Abstract
Genetic variation within the HLA-B locus has the strongest impact on HIV disease progression of any polymorphisms within the human genome. However, identifying the exact mechanism involved is complicated by several factors. HLA-Bw4 alleles provide ligands for NK cells and for CD8 T cells, and strong linkage disequilibrium between HLA class I alleles complicates the discrimination of individual HLA allelic effects from those of other HLA and non-HLA alleles on the same haplotype. Here, we exploit an experiment of nature involving two recently diverged HLA alleles, HLA-B*42:01 and HLA-B*42:02, which differ by only a single amino acid. Crucially, they occur primarily on identical HLA class I haplotypes and, as Bw6 alleles, do not act as NK cell ligands and are therefore largely unconfounded by other genetic factors. We show that in an outbred cohort (n = 2,093) of HIV C-clade-infected individuals, a single amino acid change at position 9 of the HLA-B molecule critically affects peptide binding and significantly alters the cytotoxic T lymphocyte (CTL) epitopes targeted, measured directly ex vivo by gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay (P = 2 × 10−10) and functionally through CTL escape mutation (P = 2 × 10−8). HLA-B*42:01, which presents multiple Gag epitopes, is associated with a 0.52 log10 lower viral-load set point than HLA-B*42:02 (P = 0.02), which presents no p24 Gag epitopes. The magnitude of this effect from a single amino acid difference in the HLA-A*30:01/B*42/Cw*17:01 haplotype is equivalent to 75% of that of HLA-B*57:03, the most protective HLA class I allele in this population. This naturally controlled experiment represents perhaps the clearest demonstration of the direct impact of a particular HIV-specific CTL on disease control.
INTRODUCTION
The genetic polymorphisms that have the greatest impact on the HIV load set point and therefore on the rate of HIV disease progression are those occurring within the HLA class I region (15, 16, 19, 44, 46), with particular HLA-B polymorphisms exerting the biggest impact (28, 35). However, despite these data, the underlying mechanism(s) is still debated. The most likely explanation, given the role of HLA in CD8+ T-cell recognition of virus-infected cells, is that CD8+ T-cell epitope specificity might be critical to immune control (29, 30, 42, 43). However, HLA class I molecules can also act as NK cell ligands, and some studies find a strong impact of HLA-Bw4-80I molecules in combination with particular KIR allotypes on the NK-mediated control of HIV (17, 39, 40). In addition, HLA class I alleles are often in very strong linkage disequilibrium, particularly between the HLA-B and Cw loci, which can make it difficult to determine which allele in a haplotype is primarily responsible for any observed effect. Importantly, polymorphism occurring between the HLA loci may also get transmitted with particular alleles, as is the case for the protective −35 single-nucleotide polymorphism (SNP) strongly linked to HLA-B*57 (33, 51). This may explain the protective effect observed for certain HLA haplotypes (11, 31, 35, 41). Here, we study two closely related alleles, which provide a unique opportunity to minimize the factors that typically confound studies of HLA-associated control of HIV.
HLA-B*42:01 and B*42:02 are two closely related HLA class I alleles that differ only by a single amino acid within the peptide-binding groove on the α1 helix, namely, Tyr and His at HLA residue 9 in HLA-B*42:01 and HLA-B*42:02, respectively. The amino acid at HLA residue 9 is one that lines the B and C pockets of the HLA class I binding site (38) and therefore plays an important part in determining the peptides presented by different HLA class I molecules. The phenotypic frequencies of HLA-B*42:01 and B*42:02 alleles in South African cohort studies (26, 28–30, 35, 42) are 19% and 2%, respectively. Both are HLA-Bw6 alleles, and therefore, they do not act as KIR ligands (39). HLA-B*42:01/42:02 are part of the B7 superfamily, and in spite of the Tyr/His polymorphism arising at HLA residue 9, the peptide-binding motifs for HLA-B*42:01 and HLA-B*42:02 are very similar (see below). Thus, these alleles might be expected to perform similarly in relation to intrathymic negative selection of self-reactive T cells, according to the model proposed by Kosmrlj et al. in relation to HLA-B*0702 and HLA-B*3501 (32). Crucially, both HLA-B*42 alleles are in very strong linkage disequilibrium with HLA-A*3001 and HLA-Cw*1701 (35), with the rarer HLA-B*42:02 occurring almost exclusively on this haplotype.
HLA-B*42:01 and B*42:02 therefore represent an invaluable natural experiment allowing dissection of the clinical impact of CD8+ T-cell responses in a large cohort of untreated C-clade-infected individuals, with the minimum possible impact of other confounding factors, such as HLA linkage disequilibrium, KIR interaction, or T-cell receptor (TCR) availability.
MATERIALS AND METHODS
Study subjects.
We studied a total of 2,093 adults with chronic antiretroviral therapy (ART)-naïve C-clade HIV-1 infection recruited from four cohorts, as follows: (i) Durban, South Africa (n = 1,263), as previously described (12, 14, 28, 42); (ii) Gaborone, Botswana (n = 525), via the Mma Bana study; (iii) Bloemfontein, Free State, South Africa (n = 261), as previously described (26); and (iv) Kimberley, South Africa (n = 44). Data describing viral loads were available for 1,972, with DNA sequences available from 1,482 for whom HLA typing to four-digit resolution had been determined, including 386 HLA-B*42:01- and 44 HLA-B*42:02-positive individuals and excluding 3 individuals coexpressing HLA-B*42:01 and B*42:02. The viral load was measured using the Roche Amplicor version 1.5 assay. Informed consent was obtained from all participating individuals, and institutional review boards at the University of KwaZulu-Natal, Massachusetts General Hospital, and the University of Oxford approved the study.
IFN-γ ELISPOT.
For 1,010 HIV C-clade-infected subjects, the HIV-specific CD8+ T-cell responses were determined in gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays. Responses were determined to a set of 410 overlapping peptides (OLPs) whose sequences were based on the 2001 C-clade consensus arranged in a matrix system with 11 to 12 peptides in each pool. Responses to the matrix pools were deconvoluted by subsequent testing with the individual 18-mer peptides within each pool, and the identities of the individual 18-mers recognized were thus confirmed, as previously described (28). Associations of expression of HLA-B*42:01 and B*42:02 and OLP targeting were calculated from 154 HLA-B*42:01 and 16 B*42:02 individuals from a total of 1,010 screened individuals. To determine the percent targeting frequency of HLA-B*42:01- and B*42:02-restricted epitopes, individuals coexpressing HLA-B*0702/3910/8101 were excluded from analysis, as the restriction element in these individuals could not be exclusively attributed to HLA-B*42:01 and B*42:02.
HLA class I typing.
Four-digit high-resolution typing of HLA-A, HLA-B, and HLA-C alleles were performed using the Dynal Realtime reverse sequence-specific oligonucleotide (SSO) kits as previously described (24) for 914 subjects in the Durban cohort. HLA typing for the remaining 1,179 study subjects was undertaken by direct sequencing. No HLA-B*42 subtype other than HLA-B*42:01/42:02 was identified through either method of HLA typing.
HLA-peptide binding, stability assays, and peptide-binding motifs.
The measurement of peptide major histocompatibility complex class I (MHC-I) stability was determined with a dissociation assay based on radiolabeled β2m and biotinylated MHC, as recently described (22), and peptide MHC-I affinity interactions were undertaken using the AlphaScreen technology, as previously described (21).
Peptide-binding motifs for HLA-B*42:01 and HLA-B*42:02 were determined using positional scanning combinatorial peptide libraries (PSCPL) (50) in combination with the dissociation assay (22). The PSCPL approach is based on sublibraries in which 1 amino acid is kept constant in one position, whereas all other positions contain mixtures of all amino acids. A PSCPL library based on 9-amino-acid-long peptides results in 20 × 9 different sublibraries that have to be tested. Each sublibrary is compared to a completely random sample containing a mixture of all amino acids in all 9 positions (denoted X9), and a relative binding (RB) value is calculated as follows: RB = stabilitysublibrary/stabilityX9. If a sublibrary gives a more stable interaction than the completely random X9, the amino acid fixed in this position will have an RB value greater than 1, and vice versa.
Tetramer synthesis and tetramer staining.
Tetramers were generated as previously described (2). Briefly, HLA-B*42:01 heavy chain (HC) was expressed in Rosetta(DE3)pLysS (Novagen), purified, and refolded around the peptide of interest in the presence of human β2m light chain. The unrefolded HC and peptide were separated from refolded MHC-peptide monomer complexes using fast protein liquid chromatography (FPLC) prior to tetramerization of monomers and conjugation to R-phycoerythrin (PE) (Extravidin PE; Sigma) to obtain PE-labeled HLA-B*42:01 tetramers.
For staining of PE- or allophycocyanin (APC)-conjugated tetramer-specific cells, peripheral blood mononuclear cells (PBMCs) were thawed in R20 medium (RPMI medium, 20% fetal calf serum [FCS], 1% penicillin/streptomycin, 1% glutamine) rested for 1 h at 37°C, 5% CO2; stained with HLA class I tetramer for 30 min at room temperature; washed; surface stained with CD8 AlexaFlour 750 (eBioscience), CD3 AlexaFlour 700 (BD), and a live/dead violet cell stain kit (Invitrogen) for 30 min; washed in phosphate-buffered saline (PBS); and fixed in 2% paraformaldehyde. Samples were acquired on an LSR II (BD) flow cytometer within 12 h of staining and analyzed using FlowJo version 8.8.6. The cells were hierarchically gated on singlets, lymphocytes, live cells, and CD3+ T cells and gated for a distinct tetramer-specific CD8-positive population.
Sequencing of proviral DNA.
Sequences from Gag, Pol, and Nef were generated by extraction of genomic DNA from peripheral blood mononuclear cells (PBMCs) and amplified by nested PCR using previously published primers (24), and the PCR product was purified as previously described (42). Sequencing was undertaken using the BigDye ready reaction mix as described previously (14, 34), and in this way, HIV Gag, Pol, and Nef sequences were obtained from 1,209, 446, and 436 study subjects, respectively (42), and Vif, Vpr, and Env sequences were obtained from HIV full-length sequences available for 248 study subjects, as previously described (48).
Statistical analysis.
Associations between HLA-B*42:01 and B*42:02 expression and 18-mer peptide (OLP) response were determined as previously described (30). Briefly, we used a decision tree based on Fisher's exact test to identify associations between the recognition of individual 18-mer peptides and the expression of HLA-B*42:01. For each 18-mer peptide, we computed the Fisher exact test P value against all 4-digit HLA alleles observed in the cohort and added the most significant HLA allele to the decision tree. We next removed all individuals who expressed that allele and repeated the process until the most significant HLA allele had a P value of >0.2. False-discovery rates (5) were calculated using a procedure specific to Fisher's exact test that analytically computes the null distribution for all permutations of the data, as previously described (8).
The two-tailed Mann-Whitney U test was used to compare median viral loads between HLA-B*42:01-, B*42:02-, and B*42-negative individuals for all haplotypes and for HLA-A*30:01, B*42:01/02, and Cw17:01 haplotypes only and to compare immunogenic and nonimmunogenic peptides in the peptide-binding stability assay. P values are shown on the graphs and were calculated using GraphPad Prism version 5.
Differences in OLP and optimal epitope targeting and p17-RM9, p24-TL9, and RT-YL9 sequence polymorphisms between HLA-B*42:01 and B*42:02-positive and -negative individuals were calculated using Fisher's exact test.
RESULTS
HLA-B*42:01 is associated with a lower viral load than HLA-B*42:02.
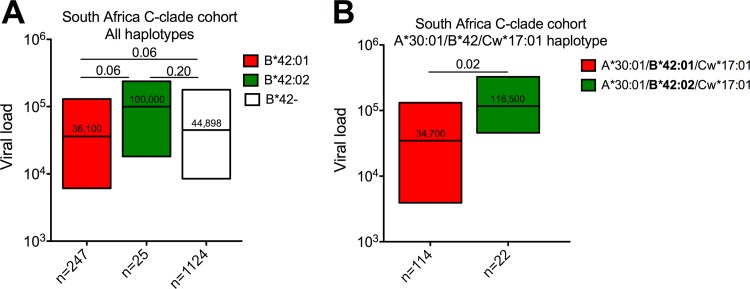
We studied a large C-clade cohort of 1,396 individuals chronically infected with HIV in South Africa and observed that HLA-B*42:01 expression (n = 247) shows a strong trend toward an association with lower viral loads than HLA-B*42:02 expression (n = 25; P = 0.06) and HLA-B*42:01/B*42:02 nonexpression (n = 1,124; P = 0.06) (Fig. 1A). Next, to minimize the impacts of other variables, we included only individuals expressing the HLA-A*30:01/B*42:01-02/Cw*17:01 haplotype, thereby excluding 133 HLA-B*42:01- and 3 HLA-B*42:02-expressing individuals. In this smaller data set, HLA-B*42:01 was associated with more clear-cut immune control over HLA-B*42:02 (median viral loads, 34,700 [4.54 log10] versus 116,500 [5.06 log10], respectively; P = 0.02) (Fig. 1B). These differences remained significant when pooling data from 4 different C-clade-infected cohorts from Botswana, Zimbabwe, and South Africa (Durban and Bloemfontein), involving 1,972 individuals. They included 189 individuals carrying HLA-A*30:01/B*42:01/Cw*17:01 and 38 carrying HLA-A*30:01/B*42:02/Cw*17:01 (P = 0.01). The differences were also maintained when the 30 HLA alleles with the overall greatest impact on the viral load were excluded (P = 0.01) (reference 9 and data not shown). Thus, in these individuals, a single-nucleotide polymorphism on the HLA class I locus is associated with significant differences in HIV disease control.
Fig 1.
Association of HLA-B*42 allele expression with the steady-state viral load in a cohort size of 1,396 C-clade chronically infected individuals from South Africa. The y axes show the association with the viral load (RNA copies/ml) for all haplotypes (A) and for HLA-A*30:01, B*42:01/02, and Cw17:01 haplotypes only (B) expressed as interquartile ranges, with the median value shown inside the box and the number of individuals included in the analysis for each parameter shown on the x axis. Significant differences between groups are indicated by the P values shown above the lines (Mann-Whitney U test).
HLA-B*42:01 and HLA-B*42:02 have very similar peptide-binding motifs.
HLA-B*42:01 and HLA-B*42:02 differ by only a single amino acid at position 9 (P9) (Tyr and His, respectively), which is 1 of 11 HLA residues (HLA residues 7, 9, 24, 25, 34, 45, 63, 66, 67, 70, and 99) located within the B pocket of the MHC-I molecule likely to anchor the amino acid at P2 of the binding peptide (38). It has previously been established that HLA-B*42:01 has a strong preference for Pro at P2 and for medium-size hydrophobic residues, such as Leu and Ile, at the carboxy-terminal position (PC) of binding peptides (25). To investigate the impact of the Tyr9His substitution, we determined the peptide-binding motifs for HLA-B*42:01 and HLA-B*42:02 by using PSCPL (50) in combination with a dissociation assay based on radiolabeled β2m and biotinylated MHC (22). In this way, the contribution to peptide-MHC stability was determined for all amino acids at P1 to P9 (see Fig. S1 and Table S1 in the supplemental material). The peptide-binding motif determined in this manner was exactly consistent with the motif previously defined for HLA-B*42:01 (25). These studies also showed no substantial difference in the peptide-binding motif between HLA-B*42:01 and HLA-B*42:02, with an equally strong preference for Pro in both and very similar C-terminal preferences for Leu, Met, Ile, and Phe bound in the F pocket.
HLA-B*42:01 presents more Gag epitopes than HLA-B*42:02.
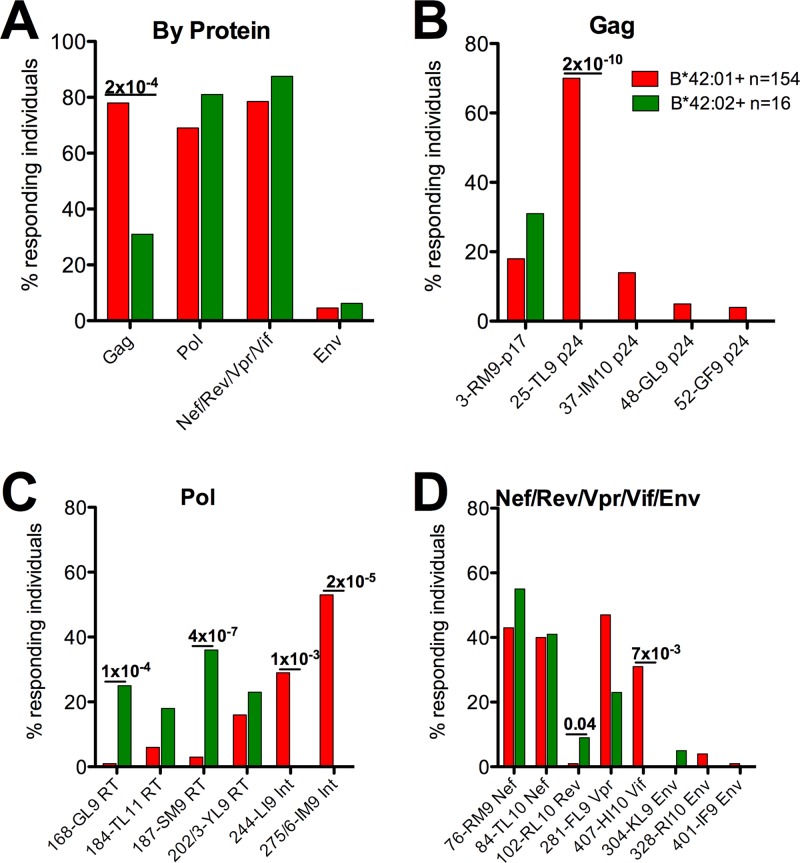
To determine the HLA-B*42:01- and HLA-B*42:02-restricted HIV-specific epitopes significantly targeted in infected cohorts, we tested recognition of a set of 410 overlapping peptides spanning the entire HIV C-clade proteome (29) in a cohort of 1,010 C-clade-infected study subjects in Durban, South Africa, from which we excluded the individuals expressing HLA-B*07:02, B*39:10, and B*81:01, as these HLA alleles have been shown in some cases to represent the same peptides that are HLA-B*42-restricted epitopes (36) (Fig. 2). Overall, there was no difference in targeting of Pol, Nef, or Env, but Gag epitopes were targeted in a significantly higher proportion of HLA-B*42:01-positive subjects than HLA-B*42:02-positive subjects (n = 122/154 [79%] versus 5/16 [31%]; P = 2 × 10−4) (Fig. 2A). The immunodominant HLA-B*42:01-restricted Gag-TL9 (TPQDLNTML; Gag 180 to 189) response, seen in 74% of HLA-B*42:01 individuals, was recognized by 0% of HLA-B*42:02-positive individuals (P = 2 × 10−10) (Fig. 2B), a difference that remained significant when we compared individuals expressing the same HLA-A*30:01/B*42:01/02/Cw*17:01 haplotype (69% versus 0%; P = 2 × 10−7) (data not shown). Of a total of 19 HLA-B*42-restricted 18-mer peptides presented by HLA-B*42:01 and/or HLA-B*42:02, 7 epitopes were differentially targeted according to HLA-B*42 subtype. In a subset of 100 individuals, we tested recognition of 12 optimally defined epitopes and confirmed the differential targeting for 3 of the epitopes (see Fig. S2 in the supplemental material). Responses to the optimal epitopes were strongly correlated with responses to the 18-mer peptides containing the optimal epitopes (r = 0.89; P < 0.0001) (data not shown). Thus, position 9 on the HLA-B locus differentiates between HLA-B*42:01 and HLA-B*42:02 with respect to the number of Gag-specific CD8+ T-cell responses made, in spite of the similar peptide-binding motifs shown above.
Fig 2.
IFN-γ ELISPOT responses to 19 HIV CD8+ T-cell epitopes. (A) Protein-specific responses were obtained by pooling responses from individual peptides within Gag, Pol, Nef/Rev/Vpr/Vif, and Env proteins. (B to D) Associations of IFN-γ ELISPOT responses to 18-mer peptides in HLA-B*42:01- and B*42:02-expressing individuals (n = 154 and n = 16, respectively) expressed as percent responders (y axis) to 19 individual 18-mer peptides grouped according to HLA-B*42:01 and B*42:02 expression, with the 18-mer peptide number and epitope abbreviation shown on the x axis. HLA-B*07:02, B*39:10, and B*81:01 individuals were excluded from the analysis, as they present a subset of these peptides, too. Significant P values (P < 0.05) between groups are shown above the bars for 18-mer peptide recognition comparing HLA-B*42:01 to B*42:02 individuals (Fisher's exact test).
Differences in p24-TL9 Gag epitope targeting are not a result of p24-TL9 escape mutations but are explained by peptide-HLA stability.
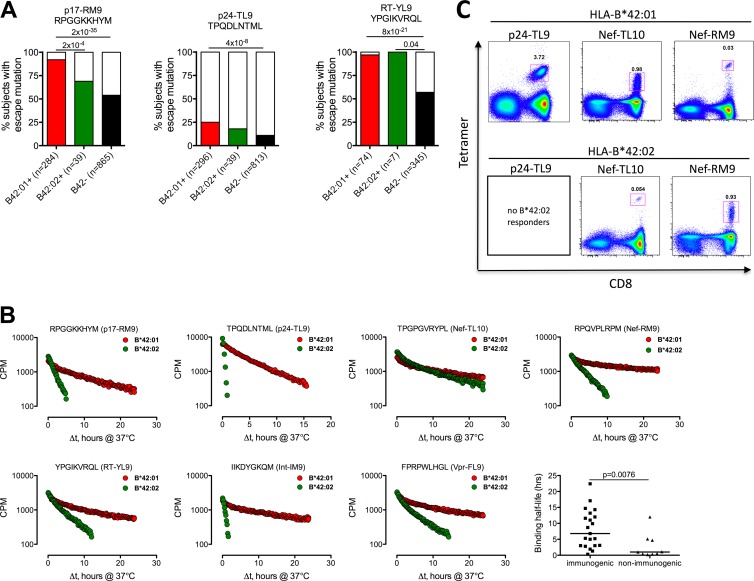
In order to determine whether the distinct targeting of epitopes observed was explained by different selections of escape mutations, we next undertook sequence analysis of Gag, Pol, Nef, and Acc/Reg/Env proteins in 1,188, 426, 443, and 247 C-clade-infected individuals, respectively, from Durban, South Africa (30, 42, 48). Figure 3A shows that the striking difference in p24-TL9 Gag targeting described above is not explained by selection of escape in p24-TL9, as there is no evidence of significant escape mutation above background in this epitope in HLA-B*42:02-positive individuals. An inverse association between the frequency of escape and epitope targeting is seen in the p17-RM9 Gag epitope, for which there are more HLA-B*42:02-positive responders than HLA-B*42:01-positive responders in chronic infection, which may be the result of stronger selection pressure and therefore more escape from the HLA-B*42:01-retricted p17-RM9 response (Fig. 3A; see Table S2 in the supplemental material). Importantly, sequences from the RT-YL9 epitope, in which both HLA-B*42:01 and B*42:02 select escape (P = 8 × 10−21 and P = 0.04, respectively), show that HLA-B*42:02-restricted responses are capable of selecting escape and therefore are functional in vivo. Overall, HLA-B*42:01 selects escape in a minority of cases in the p24-TL9 epitope but in 75% or more of HLA-B*42:01-positive individuals within p17-RM9, RT-YL9, Int-IM9, Vpr-FL9, and Vif-HI10 epitopes (see Fig. S3 in the supplemental material). In contrast, HLA-B*42:02 selects escape only in the RT-YL9 epitope. These data suggest that stronger selection pressure is exerted on HIV by HLA-B*42:01-restricted CD8+ T-cell responses than by those restricted by HLA-B*42:02.
Fig 3.
(A) Differential selection of HIV sequence polymorphisms between HLA-B*42:01 and B*42:02 individuals. The percentages of individuals with sequence variation from the wild type (% subjects with escape mutation) are expressed on the y axis and grouped according to HLA-B*42:01, B*42:02, and B*42 negative expression on the x axis, with the number of sequences analyzed shown in parentheses. B*81:01-positive subjects were removed from p24-TL9 analysis. (B) p17-RM9, p24-TL9, Nef-TL10, Nef-RM9, RT-YL9, Int-IM9, and Vpr-FL9 epitope binding half-life (stability), shown as the HLA-peptide-binding half-life (Δt, h) for immunogenic and nonimmunogenic peptides pooled for B*42:01 and B*42:02, as shown in Table 1. The P value was determined by Fisher's exact test. (C) Use of HLA-class I tetramers to rapidly define two immunodominant novel HLA-B*42:02-restricted HIV-1 CD8+ T-cell epitopes. HLA-B*42:01 tetramers were loaded with the p24-TL9, Nef-TL10, or Nef-RM9 optimal peptide and stained against ex vivo donor PBMCs from HLA-B*42:01- and HLA-B*42:02-positive individuals. The numbers in the gates are the percentages of tetramer-specific cells gated on live/CD3+ lymphocytes shown as CD8 versus tetramer.
To further investigate the observed difference in Gag-TL9 targeting by subjects with HLA-B*42:01 and HLA-B*42:02, which as described above is not explained by differential escape, we directly tested the peptide-MHC binding affinity (21) and binding stability (22). In spite of the similar peptide-binding motifs, the peptide-binding affinity for TL9 with HLA-B*42:02 was more than 10-fold lower than for HLA-B*42:01-TL9 (Kd 50% inhibitory concentration [IC50; i.e., the concentration of the competing peptide needed for a 50% inhibition of the signal], 1,106 nM versus 82 nM, respectively) (data not shown). The peptide-MHC binding stability measurements showed that HLA-B*42:01 is 16 times more stable in complex with the TL9 peptide (binding half-life, 0.22 versus 3.56 h) (Fig. 3B). In contrast, the Nef-TL10 epitope targeted by >50% of both HLA-B*42:01 and B*42:02 individuals had similar peptide-MHC binding stability (14.3 versus 6.8 h). Although a shorter binding half-life of the Nef-RM9 epitope for B*42:02 than for B*42:01 (2.8 h versus 22.4 h, respectively) was detected, we unequivocally confirmed this response in an HLA-B*42:02-positive individual by using HLA class I tetramers (Fig. 3C), along with the Nef-TL10 response, thus confirming the ELISPOT responses observed previously. This is also consistent with a sufficient peptide-binding half-life of the RT-YL9 epitope to HLA-B*42:02 (3.0 h), in which escape is selected by HLA-B*42:02 individuals, thus exemplifying a functional CD8+ T-cell response through the HLA-B*42:02 allele. In contrast to HLA-B*42:01, absence of Int-IM9 targeting by HLA-B*42:02 individuals was consistent with a very short binding half-life to B*42:02 compared to B*42:01 (0.5 versus 14.7 h), while the shorter binding half-life of p17-RM9 for HLA-B*42:02 than for B*42:01 (1.3 versus 8.7 h) is reflected in higher escape frequencies in HLA-B*42:01-positive subjects. When we applied this assay to all of the epitopes targeted by HLA-B*42:01- and/or B*42:02-positive subjects (Table 1), as expected, we observed a significantly longer binding half-life for immunogenic versus nonimmunogenic epitopes (median, 6.8 versus 1.1 h; P = 0.0076) (Fig. 3B), consistent with previous findings indicating that a peptide-MHC binding half-life of >1 h is typically required for a peptide to be immunogenic (23, 30).
Table 1.
Summary of HIV-1-derived epitopes presented by HLA-B*42:01 and HLA-B*42:02 alleles
| Protein | OLP no. | Epitope name | HXB2 location | Amino acids at positions: |
|||
|---|---|---|---|---|---|---|---|
| HLA-B*42:01a |
HLA-B*42:02a |
||||||
| 1–8 | C | 1–8 | C | ||||
| p17 | 3 | p17-RM9 | p17-Gag22–30 | RPGGKKHY | Mb | RPGGKKHY | Mb |
| p24 | 25 | p24-TL9 | p24-Gag 180–188 | TPQDLNTM | L | ||
| 48 | p24-GL9 | p24-Gag355–363 | GPSHKARV | L | |||
| 52 | p24-GF9 | p24-Gag385–393 | GPKRIVKC | Fb | |||
| RT | 168 | RT-GL9 | RT-Pol18–26 | GPKVKQWP | Lb | ||
| 184 | RT-TL11 | RT-Pol139–149 | TPGIRYQYNV | Lb | |||
| 187 | RT-SM9 | RT-Pol146–154 | SPAIFQSS | Mb | |||
| 202/3 | RT-YL9 | RT-Pol271–278 | YPGIKVRQ | L | YPGIKVRQ | Lb | |
| Int | 244 | Int-LI9 | Int-Pol28–36 | LPPIVAKE | I | ||
| 275/6 | Int-IM9 | Int-Pol267–276 | IIKDYGKQ | Mb | |||
| Nef | 76 | Nef-RM9 | Nef70–78 | RPQVPLRP | M | RPQVPLRP | Mb |
| 77 | Nef-RF9 | Nef77–85 | RPMTFKGA | F | RPMTFKGA | Fb | |
| 84 | Nef-TL10 | Nef128–137 | TPGPGVRYP | L | TPGPGVRYP | Lb | |
| Rev | 102 | Rev-RL10 | Rev66–75 | RPAEPVPLQ | Lb | ||
| Vpr | 281 | Vpr-FL9 | Vpr34–42 | FPRPWLHG | L | FPRPWLHG | Lb |
| Vif | 407 | Vif-HI10 | Vif48–57 | HPKVSSEVHIa | |||
| Env | 304 | Env-KL9 | Env117–125 | KPCVKLIP | Lb | ||
| 328 | Env-RI10 | Env298–307 | RPNNNTRKS | Ib | RPNNNTRKS | Ib | |
| 401 | Env-IF9 | Env843–851 | IPRRIRQG | Fb | |||
The underlined amino acids indicate sites of escape sequence polymorphism from the wild-type epitope.
New epitope not listed in the Los Alamos A list database, http://www.hiv.lanl.gov.
DISCUSSION
In this study, we examined the ability of a single amino acid change in an MHC molecule to significantly alter the pattern of epitope presentation, selection pressure on the virus, and ultimately immune control of HIV. In the cases of HLA-B*42:01 and HLA-B*42:02, the impact of other HLA alleles expressed on the same haplotype is largely removed because both HLA-B*42 alleles are in strong linkage disequilibrium with the same HLA-A and HLA-C alleles, and therefore, we were able to restrict our analysis to individuals expressing B*42:01/42:02 on the same HLA class I haplotype (HLA-A*30:01:B*42:01/42:02:Cw*17:01). This natural experiment allowed us to minimize the possible confounding effects of other HLA alleles or other polymorphisms in the HLA class I locus or elsewhere in the genome that might influence immune control of HIV (10, 33, 51). In addition, as both B*42 alleles possess the Bw6 motif, they do not contribute to NK-mediated control of HIV (1). In light of this, the finding that HLA-B*42:01-positive individuals have significantly better control of HIV (measured by the viral load) than those expressing HLA-B*42:02 can be strongly linked with the broader Gag-specific CD8+ T-cell activity and stronger HIV-specific selection pressure mediated through HLA-B*42:01.
These findings are consistent with recent studies of the impact of HLA micropolymorphism in relation to HLA-B*57 alleles (30). Here also, the critical factor explaining differential HIV disease outcomes for the respective HLA alleles was the number of Gag responses capable of driving selection pressure on the virus, although in that study, factors such as the impacts of other HLA and non-HLA genes in linkage disequilibrium with HLA-B*57 alleles (35, 41), in addition to the role of HLA-B57 molecules as NK ligands (1, 6, 40, 45), were potential confounders. The studies reported here in relation to HLA-B*42 alleles are also consistent with previous studies of HLA-B*35 subtypes that have shown differential disease progression linked to single amino acid differences between HLA-B*35 alleles (18). Although the mechanism underlying these observations has not been defined at the CD8+ T-cell epitope level, the frequency of Gag-specific responses in subjects expressing HLA-B*35:01, but not in subjects expressing the B35-Px alleles HLA-B*35:02, HLA-B*35:03, and HLA-B*53:01, was inversely related to the viral load (27). In the present study, the roles of the specific responses restricted by the two HLA-B*42 alleles in contributing to the differential impacts on the viral set point are not clear-cut, although responses to three of the HLA-B*42:01-restricted epitopes (p17-RM9, Vif-HI10, and Nef-TL10) are associated with significant differences in the viral-load set point between HLA-B*42:01 responders and nonresponders. p17-RM9-responding individuals were associated with lower viral loads, and Vif-HI10 and Nef-TL10 responders were associated with higher viral-load set points, whereas for HLA-B*42:02, we did not observe any significant impact on the viral-load set point (data not shown).
These studies also underline the challenges of epitope prediction approaches and the limitations of the peptide-binding motif to distinguish reliably between peptides that will or will not be bound stably to closely related HLA molecules. In the current study, the Tyr/His polymorphism at HLA residue 9 did not appear to affect the specificity of amino acids binding at P2 or P3 in peptides binding to HLA-B*42:01/42:02 (see Fig. S1 and Table S1 in the supplemental material), even though this HLA residue is one that is likely to affect both the B and C pockets of the peptide-binding groove (35). The failure of Gag-TL9 to bind adequately to HLA-B*42:02 could not be predicted using in silico-based prediction programs (13, 47) or by experimentally determined peptide-binding motifs (25).
There is no other example of closely related HLA molecules that differ only by the single Tyr→His change at HLA residue 9 (38). However, it is clear that the charge and size of the residue at this position has an important bearing on the amino acid occupying the B pocket of the HLA molecule. Comparing HLA-C molecules, which are less diverse than HLA-B, those with a negatively charged residue (e.g., Asp at HLA residue 9), such as HLA-Cw*06:02, HLA-Cw*07, and HLA-Cw*18, tend to present epitopes that carry a positively charged residue at P2 in the binding peptide (25, 38; http://www.hiv.lanl.gov/). Those with Tyr at HLA position 9, such as HLA-Cw*03, Cw*05, HLA-Cw*08, and HLA-Cw*12, tend to present epitopes carrying a small hydrophobic residue, like Pro or Ala, at P2 in the binding peptide (25, 38; http://www.hiv.lanl.gov/). HLA-Cw*04:01, which has Ser at HLA residue 9, can accommodate large hydrophobic residues, such as Tyr and Phe, at P2 in binding peptides (38). With reference to HLA-B*42:01 and HLA-B*42:02, at physiological pH, histidine (pKa = 6.5) may carry a positive charge, whereas tyrosine is likely to carry no charge. There is also a moderate size difference (molecular weight [MW], 155 versus 181, respectively) between the two amino acids. These differences in charge and size are likely to affect the interactions that determine peptide-MHC stability. One might speculate, therefore, that the hydrophobic residues proline and isoleucine, which form the anchor binding residue at P2 for the peptides binding HLA-B*42:01 and HLA-B*42:02, would be stabilized better by the tyrosine than by the less hydrophobic histidine at HLA residue 9. However, in the absence of a solved peptide-MHC crystal structure for either HLA-B*42:01 or HLA-B*42:02 bound to a peptide, speculation beyond this is likely to be of limited value.
Previous studies of related HLA molecules differing by only a single amino acid support the findings presented here that apparently minor differences between HLA molecules may have a major impact on antiviral immunity (3, 4, 7, 18, 20, 26, 29, 37, 49, 52). In relation to HLA-mediated control of HIV, we have previously demonstrated the increased repertoire of HIV-specific epitope peptides presented by HLA-B*57:03 compared to HLA-B*57:02, HLA molecules that differ by a single change at HLA residue 156 (Leu→Arg). As with HLA-B*42:01, this increased repertoire of Gag epitopes presented was associated with a lower viral set point. In a comparison of HLA-B*44:02 and B*44:03, molecules with virtually identical peptide-binding motifs (37) that also differ only at HLA residue 156 (Asp→Leu), while an estimated >95% of peptides eluted from the two closely related HLA-B*44 molecules were identical, the majority of the peptides uniquely presented by one or the other allele were presented by HLA-B*44:03. Factors maintaining closely related alleles with divergent peptide-binding repertoires in the same populations may include the balancing selection pressures of increased antiviral immunity with increased autoimmunity.
We observed a clear difference in the immunodominant CD8+ T-cell targeting of epitopes between HLA-B*42:01 and B*42:02, exemplified by p24-TL9 and Int-IM9 being dominantly targeted by HLA-B*42:01 but not targeted by HLA-B*42:02 individuals at all, whereas HLA-B*42:02 individuals target RT-GL9 and RT-SM9 significantly more than HLA-B*42:01 individuals. The difference in p24-TL9 and Int-IM9 targeting may result from the respective HLA-peptide stabilities (binding half-life). However, this cannot explain the lack of RT-GL9 and RT-SM9 targeting through HLA-B*42:01, which in part may be a consequence of the preferential immunodominant targeting of p24-TL9, Int-IM9, Int-LI9, and Vif-HI10.
In conclusion, the improved immune control of HIV from HLA-B*42:01 over HLA-B*42:02 is a direct consequence of a single amino acid substitution critical for accommodation of Gag epitopes, which translates into broader targeting of Gag epitopes in individuals expressing HLA-B*42:01 and also with stronger selection pressure on the virus imposed by HLA-B*42:01-restricted responses. This study is the first to show distinct outcomes of HIV immune control from two closely related alleles on identical HLA class I haplotypes, a magnitude of difference almost equivalent to the effect of HLA-B*57:03, the most protective allele in this population, and therefore substantiates previous work indicating that Gag-specific responses in particular are critical to the CD8-mediated immune control of HIV.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Wellcome Trust (P.G.) and National Institutes of Health grant RO1 AI46995. This project has been funded in whole or in part with federal funds from the Frederick National Laboratory for Cancer Research under contract no. HHSN261200800001E. This research was supported in part by the Intramural Research Program of the NIH, Frederick National Laboratory, Center for Cancer Research.
The contributions of C-clade sequence data made by the Seattle CFAR Computational Biology Core are gratefully acknowledged.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Published ahead of print 15 August 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Altfeld M, Goulder P. 2007. ‘Unleashed’ natural killers hinder HIV. Nat. Genet. 39:708–710 [DOI] [PubMed] [Google Scholar]
- 2. Altman JD, et al. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94–96 [PubMed] [Google Scholar]
- 3. Archbold JK, et al. 2009. Natural micropolymorphism in human leukocyte antigens provides a basis for genetic control of antigen recognition. J. Exp. Med. 206:209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bade-Doding C, et al. 2010. The impact of human leukocyte antigen (HLA) micropolymorphism on ligand specificity within the HLA-B*41 allotypic family. Haematologicae 96:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. 57:289–300 [Google Scholar]
- 6. Brackenridge S, et al. 2011. An early HIV mutation within an HLA-B*57-restricted T cell epitope abrogates binding to the killer inhibitory receptor 3DL1. J. Virol. 85:5415–5422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burrows JM, et al. 2007. The impact of HLA-B micropolymorphism outside primary peptide anchor pockets on the CTL response to CMV. Eur. J. Immunol. 37:946–953 [DOI] [PubMed] [Google Scholar]
- 8. Carlson J, Heckerman D, Shani G. 2009. Estimating false discovery rates for contingency tables. Microsoft, Redmond, WA [Google Scholar]
- 9. Carlson JM, et al. 2012. Widespread impact of HLA restriction on immune control and escape pathways in HIV-1. J. Virol. 86:5230–5243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carrington M, Walker BD. 2012. Immunogenetics of spontaneous control of HIV. Annu. Rev. Med. 63:131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Catano G, et al. 2008. HIV-1 disease-influencing effects associated with ZNRD1, HCP5 and HLA-C alleles are attributable mainly to either HLA-A10 or HLA-B*57 alleles. PLoS One 3:e3636 doi:10.1371/journal.pone.0003636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crawford H, et al. 2007. Compensatory mutation partially restores fitness and delays reversion of escape mutation within the immunodominant HLA-B*5703-restricted Gag epitope in chronic human immunodeficiency virus type 1 infection. J. Virol. 81:8346–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Erup Larsen M, et al. 2011. HLArestrictor—a tool for patient-specific predictions of HLA restriction elements and optimal epitopes within peptides. Immunogenetics 63:43–55 [DOI] [PubMed] [Google Scholar]
- 14. Feeney ME, et al. 2004. Immune escape precedes breakthrough human immunodeficiency virus type 1 viremia and broadening of the cytotoxic T-lymphocyte response in an HLA-B27-positive long-term-nonprogressing child. J. Virol. 78:8927–8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fellay J, et al. 2009. Common genetic variation and the control of HIV-1 in humans. PLoS Genet. 5:e1000791 doi:10.1371/journal.pgen.1000791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fellay J, et al. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317:944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flores-Villanueva PO, et al. 2001. Control of HIV-1 viremia and protection from AIDS are associated with HLA-Bw4 homozygosity. Proc. Natl. Acad. Sci. U. S. A. 98:5140–5145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gao X, et al. 2001. Effect of a single amino acid change in MHC class I molecules on the rate of progression to AIDS. N. Engl. J. Med. 344:1668–1675 [DOI] [PubMed] [Google Scholar]
- 19. Goulder PJ, Watkins DI. 2008. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat. Rev. Immunol. 8:619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Green KJ, et al. 2004. Potent T cell response to a class I-binding 13-mer viral epitope and the influence of HLA micropolymorphism in controlling epitope length. Eur. J. Immunol. 34:2510–2519 [DOI] [PubMed] [Google Scholar]
- 21. Harndahl M, et al. 2009. Peptide binding to HLA class I molecules: homogenous, high-throughput screening, and affinity assays. J. Biomol. Screen. 14:173–180 [DOI] [PubMed] [Google Scholar]
- 22. Harndahl M, Rasmussen M, Roder G, Buus S. 2011. Real-time, high-throughput measurements of peptide-MHC-I dissociation using a scintillation proximity assay. J. Immunol. Methods 374:5–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harndahl M, et al. 2012. Peptide-MHC class I stability is a better predictor than peptide affinity of CTL immunogenicity. Eur. J. Immunol. 42:1405–1416 [DOI] [PubMed] [Google Scholar]
- 24. Honeyborne I, et al. 2007. Control of human immunodeficiency virus type 1 is associated with HLA-B*13 and targeting of multiple gag-specific CD8+ T-cell epitopes. J. Virol. 81:3667–3672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Honeyborne I, et al. 2006. Motif inference reveals optimal CTL epitopes presented by HLA class I alleles highly prevalent in southern Africa. J. Immunol. 176:4699–4705 [DOI] [PubMed] [Google Scholar]
- 26. Huang KH, et al. 2011. Progression to AIDS in South Africa is associated with both reverting and compensatory viral mutations. PLoS One 6:e19018 doi:10.1371/journal.pone.0019018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin X, et al. 2002. Human immunodeficiency virus type 1 (HIV-1)-specific CD8+-T-cell responses for groups of HIV-1-infected individuals with different HLA-B*35 genotypes. J. Virol. 76:12603–12610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kiepiela P, et al. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432:769–775 [DOI] [PubMed] [Google Scholar]
- 29. Kiepiela P, et al. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46–53 [DOI] [PubMed] [Google Scholar]
- 30. Kloverpris HN, et al. 2012. HLA-B*57 micropolymorphism shapes HLA allele-specific epitope immunogenicity, selection pressure and HIV immune control. J. Virol. 86:919–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koehler RN, et al. 2010. Class I HLA-A*7401 is associated with protection from HIV-1 acquisition and disease progression in Mbeya, Tanzania. J. Infect. Dis. 202:1562–1566 [DOI] [PubMed] [Google Scholar]
- 32. Kosmrlj A, et al. 2010. Effects of thymic selection of the T-cell repertoire on HLA class I-associated control of HIV infection. Nature 465:350–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kulkarni S, et al. 2011. Differential microRNA regulation of HLA-C expression and its association with HIV control. Nature 472:495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leslie A, et al. 2005. Transmission and accumulation of CTL escape variants drive negative associations between HIV polymorphisms and HLA. J. Exp. Med. 201:891–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leslie A, et al. 2010. Additive contribution of HLA class I alleles in the immune control of HIV-1 infection. J. Virol. 84:9879–9888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leslie A, et al. 2006. Differential selection pressure exerted on HIV by CTL targeting identical epitopes but restricted by distinct HLA alleles from the same HLA supertype. J. Immunol. 177:4699–4708 [DOI] [PubMed] [Google Scholar]
- 37. Macdonald WA, et al. 2003. A naturally selected dimorphism within the HLA-B44 supertype alters class I structure, peptide repertoire, and T cell recognition. J. Exp. Med. 198:679–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Marsh SGE, Parham P, Barber LD. 2000. The HLA facts book. Academic Press, London, United Kingdom [Google Scholar]
- 39. Martin MP, et al. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31:429–434 [DOI] [PubMed] [Google Scholar]
- 40. Martin MP, et al. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39:733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Matthews PC, et al. 2011. HLA-A*7401-mediated control of HIV viremia is independent of its linkage disequilibrium with HLA-B*5703. J. Immunol. 186:5675–5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Matthews PC, et al. 2008. Central role of reverting mutations in HLA associations with human immunodeficiency virus set point. J. Virol. 82:8548–8559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mothe B, et al. 2011. Definition of the viral targets of protective HIV-1-specific T cell responses. J. Transl. Med. 9:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. O'Brien SJ, Gao X, Carrington M. 2001. HLA and AIDS: a cautionary tale. Trends Mol. Med. 7:379–381 [DOI] [PubMed] [Google Scholar]
- 45. Pelak K, et al. 2011. Copy number variation of KIR genes influences HIV-1 control. PLoS Biol. 9:e1001208 doi:10.1371/journal.pbio.1001208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pereyra F, et al. 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rapin N, Hoof I, Lund O, Nielsen M. 2008. MHC motif viewer. Immunogenetics 60:759–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rousseau CM, et al. 2006. Large-scale amplification, cloning and sequencing of near full-length HIV-1 subtype C genomes. J. Virol. Methods 136:118–125 [DOI] [PubMed] [Google Scholar]
- 49. Smith KD, Epperson DF, Lutz CT. 1996. Alloreactive cytotoxic T-lymphocyte-defined HLA-B7 subtypes differ in peptide antigen presentation. Immunogenetics 43:27–37 [DOI] [PubMed] [Google Scholar]
- 50. Stryhn A, et al. 1996. Peptide binding specificity of major histocompatibility complex class I resolved into an array of apparently independent subspecificities: quantitation by peptide libraries and improved prediction of binding. Eur. J. Immunol. 26:1911–1918 [DOI] [PubMed] [Google Scholar]
- 51. Thomas R, et al. 2009. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat. Genet. 41:1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zernich D, et al. 2004. Natural HLA class I polymorphism controls the pathway of antigen presentation and susceptibility to viral evasion. J. Exp. Med. 200:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.