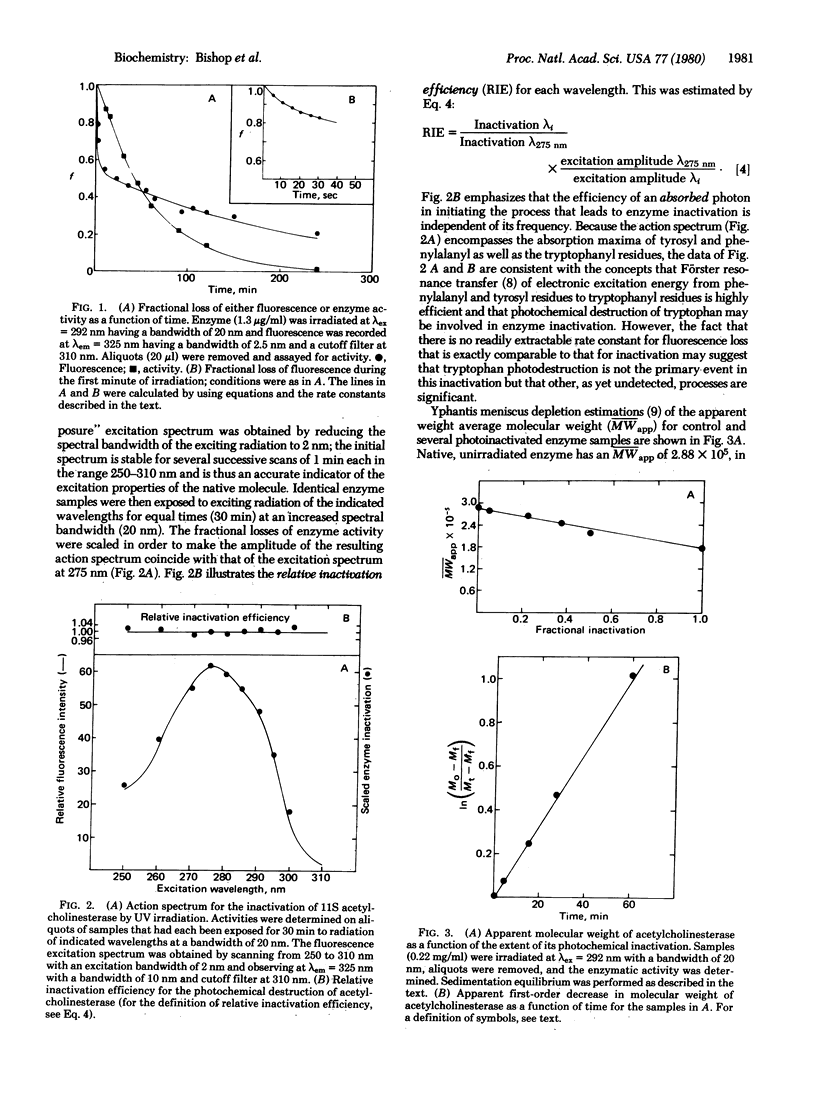
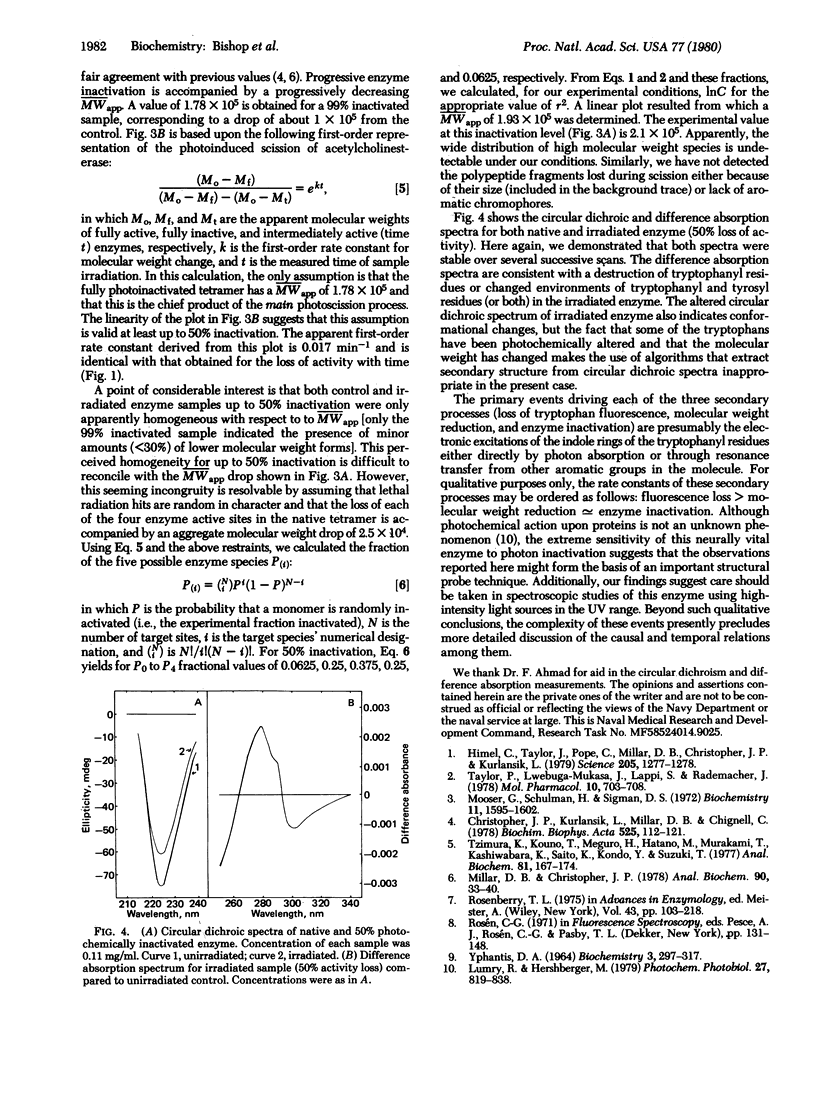
Ultraviolet irradiation of 11S acetylcholinesterase (acetylcholine acetylhydrolase, EC 3.1.1.7) produces a loss of tryptophan fluorescence which is best described as the sum of two separable first-order processes, one much more rapid than the other. In addition, the enzyme undergoes an all-or-none inactivation that is monotonically first order. Simultaneous with activity loss, photoscission takes place and results in a molecular weight drop of 1 x 10(5); this decrease is first order with a rate constant identical to that for enzymatic inactivation. These processes are accompanied by apparent conformational changes, as shown by circular dichroic and difference absorption spectra. The relative photochemical inactivation efficiency of incident light is unity when corrected for the wavelength dependence of fluorescence excitation, which is consistent with an efficient Förster resonance transfer of energy among the aromatic chromophores. The extreme sensitivity of acetylcholinesterase to photodestruction upon photon absorption and the several events that follow it not only suggest that these findings might be a basis for a useful molecular probe of the structure of this enzyme, but also indicate that additional care should be taken when conducting spectroscopic studies in the UV region.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christopher J. P., Kurlansik L., Millar D. B., Chignell C. On the homogeneity of 11-S acetylcholinesterase. Biochim Biophys Acta. 1978 Jul 7;525(1):112–121. doi: 10.1016/0005-2744(78)90205-x. [DOI] [PubMed] [Google Scholar]
- Himel C. M., Taylor J. L., Pape C., Millar D. B., Christopher J., Kurlansik L. Acridine araphanes: a new class of probe molecules for biological systems. Science. 1979 Sep 21;205(4412):1277–1279. doi: 10.1126/science.472743. [DOI] [PubMed] [Google Scholar]
- Millar D. B., Christopher J. P. Coping with convection in the ultracentrifuge. Anal Biochem. 1978 Oct 1;90(1):33–40. doi: 10.1016/0003-2697(78)90005-2. [DOI] [PubMed] [Google Scholar]
- Mooser G., Schulman H., Sigman D. S. Fluorescent probes of acetylcholinesterase. Biochemistry. 1972 Apr 25;11(9):1595–1602. doi: 10.1021/bi00759a008. [DOI] [PubMed] [Google Scholar]
- Rosenberry T. L. Acetylcholinesterase. Adv Enzymol Relat Areas Mol Biol. 1975;43:103–218. doi: 10.1002/9780470122884.ch3. [DOI] [PubMed] [Google Scholar]
- Tuzimura K., Konno T., Meguro H., Hatano M., Murakami T. A critical study of the measurement and calibration of circular dichroism. Anal Biochem. 1977 Jul;81(1):167–174. doi: 10.1016/0003-2697(77)90610-8. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]



