Abstract
Branched-chain aminotransferases (BCAT), which utilize pyridoxal 5′-phosphate (PLP) as a cofactor, reversibly catalyze the transfer of the α-amino groups of three of the most hydrophobic branched-chain amino acids (BCAA), leucine, isoleucine, and valine, to α-ketoglutarate to form the respective branched-chain α-keto acids and glutamate. The BCAT from Deinococcus radiodurans (DrBCAT), an extremophile, was cloned and expressed in Escherichia coli for structure and functional studies. The crystal structures of the native DrBCAT with PLP and its complexes with l-glutamate and α-ketoisocaproate (KIC), respectively, have been determined. The DrBCAT monomer, comprising 358 amino acids, contains large and small domains connected with an interdomain loop. The cofactor PLP is located at the bottom of the active site pocket between two domains and near the dimer interface. The substrate (l-glutamate or KIC) is bound with key residues through interactions of the hydrogen bond and the salt bridge near PLP inside the active site pocket. Mutations of some interaction residues, such as Tyr71, Arg145, and Lys202, result in loss of the specific activity of the enzymes. In the interdomain loop, a dynamic loop (Gly173 to Gly179) clearly exhibits open and close conformations in structures of DrBCAT without and with substrates, respectively. DrBCAT shows the highest specific activity both in nature and under ionizing radiation, but with lower thermal stability above 60°C, than either BCAT from Escherichia coli (eBCAT) or from Thermus thermophilus (HB8BCAT). The dimeric molecular packing and the distribution of cysteine residues at the active site and the molecular surface might explain the resistance to radiation but small thermal stability of DrBCAT.
INTRODUCTION
Branched-chain aminotransferase (BCAT), which contains pyridoxal 5′-phosphate (PLP) as a cofactor, is the key enzyme in the biosynthetic path of hydrophobic amino acids, e.g., leucine, isoleucine, and valine, to α-ketoglutarate to form the respective branched-chain α-keto acids and glutamate (27, 53). BCAT enzymes are distributed widely in many species (10, 28), from bacteria containing a single BCAT enzyme (33) to mammals with these enzymes in both mitochondrial and cytosolic forms (24, 26). In plants, BCAT might be restrained by hormone treatment, resulting in the regulation of endogenous hormones by affecting specific genes (18). Among four classified families with PLP-dependent enzymes of varied fold types based on similarities in the sequence, secondary structure, and hydrophobicity profiles, BCAT belongs to the family type IV that transfers protons on the re-face of the PLP cofactor (30, 60). BCAT can be used to synthesize l-tert-leucine and other branched-chain or unnatural amino acids from their respective keto acids; they have a broad applicability in the synthesis of fine chemicals and pharmaceuticals (52). The enzyme functions via a ping-pong kinetic mechanism that comprises two half reactions, each with three main steps, shown as follows (34).
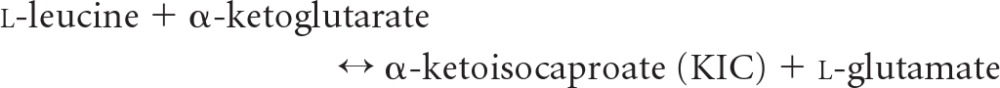 |
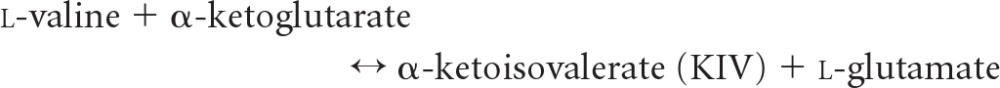 |
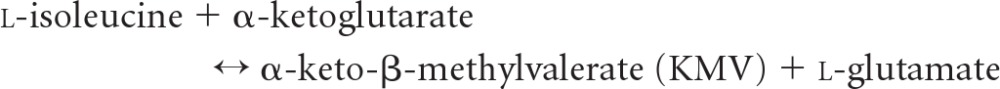 |
Deinococcus radiodurans R1 is a Gram-positive, extremophile, red-pigmented, nonmotile bacterium that is well-known for its extreme resistance to the lethal effects of ionizing radiation, UV radiation, and hydrogen peroxide (40, 56). D. radiodurans has been described as the organism most resistant to radiation: exponentially growing cells were 200 times as resistant to ionizing radiation and 20 times as resistant to UV irradiation as Escherichia coli (56). It was originally identified in irradiated canned meat and has been found widely from environments rich in organic nutrients, including soil, animal feces, and processed meats, and from dry, nutrient-poor environments, including weathered granite in a dry Antarctic valley, room dust, and irradiated medical instruments (38). The complete genome sequence of D. radiodurans R1 has been determined (37, 56); it reveals the related chromosomes and megaplasmid of a bacterium able to survive under conditions of starvation, oxidative stress, and extensive DNA damage. The mechanism of repair of DNA double-strand breaks was studied intensively (16, 17, 36, 39). The protein components of D. radiodurans were valuable sources for the investigation of structure-function relations possibly due to their unique structures and amino acid compositions that might confer special properties, such as thermal stability or radiation resistance.
DrBCAT (BCAT from D. radiodurans), comprising 358 amino acids, contains PLP as a cofactor for its function. The enzyme was expected to have particular activity and stability in an environment of ionizing radiation in terms of the three-dimensional (3-D) structure, amino acid sequence, and molecular assembly. Several structures of BCAT from various species were previously determined, including human mitochondrial BCAT (hBCATm) (59) and human cytosolic BCAT (hBCATc) from human source (23), BCAT from E. coli (eBCAT) (42), HB8BCAT from Thermus thermophilus (PDB accession no. 1WRV [unpublished work]), BCAT from Mycobacterium tuberculosis (MtBCAT) (6), and BCAT from Mycobacterium smegmatis (MsBCAT) (6). The hBCATm is expressed in body tissues, whereas hBCATc was localized primarily in the central nervous system (11, 25, 61). The mammalian BCATs were commonly found as dimers with molecular masses of 41.3 and 43 kDa of the hBCATm and hBCATc monomers, respectively. eBCAT and HB8BCAT were hexamers with molecular masses of ∼34 kDa for each monomer, whereas both MtBCAT and MsBCAT were dimers with similar molecular masses of 40 kDa for each monomer. Among these structures, DrBCAT shows fairly small sequence identities of 32.0, 33.0, 32.5, 29.1, 42.6, and 42.6% in comparison with hBCATm, hBCATc, eBCAT, HB8BCAT, MtBCAT, and MsBCAT, respectively. Moreover, the numbers of cysteine (Cys) residues in DrBCAT (6 [the number of Cys residues shown in parentheses after the enzyme]), hBCATm (6), hBCATc (10), eBCAT (3), HB8BCAT (1), MtBCAT (3), and MsBCAT (2) vary significantly with the distribution in the corresponding sequences.
In this work, we have expressed and purified the DrBCAT to study the activity assay and structure, which is the first reported BCAT from a radiation-resistant organism. We describe the crystal structures of DrBCAT and its complexes with α-ketoisocaproate and l-glutamate, for the reaction shown in Fig. 1.
Fig 1.
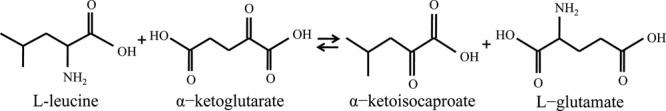
Ping-pong reaction mechanism of BCAT.
We surmise that a comparison of amino acid sequences, structures, and enzymatic activities among those BACT enzymes from various species might provide a structural basis for a radiation-resistant mechanism and a specific activity of DrBCAT greater than those of eBCAT and HB8BCAT.
MATERIALS AND METHODS
Protein purification.
The details of protein purification of DrBCAT were described (8). In brief, the pQE30 expression vector (Qiagen) containing the DrBCAT cDNA was transformed into E. coli JM109 cells for overexpression in Luria-Bertani (LB) broth (200 ml) containing ampicillin (50 μg/ml) for incubation overnight at 37°C. Isopropyl-β-d-thiogalactopyranoside (IPTG) (final concentration of 1 mM) was added to the culture for induction at 20°C for 18 h before the cells were harvested on centrifugation (6,800 × g). The medium was discarded, and the cell pellet was resuspended with binding buffer (250 ml) containing NaCl (0.5 M), imidazole (5 mM), and Tris-HCl (20 mM; pH 7.9), and subjected to cell disruption by ultrasonication and a high-pressure homogenizer. The suspension was collected on centrifugation (24,000 × g) at 4°C for 30 min. The soluble protein extract was passed through a His-tag Ni2+-nitrilotriacetic acid (NTA) column twice to increase its homogeneity. The protein was concentrated on Amicon (molecular weight cutoff [MWCO] of 10,000; Millipore), and the buffer was concurrently changed to Tris-HCl buffer (20 mM; pH 7.0).
Activity assay.
BCAT catalyzes the process from branched-chain 2-oxo acid-dependent transamination of l-glutamate that is coupled to l-leucine and 2-oxoglutarate. The process is further catalyzed by l-aspartate aminotransferase (AspAT) (EC 2.6.1.1) from 2-oxoglutarate to oxaloacetate. l-Malate dehydrogenase (MDH) (EC 1.1.1.37) was used to catalyze the reaction from oxaloacetate to l-malate through oxidation of NADH to NAD+ as the indicator system (49). The specific measurement of the decrease of NADH absorbance at 340 nm can monitor the rate of transamination that can transform to become the activity of the enzyme. The BCAT activity was determined by monitoring the temporal course of NADH consumption at 340 nm. The amounts of NADH change were recorded every minute with a spectrophotometer (model U-2010; Hitachi). A thermostated cuvette holder (model B401D; First Scientific) was used to control the reaction temperature at 25°C. The standard assay conditions as given in Table 1 were applied. The enzyme activity concentration in the assays is limited to ≤25 U/liter (1 unit [U] is 1 μmol of oxaloacetate per unit volume and unit time).
Table 1.
Standard assay conditions for spectrophotometric determination of BCAT activity
| Component or enzyme | Component concn (mM) or enzyme activity in assay (μmol × min−1 × liter−1) |
|---|---|
| Components | |
| Tris-HCl (pH 8.3) | 100 |
| KIC | 2.1 |
| l-Glutamate | 300 |
| NADH | 0.2 |
| PLP | 0.1 |
| l-Aspartate | 200 |
| Enzymes | |
| l-Aspartate aminotransferase | 2,500 |
| l-Malate dehydrogenase | 5,000 |
| BCAT | Variable but ≤25 |
The assay volume is 1 ml; the molar extinction coefficient is εM = 6,225 M−1 cm−1. According to the Beer-Lambert law, the enzyme activity was measured under the standard conditions given above.
where ΔA is the change in absorbance, V denotes the assay volume of BCAT (in milliliters), C is the concentration of BCAT (in milligrams per milliliter), and specific activity is given in units per milligram where the unit is the activity unit (1 μmol of oxaloacetate per min−1).
Crystallization and X-ray data collection.
The crystals of native DrBCAT were obtained with the hanging-drop vapor diffusion method at a temperature of 20°C. The protein solution comprised DrBCAT (14 mg ml−1) with PLP (10 μΜ) in Tris-HCl buffer (0.1 M; pH 7.0). Crystallization drops were prepared on mixing the protein solution (1 μl) with an equal volume of reservoir solution (100 μl) containing magnesium chloride (0.15 M), polyethylene glycol 8000 (15% [wt/vol]) in Tris-HCl buffer (0.1 M; pH 7.4). The crystals of native DrBCAT with PLP were grown to the terminal size of 0.6 by 0.02 by 0.02 mm3 after 2 weeks.
Cocrystals of DrBCAT-KIC and DrBCAT–l-glutamate were also obtained with a crystallization protocol similar to that of native crystals. DrBCAT (14 mg ml−1) with α-ketoisocaproate (KIC) (10 mM), referred as DrBCAT-KIC, and DrBCAT (9 mg ml−1) with l-glutamate (10 mM), referred as DrBCAT–l-glutamate, were mixed with an equal volume (1 μl) of the corresponding reservoir solutions containing MgCl2 (0.15 M), polyethylene glycol 8000 (15% [wt/vol]), and Tris-HCl buffer (0.1 M; pH 7.3), and NaCl2 (0.2 M), polyethylene glycol 3350 (25% [wt/vol]) and bis-Tris (0.1 M; pH 6.5), respectively.
The diffraction data of the native DrBCAT crystal were collected to 2.5-Å resolution at 100 K at the Taiwan-contracted beamline BL12B2 of SPring-8 in Japan. The native crystals belong to space group P212121 with cell dimensions a = 56.37 Å, b = 90.70 Å, and c = 155.47 Å. The solvent content of crystals was estimated to be ∼47.5%, with a dimer in the asymmetric unit (AU). The data sets for DrBCAT-KIC and DrBCAT–l-glutamate crystals were collected to 3.0- and 2.0-Å resolution, respectively, at 100 K at beamline BL13B1 of the National Synchrotron Radiation Research Center (NSRRC) in Hsinchu, Taiwan. Both complex crystals belong to space group P21 but had cell dimensions a = 56.11 Å, b = 172.58 Å, and c = 80.08 Å for DrBCAT-KIC and a = 55.88 Å, b = 75.98 Å, and c = 79.12 Å for DrBCAT–l-glutamate. The DrBCAT-KIC crystals contain a tetramer in the AU with a solvent content of 45.5%, whereas the DrBCAT–l-glutamate crystals contain a dimer in the AU with a solvent content of 38.1%. Details of the crystal diffraction and structural refinement data statistics are given in Table 2.
Table 2.
Statistics of crystal diffraction and structural refinement of DrBCAT and complexes with l-glutamate and KIC
| Statistic | Valuea for: |
||
|---|---|---|---|
| Native DrBCAT alone |
DrBCAT in complexes with: |
||
| l-Glutamate | KIC | ||
| Crystal diffraction statistics | |||
| Wavelength (Å) | 1.00 | 1.00 | 1.00 |
| Temp (K) | 100 | 100 | 100 |
| Resolution range (Å) | 2.5–30 | 2.0–30 | 3.0–30 |
| Space group | P212121 | P21 | P21 |
| No. of unique reflections | 28,352 | 53,244 | 34,610 |
| Completeness (outermost shell) (%) | 98.6 (100) | 99.4 (97.7) | 97.0 (96.2) |
| I/σ (outermost shell) | 34.8 (4.5) | 26.78 (2.9) | 11.52 (3.64) |
| Average redundancy | 6.2 | 4.9 | 4.4 |
| Rsymb (%) | 3.9 | 6.0 | 14.0 |
| Mosaicity | 0.41 | 0.56 | 0.50 |
| Unit-cell parameter (Å) | |||
| a | 56.37 | 55.88 | 56.11 |
| b | 90.70 | 75.98 | 172.58 |
| c | 155.47 | 79.12 | 80.08 |
| Structural refinement statistics | |||
| Rc (%) | 23.34 | 22.10 | 20.42 |
| Rfreed (%) | 28.38 | 26.30 | 27.11 |
| RMSD | |||
| Bond length (Å) | 0.007 | 0.006 | 0.007 |
| Bond angle (°) | 1.318 | 1.270 | 1.387 |
| No. of: | |||
| Amino acids | 358 | 358 | 358 |
| Molecules per AU | 2 | 2 | 4 |
| Water molecules | 63 | 156 | 177 |
| Average B-factor (Å2) | 43.30 | 25.09 | 26.27 |
| Ramachandran plot (favored regions/allowed regions/outlier regions) (%) | 90.3/99.2/0.7 | 94.3/100.0/0.0 | 96.7/99.7/0.2 |
The values in parentheses are the values for the outermost shell.
Rsym= ∑h ∑i[|Ii(h) − </>I(h)</>|/∑h ∑I Ii(h)] where Ii is the ith measurement and </>I(h)</> is the mean weight of all measurements of I(h). The reflection cutoff (I/σ>0) was applied in generating the statistics.
R = ∑h |Fo − Fc|/∑h Fo, where Fo and Fc are the observed and calculated structure factor amplitudes of reflection h.
Rfree= ∑h ||Fobs| − |Fcalc||/∑h |Fobs| for 5% of the reserved reflections.
Determination and refinement of crystal structures.
The crystal structure of native DrBCAT was solved by the molecular replacement (MR) method using a structure of the hBCATc monomer (PDB accession no. 2COJ) as the search model (23), which has 33% sequence identity with DrBCAT. Two molecules were located in the AU, confirming the dimer form. Crystallographic refinement was performed with CNS v1.2 (3). After rigid-body refinement, the solution was refined to an R factor of 51.63% at a resolution range from 30 to 2.5 Å.
Throughout the refinement, a randomly selected 10% of the data were set aside as a “free data set,” and the model was refined against the rest of the data with F ≥ 0 (4) (working data set). The composite omit electron density maps with coefficient 2Fo–Fc (see footnote c of Table 2) were calculated and visualized using O v7.0 (31), and the model was rebuilt and adjusted iteratively as required. After several cycles of annealing and B-group refinement, the R factor and Rfree (see footnote d of Table 2) values decreased to 24.54 and 29.30%, respectively. The PICK-WATER subroutine from CNS was employed to define peaks in the difference maps (3σ cutoff level) to locate water molecules. A water molecule is accepted when the identified peak correlated with a separate peak in the corresponding Fo–Fc electron density map and when one (or more) hydrogen bond was identified. On the basis of these criteria, 63 water molecules were located automatically. The protein model and water molecules were then subjected to another run of positional, simulated annealing and B-factor refinement. For the data with resolution between 30 and 2.5 Å, the resulting model yielded a final R factor of 23.34% and Rfree of 28.38% (Table 2).
The refinement procedures of DrBCAT-KIC and DrBCAT–l-glutamate structures were similar to those of the native DrBCAT structure. The structures of DrBCAT-KIC and DrBCAT–l-glutamate were solved with the molecular replacement method using the determined native DrBCAT monomer as the search model. The tetramer and dimer molecules were located in the asymmetric unit of DrBCAT-KIC and DrBCAT–l-glutamate crystals, respectively. The models of KIC and l-glutamate with clearly observable electron densities were built at the active site. A Ramachandran plot was generated using MolProbity to examine backbone dihedral angles of amino acids in the favored, generally allowed, and disallowed regions for all refined models (9, 46). All refinement statistics are given in Table 2.
Protein structure accession numbers.
The atomic coordinates and structure factors have been deposited in the Protein Data Bank with accession numbers 3UYY, 3UZO, and 3UZB for native DrBCAT, DrBCAT–l-glutamate, and DrBCAT-KIC, respectively.
RESULTS
Enzyme activity and substrate specificity.
The purified protein with a high concentration showed a yellow color, indicating that PLP was incorporated in the active site. The activity assay showed that the recombinant native DrBCAT exhibits a specific activity of 180 U/mg using α-ketoisocaproate and l-glutamate as the substrates. The specific activity of DrBCAT is 30 to 40 times that of the HB8BCAT (4 U/mg) and eBCAT (6 U/mg) (Table 3). The K202R and R145E single mutants and the Y71A/R145A double mutant of DrBCAT exhibit no specific activity (Table 3), confirming that these amino acids are the key residues for the enzymatic activity.
Table 3.
Specific activities of DrBCAT, DrBCAT mutants, HB8BCAT, and eBCAT
| Protein | Sp act (μmol/min mg) |
|---|---|
| DrBCAT | |
| Wild type | 180 |
| Mutants | |
| Y71A/R145A | 0 |
| K202R | 0 |
| R145E | 0 |
| HB8BCAT | 4 |
| eBCAT | 6 |
We compared the catalytic powers of DrBCAT, eBCAT, and HB8BCAT with several various α-keto acid substrates, including α-ketoisocaproate (KIC) (4-methyl-2-oxopentanoate), α-keto-β-methylvalerate (KMV) (3-methyl-2-oxobutanoate), α-ketoisovalerate (KIV) (3-methyl-2-oxopentanoate), ketomethiobutyrate (KMTB), 2-ketohexanoic acid, and 2-oxovaleric acid, using l-glutamate as the common amino group donor (Table 4). Among these substrates, KIC showed the greatest relative activity as the best substrate for DrBCAT, eBCAT, and HB8BCAT. With the relative activity of KIC set at a standard 100%, the 2-ketohexanoic acid and 2-oxovaleric acid showed a catalytic power greater than 50%. For KMV, the substrate specificities of DrBCAT and HB8BCAT were less than that of eBCAT, but our data showed that the DrBCAT, eBCAT and HB8BCAT could catalyze BCAA of any kind and could also react with KMTB. These results also showed that the substrate specificity of BCAT for KMTB was small. KMTB presumably does not comprise all hydrophobic groups, making the relative activity of BCAT for KMTB less than that for KIC.
Table 4.
Substrate specificities of DrBCAT, eBCAT, and HB8BCAT
| α-Keto acid substratea |
DrBCAT |
eBCAT |
HB8BCAT |
|||
|---|---|---|---|---|---|---|
| Relative activity (%) | Sp act | Relative activity (%) | Sp act | Relative activity (%) | Sp act | |
| KIC | 100.0 | 154.44 | 100.0 | 5.26 | 100.0 | 8.45 |
| KIV | 62.5 | 96.57 | 36.5 | 1.92 | 41.6 | 3.51 |
| KMV | 34.9 | 53.95 | 61.5 | 3.24 | 28.3 | 2.39 |
| KMTB | 22.7 | 34.98 | 26.4 | 1.39 | 14.5 | 1.22 |
| 2-Ketohexanoic acid | 57.1 | 88.20 | 78.4 | 4.12 | 30.7 | 2.60 |
| 2-Oxovaleric acid | 51.0 | 78.84 | 66.9 | 3.52 | 23.5 | 1.99 |
l-Glutamate as an amino group donor reacted with α-keto acid. The activity of KIC was set at 100%. Abbreviations used: KIC, α-ketoisocaproate; KMV, α-keto-β-methylvalerate; KIV, α-ketoisovalerate; KMTB, ketomethiobutyrate.
Radiation effect and thermostability.
Comparing the rates of survival of D. radiodurans with E. coil exposed to 2.5 kGy gamma ray, D. radiodurans is extremely resistant to ionizing radiation that does not influence its survival, but the survival rate of E. coli was decreased to about 10−4 by 2.5 kGy gamma ray (12). The activities of DrBCAT, eBCAT, and HB8BCAT were measured and compared after exposure to X-rays with 718.3 rads per min for 6 h; the total dosage of X-rays for 6 h was about 2.58 kGy. When the concentrations of DrBCAT, eBCAT, and HB8BCAT were ∼0.5 mg ml−1, the activities remained ∼59, 45, and 49% after X-ray radiation, respectively (Table 5). The analysis shows that DrBCAT resists radiation better than eBCAT and HB8BCAT. Moreover, our measurements show that the thermal stability of HB8BCAT becomes greater than those of eBCAT and DrBCAT above 60°C, as shown in Fig. 2. We discuss the reason in the Discussion.
Table 5.
Remaining activities of BCAT after X-ray exposure
| Protein | Concn (mg ml−1) | Remaining activity (%)a |
|---|---|---|
| DrBCAT | 0.4978 | 58.79 |
| eBCAT | 0.4936 | 44.88 |
| HB8BCAT | 0.5217 | 48.64 |
The activity of unexposed protein is 100% for the reference.
Fig 2.
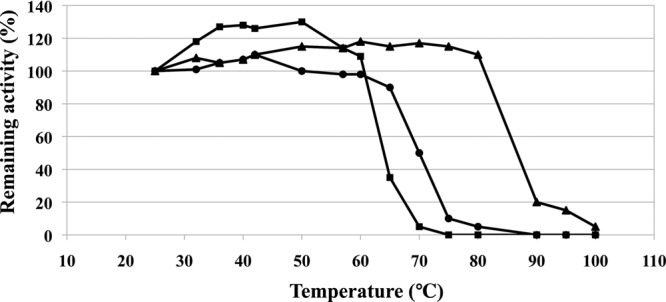
Comparison of thermal stabilities of various BCATs. The activity of untreated BCAT enzyme was set at 100%. BCATs were incubated at each temperature for 10 min before measurement. The remaining activities were determined on measuring the temporal decrease of NADH absorbance at 340 nm at 25°C. The activity data of DrBCAT (squares), eBCAT (circles), and HB8BCAT (triangles) are indicated.
Overall structure of DrBCAT.
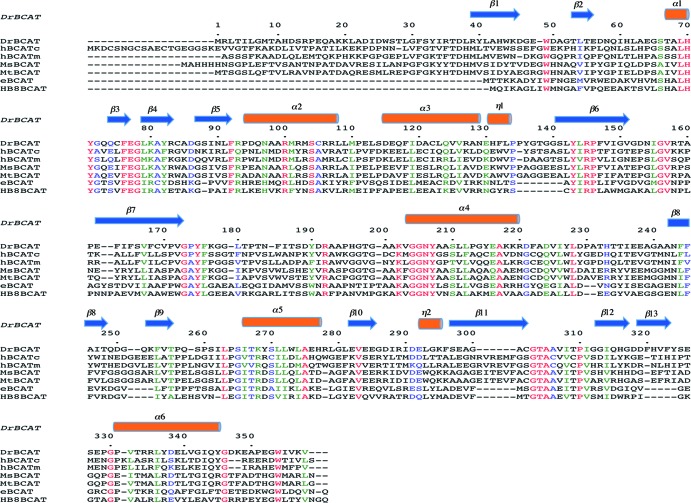
The crystal structure of native DrBCAT was determined at a resolution of 2.5 Å (Table 2). The asymmetric unit of space group P212121 contains two DrBCAT molecules with the essentially identical structure upon superimposition. The first 23 amino acids at the N-terminal region were disordered and invisible in the density map. The overall structure of the DrBCAT monomer comprises two domains—a small domain (Ile24 to Pro171) and a large domain (Ile186 to Val358), which are linked by an interdomain loop (Val172 to Phe185) located on the molecular surface (Fig. 3A). The fragment from Gly173 to Gly179 exhibits a dynamic character in the interdomain loop near the active site. The small and large domains are folded into an open α/β structure and a pseudobarrel structure, respectively. The small domain consists of 7 β-strands (β1 to β7) and 3 α-helices (α1 to α3); the large domain contains 6 β-strands (β8 to β13) and 3 α-helices (α4 to α6) (Fig. 3A). The cofactor PLP is located at the bottom of the active site that is formed by residues at the interface of both the small and large domains and the two loops (His70 to Gln74 and Val151 to Glu162) of the small domain from other monomers, which might explain why the enzyme functions as a dimer.
Fig 3.
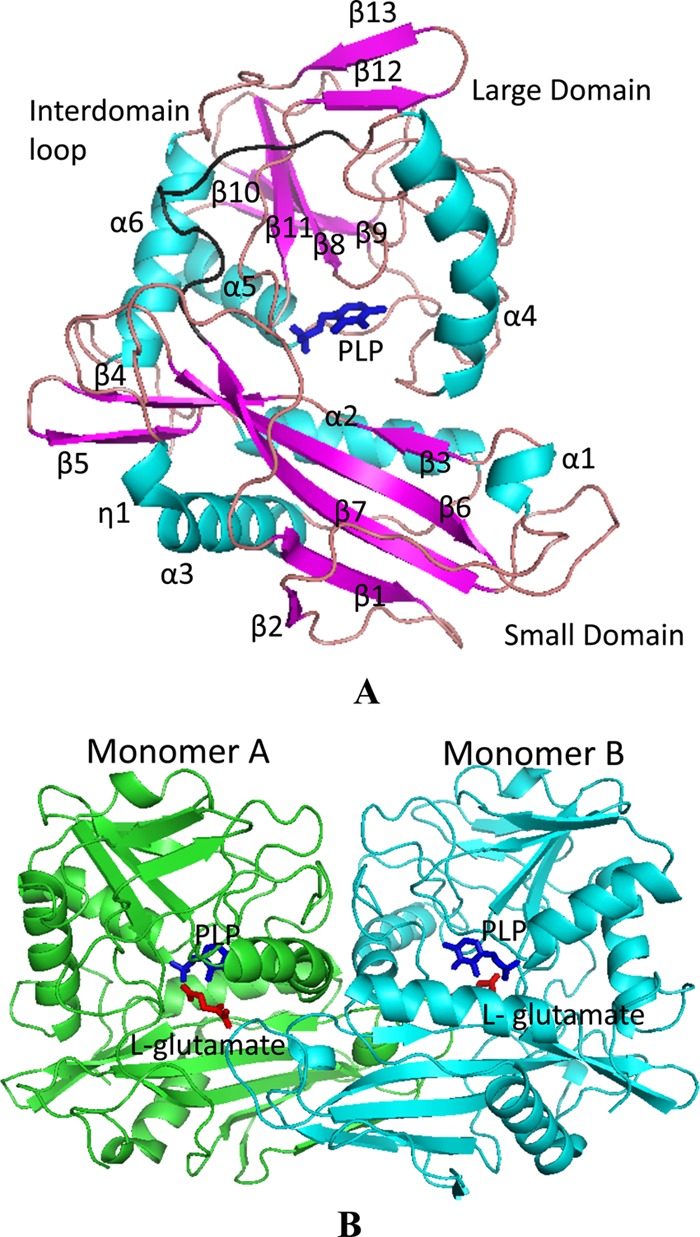
Crystal structures of DrBCAT and molecular packing. (A) The PLP-bound monomer structure of DrBCAT is shown with six α-helices (turquoise) and 13 β-strands (magenta). The overall structure comprises small and large domains, connected to the interdomain loop (Val172 to Phe185; shown in black) located on the molecular surface. The cofactor PLP (dark blue stick) is located at the bottom of the active site between the two domains. (B) The DrBCAT–l-glutamate dimer (space group P21) is shown from a direction nearly perpendicular to the 2-fold axis. The PLP (dark blue stick) and l-glutamate (red stick) are located at the bottom of the active site.
The sequence alignment reveals that DrBCAT exhibits small sequence identities of 32.0, 33.0, 32.5, 29.1, 42.6, and 42.6% in comparison with hBCATm, hBCATc, eBCAT, HB8BCAT, MtBCAT, and MsBCAT, respectively (Fig. 4). The structure of DrBCAT comprises six α-helices and 13 β-strands (6α/13β) in total. An inspection of other BCAT structures shows varied compositions of the secondary structures in hBCATm (8α/20β), hBCATc (6α/20β), eBCAT (9α/14β; refer to PDB accession no. 1A3G), HB8BCAT (11α/21β), MtBCAT (9α/17β), and MsBCAT (7α/19β). Superimposed structures of DrBCAT and other BCAT enzymes reveal structural deviations with large root mean square deviation (RMSD) values (in angstroms) for hBCATm (7.21), hBCATc (20.20), eBCAT (17.26), HB8BCAT (17.40), MtBCAT (7.21), and MsBCAT (7.18) for all main-chain atoms.
Fig 4.
Structure-based sequence alignment and comparison of BCATs from various species. From top to bottom, the BCATs were from Deinococcus radiodurans (DrBCAT), human cytosolic (hBCATc), human mitochondrial (hBCATm), Mycobacterium smegmatis (MsBCAT), Mycobacterium tuberculosis (MtBCAT), Escherichia coli (eBCAT), and Thermus thermophilus HB8 (HB8BCAT). The secondary structures are defined according to the DrBCAT structure.
Active site, essential residues, and PLP binding.
The sequence analysis and alignment of DrBCAT with other BCAT enzymes from various species indicated that the conserved residue Lys202 in DrBCAT is the catalytic residue (Fig. 4). An inspection of the DrBCAT structure reveals that this residue, Lys202, on the loop before the α4 helix, is located in the active site. The electron density analysis clearly reveals that the PLP cofactor is located at the bottom of the active site between two domains. This positively charged active site pocket with a width of 9.3 Å and depth of 9.8 Å is formed and surrounded by residues Phe76, Glu77, Gly78, Lys80, Arg100, Tyr143, Arg145, Lys202, Tyr207, Glu238, Ala241, Leu263, Thr304, Ala305, Tyr71*, and Val157* (an asterisk indicates that the residue is from another monomer), and residues Val172 and Tyr175 at the interdomain to cover partially the active site (Fig. 5A).
Fig 5.
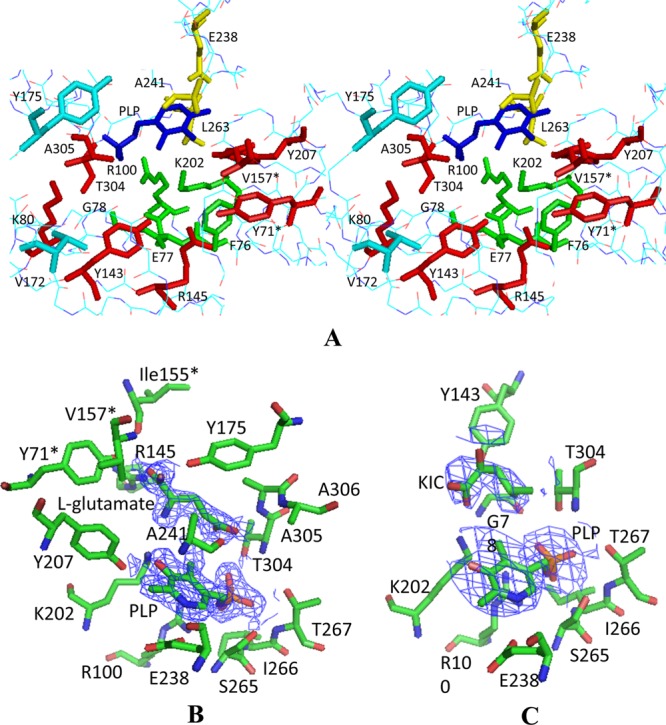
Active site of native DrBCAT. (A) Stereo view of the active site residues. The PLP (dark blue) is located at the bottom of the active site pocket surrounded by the residues from varied orientations—at the bottom (green), near the structural surface (red), at the interdomain loop (turquoise), and at the positions behind and above the aldehyde group of PLP (yellow). (B) Cofactors and substrates in the active sites of the DrBCAT–l-glutamate complex. (C) Cofactors and substrates in the active sites of DrBCAT–l-KIC. The omit electron density maps (contoured ≥ 1.0 σ) with the coefficient 2Fo–Fe were calculated for DrBCAT–l-glutamate at a resolution of 2.0 Å and for DrBCAT-KIC at a resolution of 3.0 Å, respectively.
The PLP cofactor is located between the main chain of Ala241 from the si-face of PLP and the side chain of Leu263 from the re-face of PLP, which is composed of the sandwich form, in the active site pocket. The PLP is bound to the active site pocket mainly with hydrogen bond and salt bridge interactions mediated by residues Arg100, Tyr207, Glu238, Ser265, Ile266, Thr267, and Thr304 in DrBCAT (Fig. 6A) (43, 59). These residues, except Glu238, Ser265, Ile266, and Thr267, are conserved for PLP binding in the active site based on the sequence analysis and alignment of DrBCAT with hBCATm, hBCATc, eBCAT, HB8BCAT, MtBCAT, and MsBCAT (Fig. 4). The crystal structures of native eBCAT and hBCAT with only PLP were previously determined, allowing us to compare the interaction differences between PLP and residues of DrBCAT, eBCAT (42), and hBCATm (59). In the PLP binding site of the native DrBCAT, the observed water molecules form no hydrogen bond with PLP at a resolution of 2.5 Å, which is different from eBCAT and hBCATm with some water-mediated hydrogen bonding at a similar resolution. The detailed interactions between residues and the PLP molecule are shown in Fig. 6A. The hydrogen bonding between the phosphate OP3 of PLP and the hydroxy group of Ser265 in DrBCAT was not observed in both eBCAT and hBCATm, and OP3 formed no hydrogen bond with the hydroxy group of Thr267 in the hBCATm. OP4 has a hydrogen bond with the guanidino group of Arg100, differing from eBCAT and hBCATm.
Fig 6.
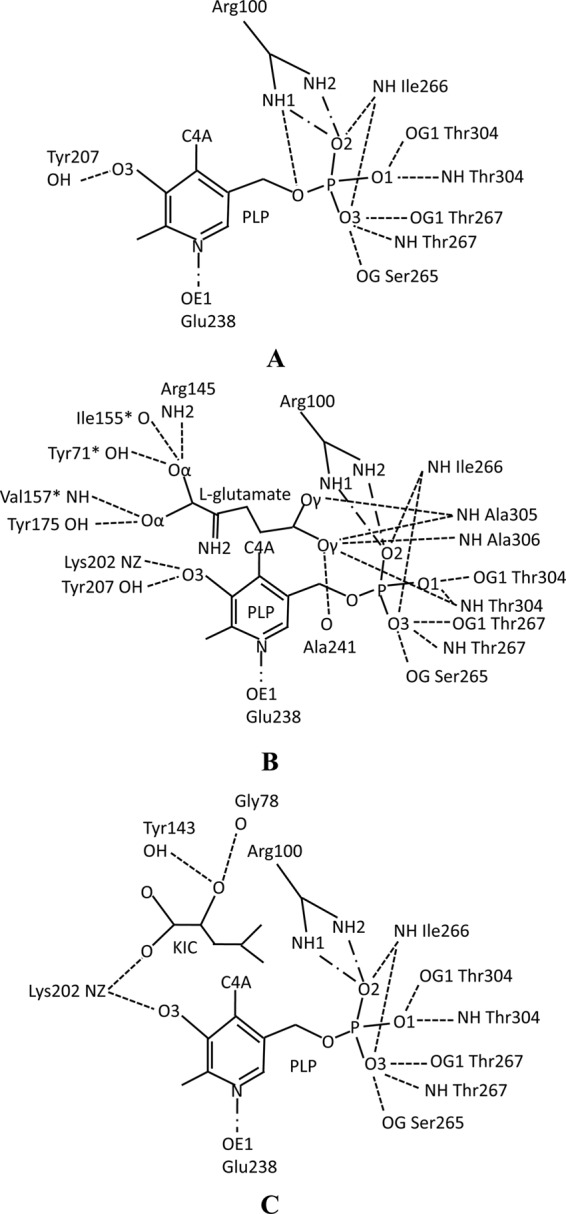
Schematic diagram of interaction networks with hydrogen bonds and salt bridges among the PLP, substrates, and residues. (A) Interactions for the native DrBCAT monomer. (B) Interactions for the DrBCAT–l-glutamate complex. (C) Interactions for the DrBCAT-KIC complex. The PLP as a cofactor was located at the bottom of the active site pocket with its re-face toward the DrBCAT. The interactions of hydrogen bonds (broken lines) and salt bridges (lines made of a long dash, a dot, and a long dash) are shown.
The NH2 of the catalytic residue Lys202 is not covalently linked to C4A (C4′) of the PLP, which differs markedly from the cases of eBCAT and hBCATm that exhibit covalent bonds of length of ∼1.37 Å between Lys and the PLP-C4A atom (59). Lys202 lies on the re-face of the PLP with the interacting distance in a range from 3.06 to 4.22 Å for all crystal structures of DrBCAT in this work. With C5-C5A (C5′) in the PLP as the axis of rotation, the PLP in DrBCAT is rotated 48° and 19° relative to PLP molecules of eBCAT and hBCATm, respectively, which primarily makes the interaction distance greater than the covalent bond between C4A of PLP and NH2 of Lys202.
Complex structures of the DrBCAT-substrate and substrate binding.
To observe the substrate molecules bound in the active site, we cocrystallized DrBCAT with various substrates. The structures of DrBCAT complexes with l-glutamate and KIC were solved at a resolution of 2.0 and 3.0 Å, respectively (Table 2). The DrBCAT–l-glutamate complex exists as a dimer in an asymmetric unit of a crystal form P21 (Fig. 3B), whereas the DrBCAT-KIC complex exhibits a tetramer in the same space group but with a longer b axis (double length). Both DrBCAT complexes clearly reveal electron densities of the l-glutamate and KIC substrates bound near the cofactor PLP inside the active site pocket (Fig. 5B and C). The enzyme-substrate complexes reveal several essential residues for the substrate binding, of which some are conserved in all BCAT enzymes from various species (Fig. 4). A structural comparison revealed that no significant conformational variations are observed between the backbone structures of DrBCAT with and without substrates, except for the dynamic loop (residues 173 to 179) that exhibits the close and open forms, which is described in the next section.
In DrBCAT–l-glutamate, Tyr71*, Ile155*, Val157*, Arg145, Tyr175, Ala241, Thr304, Ala305, and Ala306 form the substrate-binding site and share hydrogen bonds with l-glutamate. l-Glutamate contains four carboxylate oxygen atoms, of which two are α-carboxylate oxygen atoms and two are γ-carboxylate oxygen atoms, which interact with the surrounding residues mainly through hydrogen bonds. The detailed interactions between residues, PLP, and the l-glutamate molecule are shown in Fig. 6B (also data not shown).
In DrBCAT-KIC, Gly78, Tyr143, and Lys202 form hydrogen bonds with KIC at the substrate-binding site. The detailed network of hydrogen bonds between residues, PLP, and the KIC molecules is shown in Fig. 6C (also data not shown). The position and orientation of the KIC substrate in DrBCAT and hBCATm-C315A-C318A vary because different residues form hydrogen bonds with KIC in DrBCAT and hBCATm-C315A/C318A (58).
At the PLP binding site of the DrBCAT complexes, the PLP bonds are almost the same in the DrBCAT-KIC and DrBCAT–l-glutamate with exception of only one residue, Tyr207 (Fig. 6B and C). O3 of PLP forms hydrogen bonds with the NH2 of Lys202 and the hydroxyl group of Tyr207 in DrBCAT–l-glutamate. The hydrogen bond between O3 of PLP and OH of Tyr207 is absent from DrBCAT-KIC. The interactions of other residues and PLP in DrBCAT complex are similar to those in native DrBCAT (data not shown).
Interdomain loop (residues Val172 to Phe185) near the active site.
A comparison of native and complex structures (DrBCAT–l-glutamate and DrBCAT-KIC) shows that the overall structures are similar except for one dynamic loop (Gly173 to Gly179), structurally located near the active site, in the interdomain region (Val172 to Phe185) after the strain β7 to connect the small and large domains (Fig. 3A). An inspection of the distributions of the temperature factor in the structures of the native and complex forms shows high B-factors with average values of 67.47, 46.6, and 40.26 Å2 at this region for native DrBCAT, DrBCAT–l-glutamate, and DrBCAT-KIC, respectively, indicating that the loop is structurally dynamic. This loop is originally orientated away from the PLP by ∼14.1 Å (a distance measured from main-chain Cα of Tyr175 to C4A of the PLP cofactor) at the active site in the native DrBCAT (an open form for the precatalyzing stage); upon binding to the l-glutamate or KIC substrate, the loop shifts toward the PLP (a close form for the catalyzing stage) (Fig. 7A). The dynamic loop is notable because the substrate binding induced its maximum conformational shift ∼5.8 Å from the substrate-free state to the substrate-bound state (Fig. 7A). Residues Tyr175 and Phe176, located on this dynamic loop to form a substrate channel from the surface to the active site, exhibit an edge-to-face interaction in the native DrBCAT, DrBCAT-KIC, and DrBCAT–l-glutamate.
Fig 7.
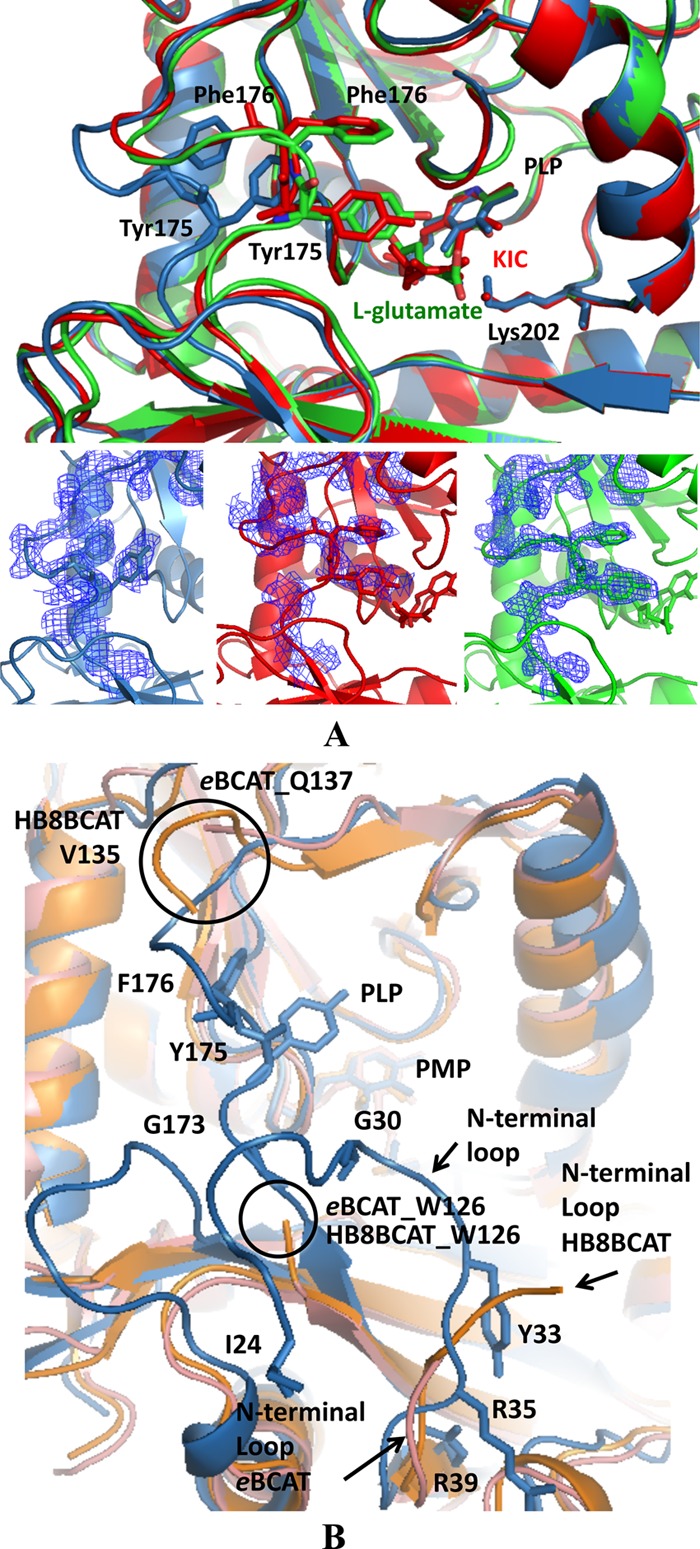
Molecular surface of the active site and interdomain loop. (A) Structural comparison of the active sites of native DrBCAT (blue), DrBCAT–l-glutamate (green), and DrBCAT-KIC (red). The active site exhibits an open form in native DrBCAT, whereas it becomes a closed form once the l-glutamate substrate or KIC binds to the active site, because of the dynamics and flexibility of the interdomain loop. The composite omit electron density maps with coefficient 2Fo–Fc of the interdomain loops are shown for native DrBCAT, DrBCAT–l-glutamate, and DrBCAT-KIC at the bottom of panel A. (B) Structural comparison of native DrBCAT-PLP, eBCAT-PLP, and HB8BCAT-PMP. Superimposing the structures of the native DrBCAT (blue), eBCAT (salmon), and HB8BCAT (orange) reveals that the interdomain loop is highly flexible and disordered without electron densities in eBCAT and HB8BCAT, whereas the loop is clearly visible with large B-factors in DrBCAT. The N-terminal loop in DrBCAT is longer than those in eBCAT and HB8BCAT. Residues Tyr175 and Phe176 exhibit an edge-to-face interaction in the interdomain loop in DrBCAT. The black circles indicate the disordered ends of the interdomain loops of eBCAT and HB8BCAT.
A comparison of the active sites and interdomain loops of DrBCAT, eBCAT, and HB8BCAT shows some varied structural features (Fig. 7B). In the native eBCAT, the interdomain loop (Trp126 to Gln137), which was not definable in the electron density map, is highly flexible. When substrates bound to the active site, the flexible loop became visibly located near the active site in the eBCAT–4-MeVA (4-MeVA stands for 4-methylvalerate) and eBCAT–l-glutamate (22, 43). A similar dynamic feature of the interdomain loop (Trp126 to Val135) in HB8BCAT was also observed. In the DrBCAT, however, the interdomain region containing the dynamic loop in our three structures—native DrBCAT, DrBCAT-KIC, and DrBCAT–l-glutamate are all definable and visible with varied conformations in the electron density maps (Fig. 7A).
DISCUSSION
Oligomerization of BCAT and structural implication for thermostability.
As mentioned previously, native DrBCAT and DrBCAT–l-glutamate complex exhibit a dimer form, whereas DrBCAT-KIC molecules pack into a tetramer form that is composed of two basic dimers in the crystals (Fig. 3B). In contrast with DrBCAT, hBCATc, hBCATm, MsBCAT, and MtBCAT that form dimers, eBCAT (42) and HB8BCAT (PDB accession no. 1WRV), which equip the shorter N terminus with ∼40 fewer residues, have been shown to be hexamers that comprise three dimers with crystallographic 3-fold axes perpendicular to 2-fold axes. Previous work showed that eBCAT is a hexamer in aqueous solution (35), whereas we demonstrated that DrBCAT and HB8BCAT are a dimer and a hexamer, respectively, in aqueous solution using a gel filtration chromatography. A comparison of the dimeric, hexameric, and tetrameric molecular packing shows that all crystal forms of BCAT have a similar dimer unit. The basic dimer molecules are greatly stabilized by the network of hydrogen bonds between monomer A and monomer B in native DrBCAT and DrBCAT–l-glutamate with 27 and 31 hydrogen bonds, respectively, of which 23 and 24, respectively, are within the range of van der Waals forces (<3.5 Å) (data not shown).
In DrBCAT-KIC, the DrBCAT tetramer is formed by monomers A, B, C, and D. There are 26 hydrogen bonds (19 van der Waals forces) at the interface between monomers A and B and 24 hydrogen bonds (18 van der Waals forces) between monomers C and D. There are three hydrogen bonds (three van der Waals forces) between monomers A and C and five hydrogen bonds (three van der Waals forces) between monomers B and D. The oligomerization might explain why the thermal stabilities in eBCAT and HB8BCAT are generally greater than that in DrBCAT (Fig. 2), because the hexamers (eBCAT and HB8BCAT) exhibit more intermolecular interactions than a dimer (native DrBCAT).
Our data show that the thermal stability of HB8BCAT is greater than that of eBCAT and DrBCAT above 60°C despite the fact that both HB8BCAT and eBCAT are hexamers. A structural comparison of DrBCAT, eBCAT, and HB8BCAT monomers revealed that HB8BCAT contains 11 α-helices, while DrBCAT has 6 α-helices and eBCAT has 9 α-helices (PDB accession no. 1A3G). A careful inspection of structures among HB8BCAT, eBCAT, and DrBCAT shows that HB8BCAT exhibits two additional small α-helices—an α-helix from Leu112 to Asn114 forms five hydrogen bonds with the neighbor loop (Gly103 to Pro111) and residue Asn115 and an α-helix from Glu299 toTrp301 forms six hydrogen bonds with the neighbor loop (Pro296 to Tyr298) and the β-strand (Leu302 to Tyr304). In contrast, there are only the loop conformations at these two corresponding α-helix regions in HB8BCAT forming fewer hydrogen bonds in DrBCAT (Thr159 to Pro161 and Pro351 to Gly353) and eBCAT. More hydrogen bonds between secondary structures could give HB8BCAT the greatest temperature of thermal denaturation and the greatest stability among the three enzymes (29, 54).
PLP binding and substrate binding of BCATs.
Comparison of the active site structures of the native DrBCAT, DrBCAT–l-glutamate, and DrBCAT-KIC complex indicates that all bonds between Lys202 and C4A of PLP with distances of 3.06, 3.16, and 4.22 Å, respectively, are not covalently linked (Fig. 8A, B, and C). The noncovalent linkage between the Lys residue and PLP was previously proposed to be an effect of radiation damage (15, 55), but we cannot exclude the possibility of a noncovalent linkage as the natural character and state for DrBCAT from the radiation-resistant D. radiodurans.
Fig 8.
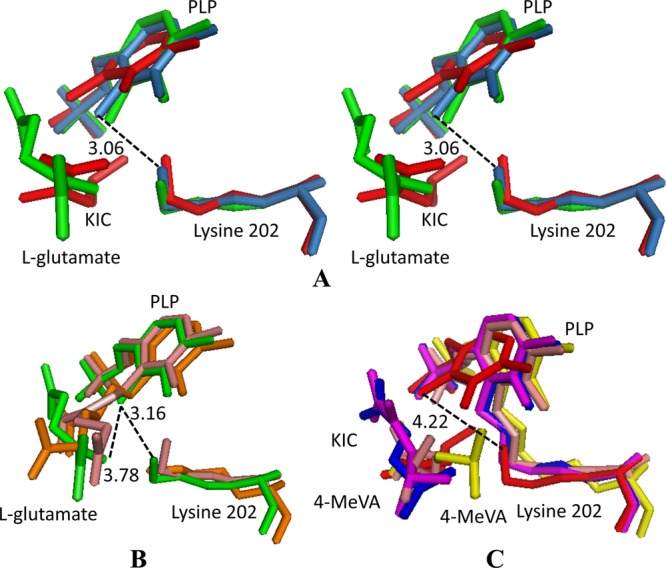
Superposition of the PLP, substrate, and lysine residues from the DrBCAT, hBCATm, hBCATc, HB8BCAT, and eBCAT. (A) Stereo view of a conformational comparison of the PLP, substrate, and Lys (PLP-substrate-Lys) in native DrBCAT (blue-gray), DrBCAT–l-glutamate (green), and DrBCAT-KIC (red) complexes. (B) Structural comparison of PLP-substrate-Lys of DrBCAT–l-glutamate (green), eBCAT–l-glutamate (salmon), and HB8BCAT–l-glutamate (orange, on the bottom). (C) Structural comparison of PLP-substrate-Lys of DrBCAT-KIC (red), hBCATm-C315A-C318A-KIC (magenta), eBCAT–4-MeVA (orange), oxidized hBCATc–4-MeVA (blue), and HB8BCAT–4-MeVA (yellow). The Lys202 is noncovalently linked to the PLP with distances of 3.06, 3.16, and 4.22 Å between C4A of PLP and NZ of Lys202 for native DrBCAT, DrBCAT–l-glutamate, and DrBCAT-KIC, respectively.
The structures of the BCAT-PLP complex with l-glutamate were previously determined in E. coli (22) and T. thermophilus (PDB accession no. 2EJ2). In our DrBCAT–l-glutamate, the guanidine group of l-glutamate does not connect with C4A (C4′) of PLP with a distance of ∼3.78 Å, which is larger than those of eBCAT (1.53 Å) and HB8BCAT (1.51 Å), as shown in Fig. 8B. Because the PLP–l-glutamate has the ketimine form in the transamination reaction in eBCAT and HB8BCAT, the guanidine group of the l-glutamate might connect with C4A (C4′) of PLP (Fig. 8B), but DrBCAT–l-glutamate is presumably in a Michaelis complex form that is C4A (C4′) of PLP without connecting with the guanidine group of l-glutamate.
The complex structures of hBCATm-C315A/C318A-KIC and hBCATm-PMP-KIV were solved previously (58). The structures of eBCAT (43), oxidized hBCATc (23), and HB8BCAT complex with substrate analogue 4-MeVA (PDB accession no. 2EIY; unpublished work) as the Michaelis complex form were also determined. The DrBCAT-KIC complex also has a Michaelis complex form in the transamination reaction. The results of comparison are shown in Fig. 8C. A Schiff base between Lys and the PLP of other BCAT enzymes (eBCAT–4-MeVA, hBCATm-C315A/C318A-KIC, oxidized hBCATc–4-MeVA, and HB8BCAT–4-MeVA) are generally observed to have a Michaelis complex form.
Putative role of the interdomain loop of BCATs.
The structural and sequence comparisons of DrBCAT, eBCAT, and HB8BCAT show that the N-terminal segment (amino acids [aa] 1 to 39) before β1 in DrBCAT is longer than the segments in eBCAT and HB8BCAT (Fig. 4 and 7B). The oxygen atom of Gly30 on the N-terminal loop forms a hydrogen bond (distance of 2.8 Å) with the main-chain NH group of Gly173 in the interdomain loop in native DrBCAT, DrBCAT-KIC, and DrBCAT–l-glutamate. The longer N-terminal loop might limit the flexibility of the dynamic interdomain loop in the native state of DrBCAT because of the spatial obstacle (Fig. 7B). Similar phenomena were observed in hBCATm, hBCATm, and MsBCAT.
The native DrBCAT containing a more stable interdomain region with the open conformation exhibits a specific activity greater than those of HB8BCAT and eBCAT (Table 3). A similar character of a definable loop was observed in native hBCATm, which also has a large specific activity (14). In contrast, the hydroxyl group of Tyr175 in the interdomain loop interacts with the α-carboxylate oxygen atom of l-glutamate in DrBCAT–l-glutamate. As a result of the above reasons, we speculate that the existence of the interdomain loop might affect the rate of reaction or electron transfer would occur between Tyr175 and the substrate. Several substrates, including KIC, KIV, KMV, KMTB, 2-ketohexanoic acid, and 2-oxovaleric acid, have been proven to be substrates of DrBCAT, despite varied substrate specificities as previously discussed. The flexibility of the interdomain loop could further explain variations of substrates for the BCAT enzyme, as the space for the substrate entrance could be adjustable.
Protective effect of cysteine and radiation resistance.
Under concentrations of ∼0.5 mg ml−1, the activities remain ∼59, 44, and 48% in DrBCAT, eBCAT, and HB8BCAT, respectively, after X-ray radiation (Table 5), implying that DrBCAT is more effectively protective against X-rays. The radiolysis of water with ionizing radiation produces electrons, free radicals (H· radicals and ·OH radicals) and hydrogen peroxide H2O2 via free radical mechanisms (50). When proteins are irradiated in aqueous solution, the reactive oxygen species, namely, ·OH free radicals, could attack specific amino acids or the groups of amino acids, such as histidine, tyrosine, tryptophan, cysteine, and methionine, in the active site, leading to protein inactivation (19, 41, 48, 51). The residues could interact with water-derived radicals because of the readily oxidizable functional groups, including indole, phenol, aromatic, and thiol groups.
Three easily oxidizable amino acids (Tyr175, Tyr207, and Tyr71*) exist in the active site of DrBCAT, whereas eBCAT and HB8BCAT contain four oxidizable residues in the active site, including the three conserved Tyr residues and one additional Trp residue that is structurally related to Val172 in DrBCAT. The hydroxyl group of the conserved Tyr207 and O3 of PLP form a hydrogen bond in DrBCAT, eBCAT, and HB8BCAT. When a readily oxidizable amino acid, such as Tyr, is irradiated, free radical ·OH might break noncovalent bonds, such as hydrogen bonds, and also damage these residues, inducing conformational modification of the secondary structure in aqueous solution (1, 48), decreasing the enzymatic activity. The additional Trp residue might make the active site more readily oxidized in eBCAT and HB8BCAT, yielding activities less than that of DrBCAT.
Cysteine serves as a powerful destroyer of free radicals or a scavenger of free radicals with a thiol-containing side chain that can protect against X-radiation-induced lethality base on the free radical metabolites in cysteine oxidation (7, 13, 21, 44, 47). After the free-radical scavengers (Cys) interact with free radicals, fewer free radicals remain in aqueous solution. As the probability of free radicals interacting with the readily oxidizable residues decreases, more Cys residues could give greater protection of BCAT activity.
The sequence inspection shows that DrBCAT contains six Cys residues in DrBCAT, including Cys75, Cys84, Cys105, Cys122, Cys169, and Cys302, whereas eBCAT has only three Cys residues and HB8BCAT has just one. We thus superimposed the BCAT enzymes from various species to reveal the positions and distributions of cysteines (Fig. 9). Among the six Cys residues, Cys75 and Cys302 located near the active site could protect the Tyr residues from oxidization in the active site of DrBCAT, whereas Cys84, Cys105, and Cys122 located on the surface of DrBCAT could reduce the free radicals on the molecular surface. Compared with other BCAT enzymes, eBCAT has two Cys residues on its surface and HB8BCAT has none. Because of the different numbers and positions of Cys residues, the activity of DrBCAT is greater than those of eBCAT and HB8BCAT after exposure to X-rays. For further comparison, hBCATc and hBCATm have five and three Cys residues located on their surfaces, respectively, and two Cys residues are found near the active sites in both enzymes (Fig. 9). We presume that hBCATc and hBCATm could also protect the enzyme with activities comparable to that of DrBCAT after irradiation.
Fig 9.
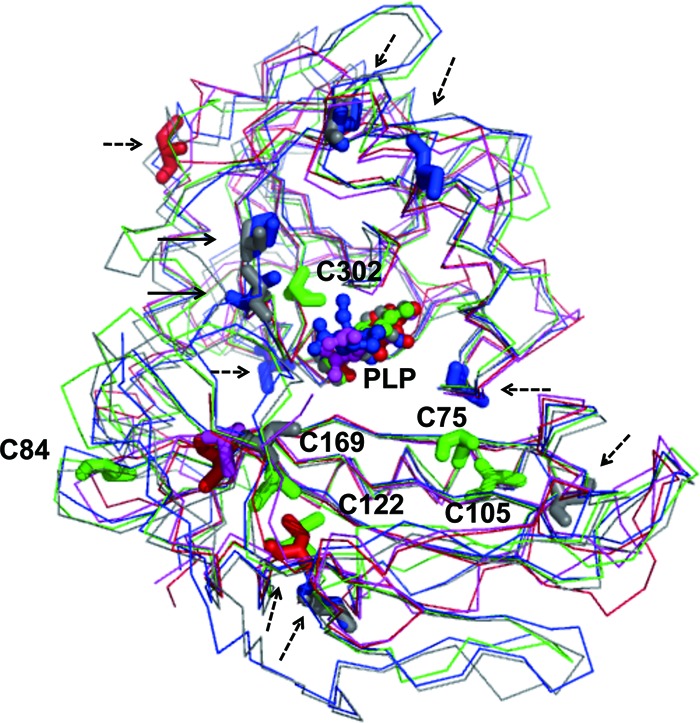
Comparison of structures and distributions of cysteine in DrBCAT, eBCAT, HB8BCAT, hBCATc, and hBCATm. The main-chain structures (thin lines), cysteine residues (sticks) and the cofactor PLP (ball-and-stick) of DrBCAT (green), eBCAT (red), HB8BCAT (purple), hBCATc (blue), and hBCATm (gray) are shown. The cysteine residues near the surface (broken-line arrow) and the active site (solid-line arrow) are indicated. Cys75 and Cys302 located near the active site might protect the Tyr residues from oxidization in the active site of DrBCAT, whereas Cys84, Cys105, and Cys122 located on the surface of DrBCAT could reduce the free radicals on the surface.
The reduction of Cys residues is irreversible only in DrBCAT. Peroxiredoxins (Prxs) are the antioxidant enzyme, of which the reduction-oxidation reactions of Cys residues are reversible (20, 57). The cysteine (RSH) in BCAT is oxidized by H2O2 to a sulfenic acid (RSOH) and sulfinic acid (RSO2H), but RSOH could be reduced to RSH with NADH-dependent enzymes (thioredoxin, AhpF, tryparedoxin, or AhpD) (5, 45), and RSO2H could reverse to RSOH with an ATP-dependent enzyme, sulfiredoxin (Srx) (2, 32), which might complete the catalytic cycle (redox reaction) in the BCAT enzyme upon irradiation.
ACKNOWLEDGMENTS
We are indebted to the supporting staffs at beamlines BL13B1 and BL13C1 at the National Synchrotron Radiation Research Center (NSRRC) and Masato Yoshimura and Hirofumi Ishii at the Taiwan contracted beamline BL12B2 at SPring-8 for technical assistance under SPring-8 proposal numbers 2007A4014 and 2012A4009. Portions of this research were carried out at the NSRRC-NCKU Protein Crystallography Laboratory at National Cheng Kung University (NCKU).
This work was supported in part by grants from the National Synchrotron Radiation Research Center (NSRRC) (97-99RSB07 and 1003RSB02) and from the National Science Council (NSC) (95-2311-B213-001-MY3 and 98-2311-B-213-001-MY3) of Taiwan to C.-J.C. and NSC 92-2317-B-002-027 to W.-C.C.
Footnotes
Published ahead of print 14 September 2012
REFERENCES
- 1. Bhattacharya D, Saha A, Mandal PC. 2000. Radiation induced modification of tryptophan and tyrosine residues in flavocytochrome b2 in dilute aqueous solution. Radiat. Phys. Chem. 59:71–80 [Google Scholar]
- 2. Biteau B, Labarre J, Toledano MB. 2003. ATP-dependent reduction of cysteine-sulphinic acid by S. cerevisiae sulphiredoxin. Nature 425:980–984 [DOI] [PubMed] [Google Scholar]
- 3. Brunger AT, et al. 1998. Crystallography and NMR system (CNS): a new software system for macromolecular structure determination. Acta Crystallogr. D 54:905–921 [DOI] [PubMed] [Google Scholar]
- 4. Brunger AT. 1992. The free R value: a novel statistical quantity for assessing the accuracy of crystal structure. Nature 355:472–474 [DOI] [PubMed] [Google Scholar]
- 5. Bryk R, Lima CD, Erdjument-Bromage H, Tempst P, Nathan C. 2002. Metabolic enzymes of mycobacteria linked to antioxidant defense by a thioredoxin-like protein. Science 295:1073–1077 [DOI] [PubMed] [Google Scholar]
- 6. Castell A, Mille C, Unge T. 2010. Structural analysis of mycobacterial branched-chain aminotransferase: implications for inhibitor design. Acta Crystallogr. D 66:549–557 [DOI] [PubMed] [Google Scholar]
- 7. Chatterjee A, Raman MJ. 1993. Protective effect of cysteine against X-ray- and bleomycin-induced chromosomal aberrations and cell cycle delay. Mutat. Res. 290:231–238 [DOI] [PubMed] [Google Scholar]
- 8. Chen C-D, et al. 2007. Purification, crystallization and preliminary X-ray crystallographic analysis of branched-chain aminotransferase from Deinococcus radiodurans. Acta Crystallogr. F 63:492–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen VB, et al. 2010. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66:12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Conway ME, Hutson SM. 2000. Mammalian branched-chain aminotransferases. Methods Enzymol. 324:355–365 [DOI] [PubMed] [Google Scholar]
- 11. Cooper AJ, Plum F. 1987. Biochemistry and physiology of brain ammonia. Physiol. Rev. 67:440–519 [DOI] [PubMed] [Google Scholar]
- 12. Daly MJ, et al. 2004. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science 306:1025–1028 [DOI] [PubMed] [Google Scholar]
- 13. Darkwa J, Mundoma C, Simoyi RH. 1998. Antioxidant chemistry reactivity and oxidation of DL-cysteine by some common oxidants. J. Chem. Soc., Faraday Trans. 94:1971–1978 [Google Scholar]
- 14. Davoodi J, et al. 1998. Overexpression and characterization of the human mitochondrial and cytosolic branched-chain aminotransferases. J. Biol. Chem. 273:4982–4989 [DOI] [PubMed] [Google Scholar]
- 15. Dubnovitsky AP, Ravelli RBG, Popov AN, Papageorgiou AC. 2005. Strain relief at the active site of phosphoserine aminotransferase induced by radiation damage. Protein Sci. 14:1498–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Englander J, et al. 2004. DNA toroids: framework for DNA repair in Deinococcus radiodurans and in germinating bacterial spores. J. Bacteriol. 186:5973–5977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frenkiel-Krispin D, Minsky A. 2006. Nucleoid organization and the maintenance of DNA integrity in E. coli, B. subtilis and D. radiodurans. J. Struct. Biol. 156:311–319 [DOI] [PubMed] [Google Scholar]
- 18. Gao F, Wang CZ, Wei C, Li Y. 2009. A branched-chain aminotransferase may regulate hormone levels by affecting KNOX genes in plants. Planta 230:611–623 [DOI] [PubMed] [Google Scholar]
- 19. Garrison WM, Jayko ME, Bennett W. 1962. Radiation-induced oxidation of protein in aqueous solution. Radiat. Res. 16:483–502 [PubMed] [Google Scholar]
- 20. Georgiou G, Masip L. 2003. An overoxidation journey with a return ticket. Science 300:592–594 [DOI] [PubMed] [Google Scholar]
- 21. Gilbert BC, Laue HAH, Norman ROC, Sealy RC. 1975. Electron spin resonance studies. Part XLVI. Oxidation of thiols and disulphides in aqueous solution: formation of RS·, RSO·, RSO2·, RSSR–RSSR−·, and carbon radicals. J. Chem. Soc., Perkin Trans. 2:892–900 [Google Scholar]
- 22. Goto M, Miyahara I, Hayashi H, Kagamiyama H, Hirotsu K. 2003. Crystal structures of branched-chain amino acid aminotransferase complexed with glutamate and glutarate: true reaction intermediate and double substrate recognition of the enzyme. Biochemistry 42:3725–3733 [DOI] [PubMed] [Google Scholar]
- 23. Goto M, et al. 2005. Structural determinants for branched-chain aminotransferase isozyme-specific inhibition by the anticonvulsant drug gabapentin. J. Biol. Chem. 280:37246–37256 [DOI] [PubMed] [Google Scholar]
- 24. Hutson SM. 1988. Subcellular distribution of branched-chain aminotransferase activity in rat tissues. J. Nutr. 118:1475–1481 [DOI] [PubMed] [Google Scholar]
- 25. Hutson SM, et al. 1998. Role of branched-chain aminotransferase isoenzymes and gabapentin in neurotransmitter metabolism. J. Neurochem. 71:863–874 [DOI] [PubMed] [Google Scholar]
- 26. Hutson SM, Fenstermacher D, Mahar C. 1988. Role of mitochondrial transamination in branched chain amino acid metabolism. J. Biol. Chem. 263:3618–3625 [PubMed] [Google Scholar]
- 27. Ichihara A, Koyama E. 1966. Transaminase of branched chain amino acids. I. Branched chain amino acids-α-ketoglutarate transaminase. J. Biochem. 59:160–169 [DOI] [PubMed] [Google Scholar]
- 28. Ichihara A, Christen P, Metzler DE. (ed). 1985. Transaminases, p 430–439. John Wiley, New York, NY [Google Scholar]
- 29. Irimia A, et al. 2004. The 2.9Å resolution crystal structure of malate dehydrogenase from Archaeoglobus fulgidus: mechanisms of oligomerisation and thermal stabilisation. J. Mol. Biol. 335:343–356 [DOI] [PubMed] [Google Scholar]
- 30. Jhee KH, et al. 2000. Stereochemistry of the transamination reaction catalyzed by aminodeoxychorismate lyase from Escherichia coli: close relationship between fold type and stereochemistry. J. Biochem. 128:679–686 [DOI] [PubMed] [Google Scholar]
- 31. Jones TA, Zou JY, Cowan SW, Kjeldgaard M. 1991. Improved methods for building protein models in electron density maps and the location errors in these models. Acta Crystallogr. A 47:110–119 [DOI] [PubMed] [Google Scholar]
- 32. Jönsson TJ, Johnson LC, Lowther WT. 2008. Structure of the sulphiredoxin-peroxiredoxin complex reveals an essential repair embrace. Nature 451:98–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kamitori S, et al. 1989. Crystallization and preliminary X-ray characterization of branched-chain amino acid aminotransferase from Escherichia coli. J. Biochem. 105:671–672 [DOI] [PubMed] [Google Scholar]
- 34. Kirsch JF, et al. 1984. Mechanism of action of aspartate aminotransferase proposed on the basis of its spatial structure. J. Mol. Biol. 174:497–525 [DOI] [PubMed] [Google Scholar]
- 35. Kuramitsu S, Ogawa T, Ogawa H, Kagamiyama H. 1985. Branched-chain amino acid aminotransferase of Escherichia coli: nucleotide sequence of the ilvE gene and the deduced amino acid sequence. J. Biochem. 97:993–999 [DOI] [PubMed] [Google Scholar]
- 36. Levin-Zaidman S, et al. 2003. Ringlike structure of the Deinococcus radiodurans genome: a key to radioresistance? Science 299:254–256 [DOI] [PubMed] [Google Scholar]
- 37. Lin J, et al. 1999. Whole-genome shotgun optical mapping of Deinococcus radiodurans. Science 285:1558–1562 [DOI] [PubMed] [Google Scholar]
- 38. Masters CI, Murray RG, Moseley BE, Minton KW. 1991. DNA polymorphisms in new isolates of ‘Deinococcus radiopugnans’. J. Gen. Microbiol. 137:1459–1469 [DOI] [PubMed] [Google Scholar]
- 39. Minsky A. 2003. Structural aspects of DNA repair: the role of restricted diffusion. Mol. Microbiol. 50:367–376 [DOI] [PubMed] [Google Scholar]
- 40. Minton KW. 1994. DNA repair in the extremely radioresistant bacterium Deinococcus radiodurans. Mol. Microbiol. 13:9–15 [DOI] [PubMed] [Google Scholar]
- 41. Moore JS, Sakhri M, Butler J. 2000. The radiolysis of protein A. Radiat. Phys. Chem. 58:331–339 [Google Scholar]
- 42. Okada K, Hirotsu K, Sato M, Hyashi H, Kagamiyama H. 1997. Three-dimensional structure of Escherichia coli branched-chain amino acid aminotransferase at 2.5 angstrom resolution. J. Biochem. 121:637–641 [DOI] [PubMed] [Google Scholar]
- 43. Okada K, Hirotsu K, Hayashi H, Kagamiyama H. 2001. Structures of Escherichia coli branched-chain amino acid aminotransferase and its complexes with 4-methylvalerate and 2-methylleucine: induced fit and substrate recognition of the enzyme. Biochemistry 40:7453–7463 [DOI] [PubMed] [Google Scholar]
- 44. Patt HM, Tyree EB, Straube RL, Smith DE. 1949. Cysteine protection against X irradiation. Science 110:213–214 [DOI] [PubMed] [Google Scholar]
- 45. Poole LB, et al. 2000. AhpF and other NADH:peroxiredoxin oxidoreductases, homologues of low Mr thioredoxin reductase. Eur. J. Biochem. 267:6126–6133 [DOI] [PubMed] [Google Scholar]
- 46. Ramachandran GN, Sasisekharan V. 1968. Conformation of polypeptides and proteins. Adv. Protein Chem. 23:283–437 [DOI] [PubMed] [Google Scholar]
- 47. Reliene R, et al. 2009. N-acetyl cysteine protects against ionizing radiation-induced DNA damage but not against cell killing in yeast and mammals. Mutat. Res. 665:37–43 [DOI] [PubMed] [Google Scholar]
- 48. Saha A, Mandal PC, Bhattacharyya SN. 1995. Radiation-induced inactivation of enzymes—a review. Radiat. Phys. Chem. 46:123–145 [Google Scholar]
- 49. Schadewaldt P, Adelmeyer F. 1996. Coupled enzymatic assay for estimation of branched-chain L-amino acid aminotransferase activity with 2-oxo acid substrates. Anal. Biochem. 238:65–71 [DOI] [PubMed] [Google Scholar]
- 50. Sophie LC. 2011. Water radiolysis: influence of oxide surfaces on H2 production under ionizing radiation. Water 3:235–253 [Google Scholar]
- 51. Stadtman ER, Levine RL. 2003. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids 25:207–218 [DOI] [PubMed] [Google Scholar]
- 52. Taylor PP, Pantaleone DP, Senkpeil RF, Fotheringham IG. 1998. Novel biosynthetic approaches to the production of unnatural amino acids using transaminases. Trends Biotechnol. 16:412–418 [DOI] [PubMed] [Google Scholar]
- 53. Taylor RT, Jenkins WT. 1966. Leucine aminotransferase. II. Purification and characterization. J. Biol. Chem. 241:4396–4405 [PubMed] [Google Scholar]
- 54. Trivedi S, Gehlot HS, Rao SR. 2006. Protein thermostability in Archaea and Eubacteria. Genet. Mol. Res. 5:816–827 [PubMed] [Google Scholar]
- 55. Watanabe N, et al. 2007. Crystal structure of LL-diaminopimelate aminotransferase from Arabidopsis thaliana: a recently discovered enzyme in the biosynthesis of L-lysine by plants and Chlamydia. J. Mol. Biol. 371:685–702 [DOI] [PubMed] [Google Scholar]
- 56. White O, et al. 1999. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science 286:1571–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wood ZA, Schröder E, Robin Harris J, Poole LB. 2003. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 28:32–40 [DOI] [PubMed] [Google Scholar]
- 58. Yennawar NH, Islam MM, Conway M, Wallin R, Hutson SM. 2006. Human mitochondrial branched chain aminotransferase isozyme: structural role of the CXXC center in catalysis. J. Biol. Chem. 281:39660–39671 [DOI] [PubMed] [Google Scholar]
- 59. Yennawar N, Dunbar J, Conway M, Hutson S, Farber G. 2001. The structure of human mitochondrial branched-chain aminotransferase. Acta Crystallogr. D 57:506–515 [DOI] [PubMed] [Google Scholar]
- 60. Yoshimura T, et al. 1993. Unique stereospecificity of D-amino acid aminotransferase and branched-chain L-amino acid aminotransferase for C-4′ hydrogen transfer of the coenzyme. J. Am. Chem. Soc. 115:3897–3900 [Google Scholar]
- 61. Yudkoff M, et al. 1983. [15N] leucine as a source of [15N] glutamate in organotypic cerebellar explants. Biochem. Biophys. Res. Commun. 115:174–179 [DOI] [PubMed] [Google Scholar]