Abstract
HIV-1 recruits members of ESCRT, the cell membrane fission machinery that promotes virus exit. HIV-1 Gag protein gains access to ESCRT directly by binding Alix, an ESCRT-associated protein that promotes budding. The Alix Bro1 and V domains bind Gag NC and p6 regions, respectively. Whereas V-p6 binding and function are well characterized, residues in Bro1 that interact with NC and their functional contribution to Alix-mediated HIV-1 budding are unknown. We mapped Bro1 residues that constitute the NC binding interface and found that they are critical for function. Intriguingly, residues involved in interactions on both sides of the Bro1-NC interface are positively charged, suggesting the involvement of a negatively charged cellular factor serving as a bridge. Nuclease treatment eliminated Bro1-NC interactions, revealing the involvement of RNA. These findings establish a direct role for NC in mediating interactions with ESCRT necessary for virus release and report the first evidence of RNA involvement in such recruitments.
INTRODUCTION
HIV-1 usurps members of the host cell fission machinery to promote virus release. Two conserved sequences located within the C-terminal p6 domain of Gag, PTAP, and LYPXnL, named late (L) domains, are utilized to fulfill such functions. They bind Tsg101 and Alix, respectively (14, 37, 40), two host cellular proteins that initiate a set of sequential interactions leading to the recruitment of members of the endosomal sorting complex required for transport (ESCRT) pathway (5, 9, 30). The latter is comprised of three multiprotein complexes, named ESCRT-I, ESCRT-II, and ESCRT-III, that facilitate membrane-modeling events critical for multivesicular body (MVB) generation (2, 3), cytokinesis (7), and autophagy (32).
Tsg101 functions in HIV-1 release as part of ESCRT-I (26) and mediates access to members of ESCRT-III, the charged MVB protein CHMP2 and CHMP4 isoforms, as well as the VPS4 ATPase (29, 38, 41). Whereas interactions that link Tsg101 (and ESCRT-I) to ESCRT-III are still unknown, Alix binds CHMP4 isoforms directly, thus linking Gag to ESCRT-III members (12, 19, 37, 39). Although the Tsg101/PTAP pathway is considered predominant in HIV-1 release, the Alix/LYPXnL pathway is also functional in 293T cells and appears to be more efficient in T lymphocytes (11–13, 37, 39). This pathway is also sufficient to drive the release of the equine infectious anemia virus (EIAV) (8, 37), a lentivirus that relies solely on cellular Alix for virus budding.
Alix structure revealed two well-ordered domains, the N-terminal boomerang-shaped Bro1 and the central V-shaped domains (12, 21); they interact with the NC and p6 domains of HIV-1 Gag, respectively (10–12, 31, 37). The binding interface between the LYPXnL motif and the V domain and its functional role have been well characterized (12). In contrast, residues in the Alix Bro1 domain that mediate interactions with NC (10, 11, 31) and their role in virus release are not known. We performed a mutational analysis and used a combination of binding and functional assays to map the Bro1-NC interface and examine its role in virus budding. Residues delineating the interface have been identified, and their nature suggested a critical role for RNA in Bro1-NC interactions.
MATERIALS AND METHODS
Proviral and expression vectors.
We used the wild-type (WT) molecular clones of HIV-1 pNL4-3 (1) and EIAVUK (24). The L-domain HIV-1 mutant PTAP− and the PTAP-RKI and PTAP-RKII mutants were previously described (15). The hemagglutinin (HA)-tagged version of full-length Alix, the Alix Bro1 domain, and the Flag-tagged CHMP4B expression constructs were previously described (10, 36). Full-length Alix was also cloned in pEXPR-IBA105 (IBA BioTAGnology, Göttingen, Germany) between EcoRI and NotI sites to obtain the Strep-tagged version. Point mutations were introduced in the Alix Bro1 domain using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA). Seventeen silent mutations that render HA-Alix resistant to short interfering RNA (siRNA) (denoted AlixRR in Fig. 3B) were introduced into the wild-type Alix coding region. Residues in Alix Bro1 were selected following solvent accessible surface (SAS) analysis using the Alix Bro1 domain crystal structure (Protein Data Bank [PDB] entry 2OEW). SAS values were calculated using the AREAIMOL program (23, 33) that is part of the CCP4 suite (43). The N-terminally Flag-tagged Nedd4.1 was described by Sette et al. (35) and the C-terminally HA-tagged APOBEC3G by Huthhoff and Malim (16). The glutathione S-transferase (GST) fusion plasmids encoding the EIAVUK NC-p9 region, HIV-1 NC-p6, and its mutants, NCRKI-p6 and NCRKII-p6, were previously described (4, 10, 36).
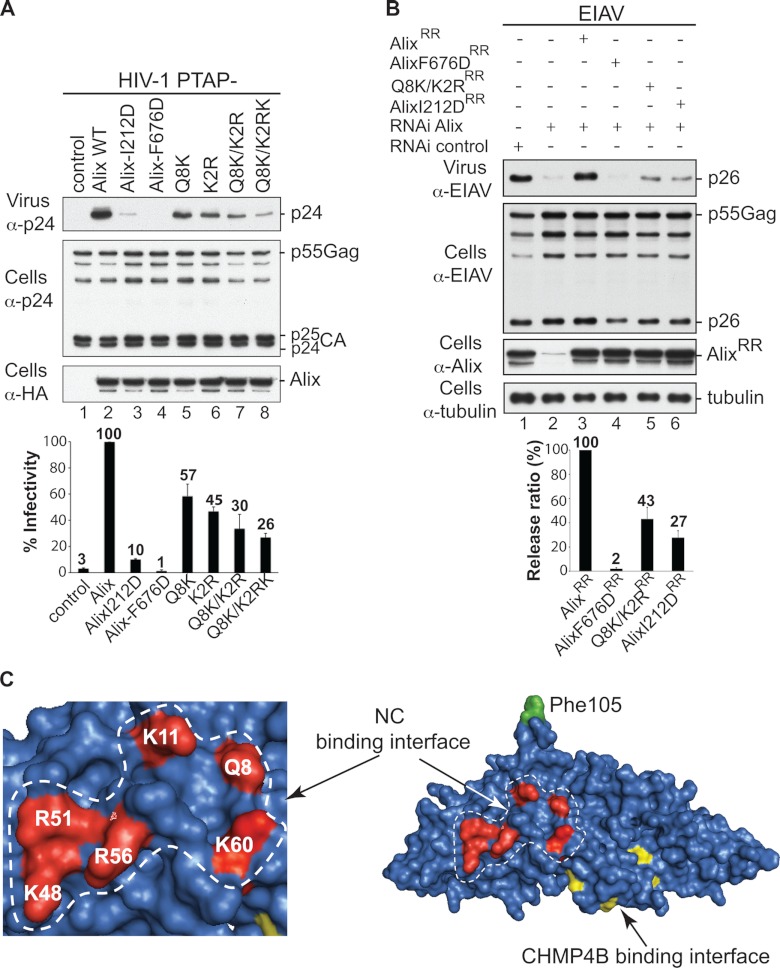
Fig 3.
Alix Bro1 interface involved in NC binding plays a critical role in virus release. (A) The Alix Bro1 mutants with a compromised NC binding interface show a decreased ability to stimulate the release of the HIV-1 PTAP− mutant. 293T cells were transfected with HIV-1 PTAP− proviral DNA alone (lane 1), with WT HA-tagged Alix (lane 2), or with the indicated Alix mutants (lanes 3 to 8). Cells and viruses were collected 24 h posttransfection, and their protein content was analyzed by SDS-PAGE and Western blotting using the indicated antibodies. Release of infectious viral particles was quantified using HeLa TZM-bl assays from five independent experiments and expressed relative to WT Alix (lane 2). Error bars represent standard deviations (SD). (B) An intact NC binding interface is required for Alix function in EIAV release. 293T cells were transfected twice with Alix RNAi oligonucleotides at 36-h intervals. At the second transfection, cells were cotransfected with EIAV provirus alone (lanes 1 and 2), with an RNAi-resistant (RR) version of Alix WT (lane 3), or with the indicated mutants (lanes 4 to 6). Cells and viruses were collected 24 h posttransfection, and their protein content was analyzed by SDS-PAGE and Western blotting using the indicated antibodies. Release ratios were calculated as described in Materials and Methods from 3 independent experiments and expressed relative to WT Alix (lane 3), ±SD. (C) Alix Bro1 residues engaged in NC interaction define a new functional interface. Residues Q8, K11, K48, R51, R56, and K60 placed in the Alix Bro1 domain crystal structure (2OEW) cluster within a positively charged exposed surface (shown in red) on one side of the Bro1 domain. For reference, the Phe105 residue (required for Alix function in HIV-1 release [36]) and the CHMP4B binding interface (27) are shown in green and yellow, respectively.
Virus release analysis.
293T cells were maintained and transfected as previously described (36). Twenty-four hours after transfection, cells and culture media were harvested and their protein content was analyzed using the protocol previously described (36). HIV-1 proteins were detected using an anti-HIV-1 p24 monoclonal antibody (clone 183-H12-5C) or NEA-9306. EIAV proteins were detected using a horse anti-EIAV serum (28). The EIAV release ratio (values are given in percentage) was calculated as virus-associated Gag divided by cell-associated Gag, as determined by densitometry analysis of Western blotting films using ImageJ software (W. S. Rasband, NIH, Bethesda, MD; http://rsb.info.nih.gov/ij). Alix, the Bro1 domain, and the respective mutants were detected using anti-HA monoclonal antibodies (Sigma, St. Louis, MO).
Infectivity assay.
Viral infectivity was quantified using TZM-bl cell assays (42). HeLa TZM-bl cells were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. They were seeded (2 × 104 cells) in 96-well plates and the following day infected in triplicate with HIV-1 PTAP-rescued virus stocks in the presence of 20 μg/ml DEAE-dextran (Sigma, St. Louis, MO). After 48 h, cells were assayed for luciferase activity using the Steady-Glo reagent kit (Promega, Madison, WI) according to the manufacturer's instructions. Luminescence was quantified using a microplate reader (Turner BioSystems, Sunnyvale, CA).
Immunoprecipitation assays.
The immunoprecipitation assays were conducted as previously described (36). Immunoprecipitation complexes and cell lysates (input fractions) were analyzed by SDS-PAGE and Western blotting using anti-HA and anti-Flag M2 antibodies (Sigma, St. Louis, MO). To perform the immunoprecipitation assays using the Strep-tagged proteins, cell lysates were incubated with Strep-Tactin Sepharose (IBA GmbH, Gottingen, Germany) for 2 h at 4°C. The matrix was washed five times in lysis buffer and eluted using SDS-PAGE loading buffer.
Pulldown assays and nuclease treatments.
The empty pGEX vector or that carrying the coding sequences of HIV-1 NC-p6 and EIAV NC-p9 were expressed in BL21(DE3) pLysS E. coli (Stratagene), and their interactions with HA-Bro1and its mutants were examined in GST pulldown assays by following the protocol previously described (36). Where indicated, protein complexes captured on beads were incubated for 30 min at 37°C in the presence or absence of 50 μg/ml RNase A (EMD Chemicals, Inc., San Diego, CA) or 75 U (0.75 U/μl) benzonase/nuclease (Novagen) in benzonase buffer (1.2 mM MgCl2, 50 mM Tris-HCl [pH 8.0]). Eluted complexes and cell lysates (input fractions) were analyzed by SDS-PAGE and Western blotting using the indicated antibodies.
Alix knockdown and reconstitution.
293T cells (2.5 × 106 cells/ml) were transfected with 250 pmol of a mixture of two RNA interference (RNAi) oligonucleotides (Invitrogen life Technologies, Grand Island, NY) against cellular Alix. After 36 h, cells were cotransfected with the same amount of RNAi, 500 ng of EIAVUK proviral DNA, and 150 ng of HA-Alix or RNAi-resistant (RR) HA-Alix mutants. Cells and virus were harvested and processed as described above.
RESULTS
Identification of the NC-Bro1 binding interface.
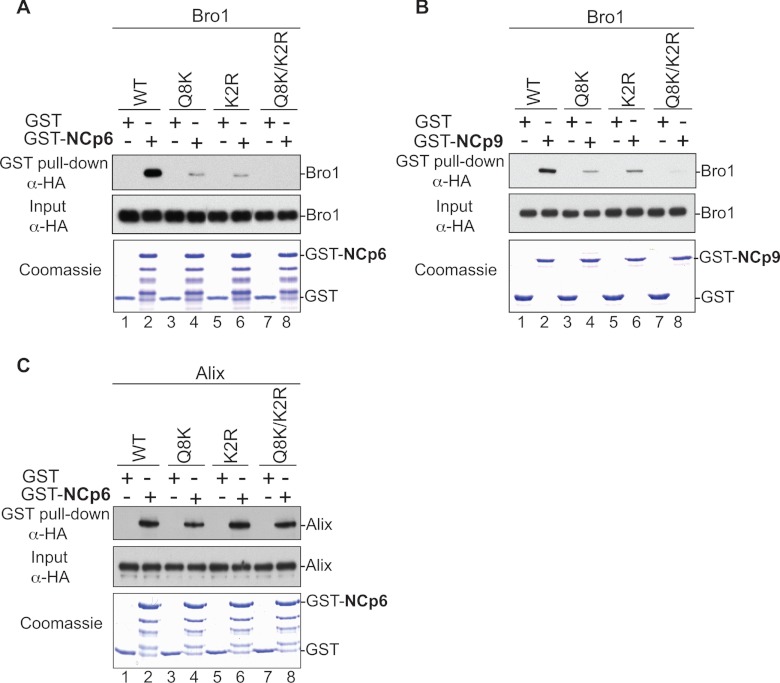
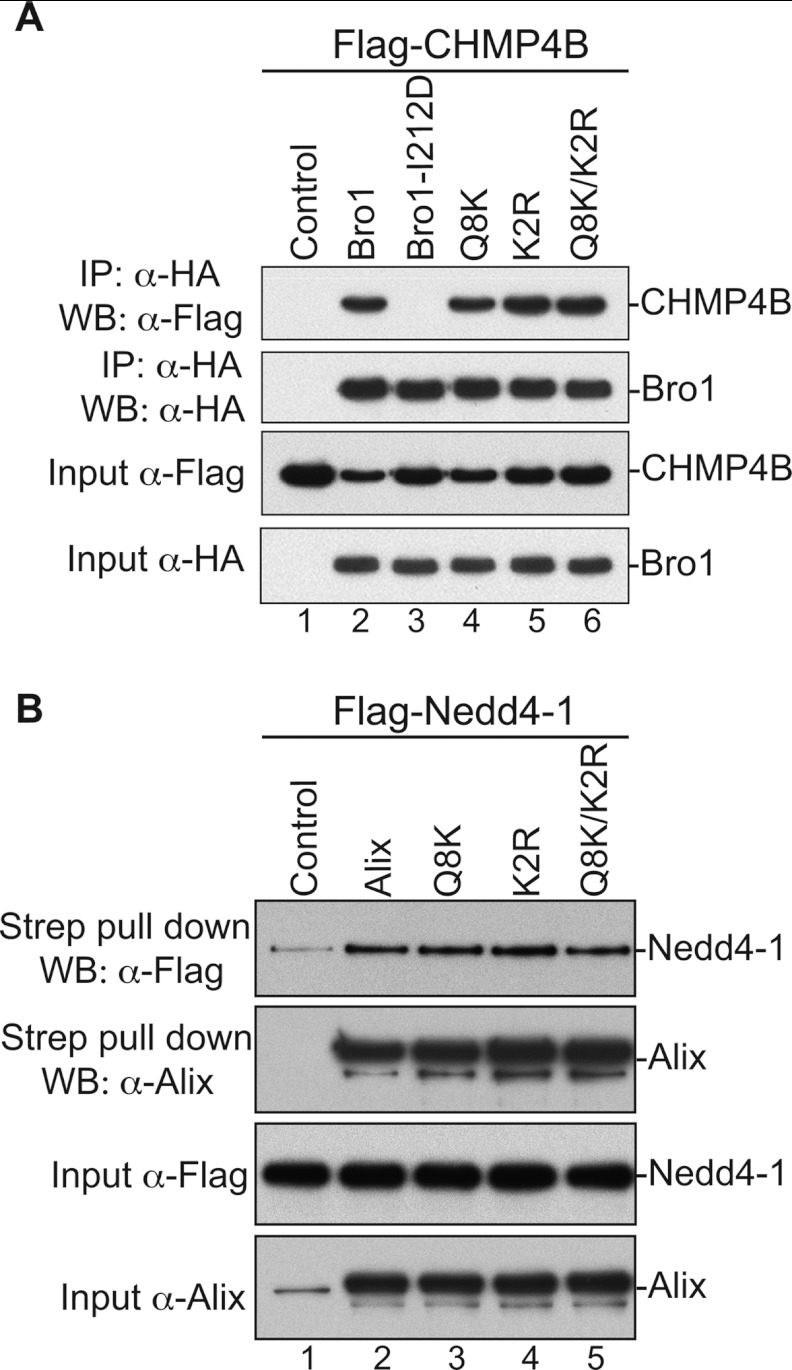
The N-terminal Alix Bro1 domain binds NC, while the central V domain binds the short conserved sequence LYPXnL late (L) domain in the p6 region (10–12, 31). Whereas binding determinants and the role of the latter have been extensively studied and characterized (12), those of the former are not known. We sought to map residues in the Alix Bro1 domain that mediate binding to HIV-1 and EIAV NC and examined their role in virus release. Residues in Bro1 that are accessible to solvent and therefore likely to be exposed and engaged in protein-protein interactions have been selected using SAS prediction for mutational analysis. More than 20 residues displaying high SAS values (see Materials and Methods), indicating exposure, were found in the first 202 residues of the Alix Bro1 domain, a fragment sufficient to bind NC (10). Specifically, residues in this region were selected for mutational and further analyses based on several criteria, including the following: (i) high SAS values compared to residues known to be exposed in the Alix Bro1 domain, such as those belonging to the Phe105 loop (36), and (ii) the ability to bind CHMP4. Mutants then were assessed for capture of NC, and the data obtained are summarized in Table 1. We found that substitutions of either Q8 (glutamine residue in position 8 in the Bro1 sequence) and K11 residues (Q8K mutant), or altering K48, R51, and R56 residues (K2R mutant) to alanines, caused a significant inhibition of the Bro1 domain interactions with NC-p6 domains in GST pulldown assays (Fig. 1A, lanes 4 and 6). Binding became undetectable when both sets of mutations were introduced in Bro1 (Q8K/K2R mutant) (Fig. 1A, lane 8; Table 1 provides nomenclature for the mutants). Similar results were obtained with EIAV NC-p9 protein (Fig. 1B). NC-Bro1 interactions had no effect on Alix V-p6 interactions, since all defective Alix Bro1 mutants retained interactions with the NC-p6 fragment (Fig. 1C). Furthermore, NC-Bro1 interactions were inhibited specifically, since all Alix mutants retained binding to their natural cellular partner CHMP4B, suggesting proper folding (Fig. 2A and Table 1). Since Alix also binds the ubiquitin ligase Nedd4-1, its cellular partner that is important for function (35), we tested the effect of mutations in the Bro1 domain on Alix interactions with Nedd4-1. Q8K, K2R, and Q8K/K2R mutants displayed a Nedd4-1 interaction pattern comparable to their WT Alix counterpart (Fig. 2B). Together, these results indicated that residues Q8, K11, K48, R51, R56, and K60 are part of the Bro1-NC binding interface.
Table 1.
List and characterization of Alix-Bro1 mutants tested in this studya
| Provisional name | Alix Bro1 domain mutants | NC binding | CHMP4B binding | SAS value (Å2) |
|---|---|---|---|---|
| Q8 | Q8/K11 | − | + | 130, 150 |
| E14/D16/K19 | ND | ND | 131, 82, 128 | |
| K23/F24/Q26/Q27 | + | + | 128, 65, 102, 145 | |
| E35/R41 | + | + | 98, 172 | |
| E44/E45 | + | + | 99, 60 | |
| K2R | K48/R51/R56 | − | + | 131, 90, 125 |
| Q8/K2R | Q8/K11/K48/R51/R56 | − | + | 130, 150, 131, 90, 125 |
| Q8/K2RK | Q8/K11/K48/R51/R56/K60 | − | + | 130, 150, 131, 90, 125, 104 |
| D59/E62 | + | + | 130, 130 | |
| F84/S85/E86 | + | + | 141, 97, 62 | |
| D100/K101 | + | + | 93, 148 | |
| L104 | + | + | 133 | |
| F105/G106/G107 | + | + | 193, 91, 59 | |
| L104/F105/G106/G107 | + | + | 133,193, 91, 59 | |
| F105/G106/G107/K110 | + | + | 193, 91, 59, 124 | |
| K110 | + | + | 124 | |
| E137/D141/N142/D143/E144 | ND | ND | 108,83, 71, 56, 109 | |
| D141/D143/E144 | + | + | 83, 56, 109 | |
| K164/E165 | + | + | 72, 134 | |
| S172/R173/E174 | + | + | 92,137,174 |
Fig 1.
Mapping of Alix Bro1 residues involved in the interaction with HIV-1 and EIAV NC domains. (A and B) GST, GST-NCp6, and GST-NCp9 fusion proteins were expressed in E. coli, captured on glutathione-conjugated beads, and subsequently incubated with lysates from 293T cells expressing WT HA-tagged Bro1 domain or the indicated Bro1 mutants (Q8K, K2R, and Q8/K2R). Captured proteins and cell lysates were analyzed by SDS-PAGE and Western blotting. GST fusion proteins were visualized by Coomassie blue staining. (C) Mutations that compromise the NC binding interface in Bro1 do not affect Alix V domain interaction with HIV-1 p6. Pulldown assays were performed as described above, with the only difference being that full-length HA-tagged Alix WT and the indicated mutants were used instead of the isolated Bro1 domain.
Fig 2.
Alix Bro1 mutants retain binding to their natural cellular partners. (A) Alix Bro1 mutants bind CHMP4B. 293T cells were cotransfected with Flag-tagged CHMP4B alone (lane 1), in combination with HA-tagged WT Bro1 (lane 2), or with the indicated mutant (lanes 3 to 6). Cells were lysed in RIPA buffer, and cleared lysates were incubated with anti-HA antibody-conjugated beads. Both input and immunoprecipitated (IP) complexes were analyzed by SDS-PAGE and Western blotting (WB) using the indicated antibody. (B) Alix Bro1 mutants bind Nedd4-1. 293T cells were cotransfected with Flag-tagged Nedd4-1 alone (lane 1), in combination with Strep-tagged WT Alix (lane 2), or with the indicated mutant (lanes 3 to 5). Cells were lysed in RIPA buffer, and cleared lysates were incubated with Strep-Tactin Sepharose. The input and the purified complexes were probed with the anti-Flag and anti-Alix antibodies.
Disruption of Bro1-NC binding inhibits Alix function in virus release.
To examine the functional significance of the Bro1-NC interface, we disrupted its residues and assessed the effect on HIV-1 release using a virus rescue assay (12, 39). Whereas ectopic expression of WT Alix rescued HIV-1 lacking access to ESCRT-I (HIV-1 PTAP− mutant) (Fig. 3A, lanes 1 and 2), Alix mutants with a compromised NC binding interface displayed diminished virus stimulation abilities that were proportional to the number of mutated residues (Fig. 3A, lanes 5 to 8). The effect of the Q8K/K2RK mutation on viral release was comparable to that of the I212D mutation, which disrupts Alix binding to the essential CHMP4 factors (Fig. 3A, compare lane 3 to lane 8). Similar results were obtained when Alix mutants were used to functionally replace cellular Alix and facilitate EIAV release (Fig. 3B). Placing residues involved in NC-Bro1 interactions in the Bro1 crystal structure (12) revealed a defined interface that exposes a cluster of basic residues on one side of the boomerang (Fig. 3C). Together, these data draw a direct correlation between the Bro1 domain's ability to bind NC and Alix function in virus release and suggest the first direct functional link between Bro1-NC interactions and virus release.
Bro1-NC binding involves RNA.
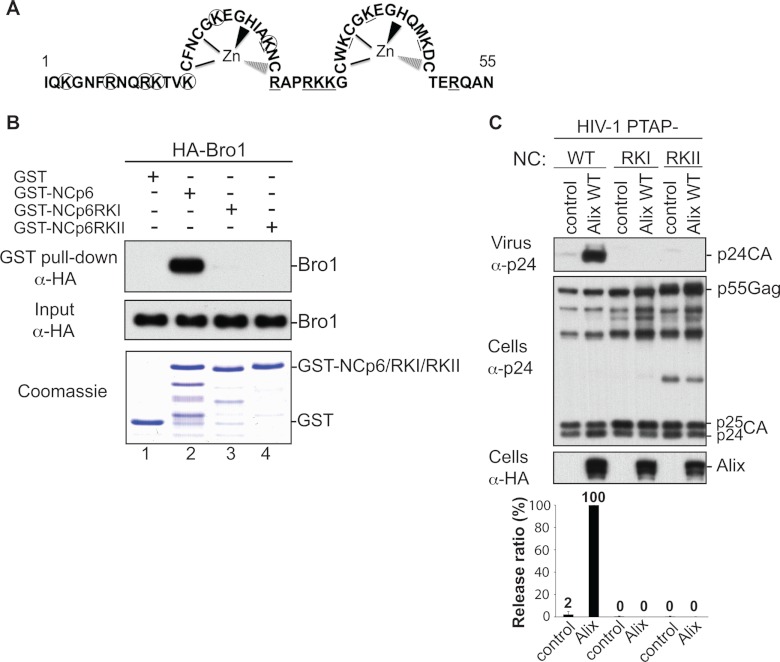
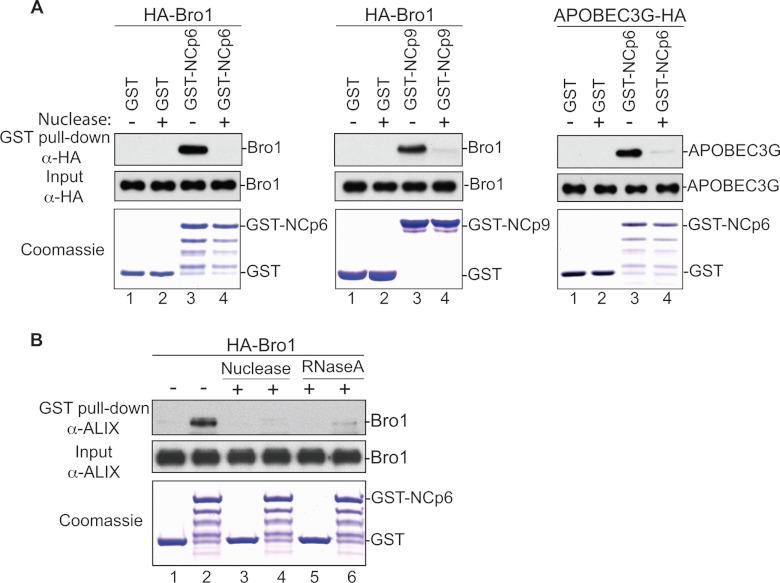
Mapping of the Bro1-NC binding interface revealed that all residues involved are positively charged (Fig. 3C). This finding was surprising because Bro1-NC interactions were previously reported to be RNA independent (31). Moreover, we previously showed that residues in NC involved in association with the Alix Bro1 domain are also positively charged. Indeed, NC mutants RKI and RKII, carrying alterations of lysine and arginine (Fig. 4A) to alanine residues, ceased to bind Bro1 and function in virus release (Fig. 4B and C and references 4, 10, 11, and 36), and these budding defects were alleviated by providing Gag with parallel access via Nedd4.2 expression to the scission-inducing members of the ESCRT pathway (11). This indicated that mutations of basic residues in NC interfered with NC-Bro1 interactions and interrupted Alix function in scission events. Involvement of positively charged residues on both NC and Bro1 sides raised the possibility that their interaction involves a negatively charged cellular factor. Since NC binds and incorporates viral (as well as cellular) RNA into virions (25), we elected to test its potential involvement in Bro1-NC interaction. GST-NC-p6 binding to the Alix Bro1 domain was tested in the presence or absence of nuclease. Whereas GST-NC-p6 captured the Bro1 domain as expected (Fig. 5A, left, lane 3), treatment of these complexes with nuclease (lane 4) or RNase A (Fig. 5B, lanes 5 and 6) abrogated binding. Similarly, GST-NC-p6 binding to APOBEC3G (34, 44), a host cell protein known to require RNA for interactions with NC (6, 20), was equally sensitive to nuclease treatment (Fig. 5A, right, lane 3) or RNase treatment (data not shown). Similar results were obtained with the EIAV NC-p9 construct (Fig. 5A, center). Collectively, these data indicate that RNA is involved and important for NC-Bro1 interactions.
Fig 4.
Basic residues in NC are required for the interaction with the Alix Bro1 domain. (A) Schematic representation of the HIV-1 NC domain (amino acids 1 to 55). Lysine (K) and arginine (R) residues replaced with alanine in the RKI and RKII mutants are circled and underlined, respectively. (B) Mutation of basic residues at the N or C terminus of NC prevents NC-Bro1 interaction. GST, GST-NCp6, GST-NCp6RKI, or GST-NCp6RKII fusion protein was expressed in E. coli, captured on glutathione-conjugated beads, and subsequently incubated with lysates from 293T cells expressing the WT HA-Bro1 domain. Captured proteins and cell lysates were analyzed by SDS-PAGE and Western blotting using an anti-HA antibody. GST fusion proteins were visualized by Coomassie blue staining. (C) Alix function in HIV-1 release requires an intact NC domain. 293T cells were transfected with HIV-1 PTAP− proviral DNA harboring a WT NC (lanes 1 and 2) or the indicated NC mutant (lanes 3 to 6) either alone (lanes 1, 3, and 5) or with WT HA-tagged Alix (lanes 2, 4, and 6). Cells and viruses were collected 24 h posttransfection, and their protein content was analyzed by SDS-PAGE and Western blotting using the indicated antibodies. Release of viral particles was quantified from two independent experiments.
Fig 5.
RNA is involved in the Alix Bro1-NC interaction. (A) GST, GST-NCp6 (left), and GST-NCp9 (center) fusion proteins expressed in E. coli and captured on glutathione beads were incubated with lysates from 293T cells expressing HA-tagged WT Bro1 domain or APOBEC3G (right), followed by incubation with or without benzonase/nuclease. (B) GST and GST-NCp6 fusion proteins purified by glutathione beads were incubated with lysates from 293T cells expressing HA-tagged WT Bro1, followed by treatment with or without benzonase (lanes 3 and 4) or RNAse A (lanes 5 and 6). In both experiments, captured proteins and cell lysates were analyzed by SDS-PAGE and Western blotting. GST fusion proteins were visualized by Coomassie blue staining.
DISCUSSION
HIV-1 interactions with the ESCRT-associated protein Alix is sufficient for virus production from T lymphocytes (11, 13). Such recruitment provides the only direct access to the cell membrane fission machinery. Indeed, the Alix Bro1 domain binds CHMP4 isoforms, which are members of ESCRT-III and play a key role in ringing and severing viral budding necks (29). Two interactions have been identified between Gag and Alix. The interaction between the Gag p6 region and the Alix V domain was identified, and its role in virus release was clearly established (12, 37). The second interaction that links NC to the Alix Bro1 domain has been recently identified (10, 31). Here, we identified residues in the Alix Bro1 domain that define the binding interface with NC and found that RNA is involved in such interactions.
Key determinants of NC-Bro1 interactions.
Since the discovery of NC-Bro1 interactions, questions regarding their role in virus release arose. Several lines of evidence underscored the importance of NC-Bro1 interactions, including the findings that a functional NC is required for Alix-mediated virus release (4, 10, 11, 31), and mutant NC viruses that fail to bind the Bro1 domain were also defective in virus budding (4, 10, 11). The role of Bro1-NC interactions in virus release was further strengthened by the identification of residues that mediate binding. Their mutational analysis revealed that those critical for binding NC lie within the first beta sheet (β1) and the second alpha helix (α2) of Bro1. Residues interacting with NC are positively charged and cluster in a well-defined interface whose disruption was sufficient to cause a dramatic loss of binding and Alix-mediated virus release (Fig. 1 and 3). This provides direct evidence of a requirement for contact between Bro1 and NC to achieve efficient virus release.
Why Alix requires binding to both NC and p6 to promote virus exit is not clear (references 12 and 39 and Fig. 2). However, clues into the necessity for interactions between NC and Bro1 come from the latter's ability to recruit CHMP4, which links Gag directly to membrane fission-inducing ESCRT-III members. Consistent with this notion, Bro1 can function as the smallest unit of Alix as its ectopic expression promoted virus release, provided it retained binding to both NC and CHMP4 (4, 10, 11). Moreover, an Alix mutant lacking binding to either NC or p6 failed to replace cellular Alix and promote EIAV budding (Fig. 3), further reaffirming a key functional role for Bro1-NC interactions in virus release.
The contribution of Bro1 versus V binding to Gag in Alix function during budding appears to differ. Indeed, disruption of interactions with V was more detrimental to virus release than that of Bro1, since the V binding-devoid AlixF676D exhibited less activity in virus release than the NC binding-defective AlixQ8/K2RK (Fig. 3). Conversely, the latter's defect mirrors interference with CHMP4 recruitment (I212D mutant), as Alix mutants lacking either determinants (Fig. 3) displayed comparable virus release defects. Interestingly, AlixF676D lost the ability to locate to sites of assembly at the membrane (18) despite retaining a functional NC binding interface, suggesting NC-Bro1 interactions take place only later in the viral egress process (i.e., in the budding neck), a role in agreement with its functional importance (Fig. 3). These findings suggest a model for Alix function in two steps (Fig. 6). First, Alix is anchored to sites of assembly via p6-V interactions and Bro1 seems to be unavailable to capture CHMP4, possibly due to a structural masking (45). Next, Bro1 binds NC, possibly in the budding neck, and becomes available to recruit CHMP4 during budding, a sequence of events that fits with the recent visualization of CHMP4 at the membrane once Gag assembly is complete (18).
Fig 6.
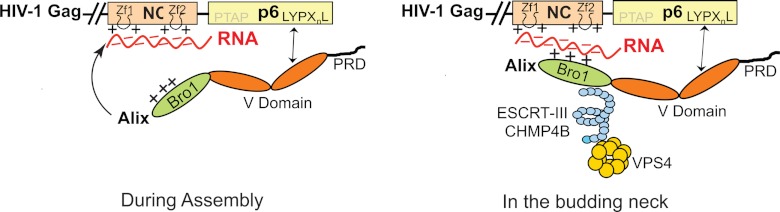
Schematic representation of a two-step model for Alix function in virus assembly and budding. In the first step, Alix is recruited by Gag to the plasma membrane during assembly through interaction between its V domain and the (L)YPXnL motif in p6 and Alix is thus anchored at the membrane (left). Whether NC binds Bro1 during this step or not is unclear, since it was insufficient to locate Alix to the membrane when the LYPXnL motif was disrupted (18). In the second step, we propose that the NC-Bro1 interaction occurs in the budding neck and is followed (or accompanied) by the recruitment of CHMP4B (right). Basic residues found in both NC and Bro1 proteins mediate binding, and RNA (in red) plays the role of the negatively charged factor that bridges this interaction.
RNA bridges interactions between NC and Bro1.
Identification of the NC binding interface in Bro1 revealed that residues involved are positively charged and delineate a well-defined interface on one side of the Bro1 boomerang domain (Fig. 3C). Moreover, mutation of basic residues in NC also eliminated binding to Bro1 (4, 10, 36), implying the involvement of a negatively charged factor. RNA, however, was excluded from Bro1-NC interactions in a previous report (31). This discrepancy is not clear but could be due to the method employed to capture Bro1 (see Materials and Methods for buffer composition in salt and detergent) and/or the fragment used. Indeed, Popov et al. (31) used NC-p1 domains to capture Alix, whereas we used NC-p1-p6 (Fig. 5). RNA nevertheless was the obvious candidate to bridge interactions between Bro1 and NC, since the latter binds genomic RNA during assembly (17, 22). In agreement with this, RNA involvement was confirmed as nuclease and RNase treatments abrogated NC-Bro1 binding. Similarly, mutations of basic residues in NC that are involved in RNA recruitment also eliminated NC-Bro1 interactions (Fig. 5) and brought Alix function to a near halt (10, 35). Recent reports employing high-resolution imaging suggested that genomic RNA localizes to the plasma membrane in the initial steps of assembly before a Gag nascent complex became visible (17, 22), whereas recruitment of Alix accompanies Gag accumulation during the early steps of assembly at the cell membrane (18). These observations and our findings suggest that Gag-RNA assembly complexes recruit Alix, which fits with the involvement of RNA in interactions with the ESCRT-binding Alix Bro1 domain and NC. The requirement for RNA in Alix Bro1-NC interactions is not surprising, as NC binding of RNA early in viral nascent particles is critical for its three-dimensional structure/folding (25), a crucial step for particle assembly as well as the subsequent steps of recruitment and utilization of ESCRT components necessary for virus budding and exit (this study).
In summary, we identified the NC binding interface in Alix Bro1. The nature of residues involved revealed a critical role for RNA. Since interactions between Gag, genomic RNA, and Alix precede CHMP4 recruitment to sites of assembly (17, 18), we propose a model (Fig. 6 right) in which an NC-RNA-Bro1 nucleoprotein complex recruits CHMP4 during budding (in the budding neck) in order for virus exit to proceed.
ACKNOWLEDGMENTS
We thank Sam T. Xiao and Jiansheng Jiang for help with SAS and Alicia Buckler-White and her team at the LMM core for sequencing.
This work was supported by the Intramural Research Program of the NIAID and in part by funds from the Office of AIDS Research (OAR), NIH.
Footnotes
Published ahead of print 15 August 2012
REFERENCES
- 1. Adachi A, et al. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Babst M, Katzmann DJ, Estepa-Sabal EJ, Meerloo T, Emr SD. 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3:271–282 [DOI] [PubMed] [Google Scholar]
- 3. Babst M, Odorizzi G, Estepa EJ, Emr SD. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1:248–258 [DOI] [PubMed] [Google Scholar]
- 4. Bello NF, et al. 2012. Budding of retroviruses utilizing divergent L domains requires nucleocapsid. J. Virol. 86:4182–4193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bieniasz PD. 2009. The cell biology of HIV-1 virion genesis. Cell Host Microbe 5:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Burnett A, Spearman P. 2007. APOBEC3G multimers are recruited to the plasma membrane for packaging into human immunodeficiency virus type 1 virus-like particles in an RNA-dependent process requiring the NC basic linker. J. Virol. 81:5000–5013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carlton JG, Martin-Serrano J. 2007. Parallels between cytokinesis and retroviral budding: a role for the ESCRT machinery. Science 316:1908–1912 [DOI] [PubMed] [Google Scholar]
- 8. Chen C, Li F, Montelaro RC. 2001. Functional roles of equine infectious anemia virus Gag p9 in viral budding and infection. J. Virol. 75:9762–9770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Demirov DG, Freed EO. 2004. Retrovirus budding. Virus Res. 106:87–102 [DOI] [PubMed] [Google Scholar]
- 10. Dussupt V, et al. 2009. The nucleocapsid region of HIV-1 Gag cooperates with the PTAP and LYPXnL late domains to recruit the cellular machinery necessary for viral budding. PLoS Pathog. 5:e1000339 doi:10.1371/journal.ppat.1000339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dussupt V, et al. 2011. Basic residues in the nucleocapsid domain of Gag are critical for late events of HIV-1 budding. J. Virol. 85:2304–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fisher RD, et al. 2007. Structural and biochemical studies of ALIX/AIP1 and its role in retrovirus budding. Cell 128:841–852 [DOI] [PubMed] [Google Scholar]
- 13. Fujii K, et al. 2009. Functional role of Alix in HIV-1 replication. Virology 391:284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garrus JE, et al. 2001. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell 107:55–65 [DOI] [PubMed] [Google Scholar]
- 15. Huang M, Orenstein JM, Martin MA, Freed EO. 1995. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 69:6810–6818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huthoff H, Malim MH. 2007. Identification of amino acid residues in APOBEC3G required for regulation by human immunodeficiency virus type 1 Vif and virion encapsidation. J. Virol. 81:3807–3815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jouvenet N, Simon SM, Bieniasz PD. 2009. Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles. Proc. Natl. Acad. Sci. U. S. A. 106:19114–19119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jouvenet N, Zhadina M, Bieniasz PD, Simon SM. 2011. Dynamics of ESCRT protein recruitment during retroviral assembly. Nat. Cell Biol. 13:394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katoh K, et al. 2003. The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J. Biol. Chem. 278:39104–39113 [DOI] [PubMed] [Google Scholar]
- 20. Khan MA, et al. 2007. Analysis of the contribution of cellular and viral RNA to the packaging of APOBEC3G into HIV-1 virions. Retrovirology 4:48 doi:10.1186/1742-4690-4-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim J, et al. 2005. Structural basis for endosomal targeting by the Bro1 domain. Dev. Cell 8:937–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kutluay SB, Bieniasz PD. 2010. Analysis of the initiating events in HIV-1 particle assembly and genome packaging. PLoS Pathog. 6:e1001200 doi:10.1371/journal.ppat.1001200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee B, Richards FM. 1971. The interpretation of protein structures: estimation of static accessibility. J. Mol. Biol. 55:379–400 [DOI] [PubMed] [Google Scholar]
- 24. Li F, Chen C, Puffer BA, Montelaro RC. 2002. Functional replacement and positional dependence of homologous and heterologous L domains in equine infectious anemia virus replication. J. Virol. 76:1569–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu K, Heng X, Summers MF. 2011. Structural determinants and mechanism of HIV-1 genome packaging. J. Mol. Biol. 410:609–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Martin-Serrano J, Zang T, Bieniasz PD. 2003. Role of ESCRT-I in retroviral budding. J. Virol. 77:4794–4804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCullough J, Fisher RD, Whitby FG, Sundquist WI, Hill CP. 2008. ALIX-CHMP4 interactions in the human ESCRT pathway. Proc. Natl. Acad. Sci. U. S. A. 105:7687–7691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Montelaro RC, Parekh B, Orrego A, Issel CJ. 1984. Antigenic variation during persistent infection by equine infectious anemia virus, a retrovirus. J. Biol. Chem. 259:10539–10544 [PubMed] [Google Scholar]
- 29. Morita E, et al. 2011. ESCRT-III protein requirements for HIV-1 budding. Cell Host Microbe 9:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morita E, Sundquist WI. 2004. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20:395–425 [DOI] [PubMed] [Google Scholar]
- 31. Popov S, Popova E, Inoue M, Gottlinger HG. 2008. Human immunodeficiency virus type 1 Gag engages the Bro1 domain of ALIX/AIP1 through the nucleocapsid. J. Virol. 82:1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rusten TE, Vaccari T, Stenmark H. 2012. Shaping development with ESCRTs. Nat. Cell Biol. 14:38–45 [DOI] [PubMed] [Google Scholar]
- 33. Saff EB, Kuijlaars ABJ. 1997. Distributing many points on a sphere. Mathematical Intelligencer 19:5–11 [Google Scholar]
- 34. Schafer A, Bogerd HP, Cullen BR. 2004. Specific packaging of APOBEC3G into HIV-1 virions is mediated by the nucleocapsid domain of the gag polyprotein precursor. Virology 328:163–168 [DOI] [PubMed] [Google Scholar]
- 35. Sette P, Jadwin JA, Dussupt V, Bello NF, Bouamr F. 2010. The ESCRT-associated protein Alix recruits the ubiquitin ligase Nedd4-1 to facilitate HIV-1 release through the LYPXnL L domain motif. J. Virol. 84:8181–8192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sette P, et al. 2011. The Phe105 loop of Alix Bro1 domain plays a key role in HIV-1 release. Structure 19:1485–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Strack B, Calistri A, Craig S, Popova E, Gottlinger HG. 2003. AIP1/ALIX is a binding partner for HIV-1 p6 and EIAV p9 functioning in virus budding. Cell 114:689–699 [DOI] [PubMed] [Google Scholar]
- 38. Stuchell-Brereton MD, et al. 2007. ESCRT-III recognition by VPS4 ATPases. Nature 449:740–744 [DOI] [PubMed] [Google Scholar]
- 39. Usami Y, Popov S, Gottlinger HG. 2007. Potent rescue of human immunodeficiency virus type 1 late domain mutants by ALIX/AIP1 depends on its CHMP4 binding site. J. Virol. 81:6614–6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. VerPlank L, et al. 2001. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55(Gag). Proc. Natl. Acad. Sci. U. S. A. 98:7724–7729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Schwedler UK, et al. 2003. The protein network of HIV budding. Cell 114:701–713 [DOI] [PubMed] [Google Scholar]
- 42. Wei X, et al. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Winn MD, et al. 2011. Overview of the CCP4 suite and current developments. Acta Crystallogr. D Biol. Crystallogr. 67:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zennou V, Perez-Caballero D, Gottlinger H, Bieniasz PD. 2004. APOBEC3G incorporation into human immunodeficiency virus type 1 particles. J. Virol. 78:12058–12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhai Q, et al. 2011. Activation of the retroviral budding factor ALIX. J. Virol. 85:9222–9226 [DOI] [PMC free article] [PubMed] [Google Scholar]