Abstract
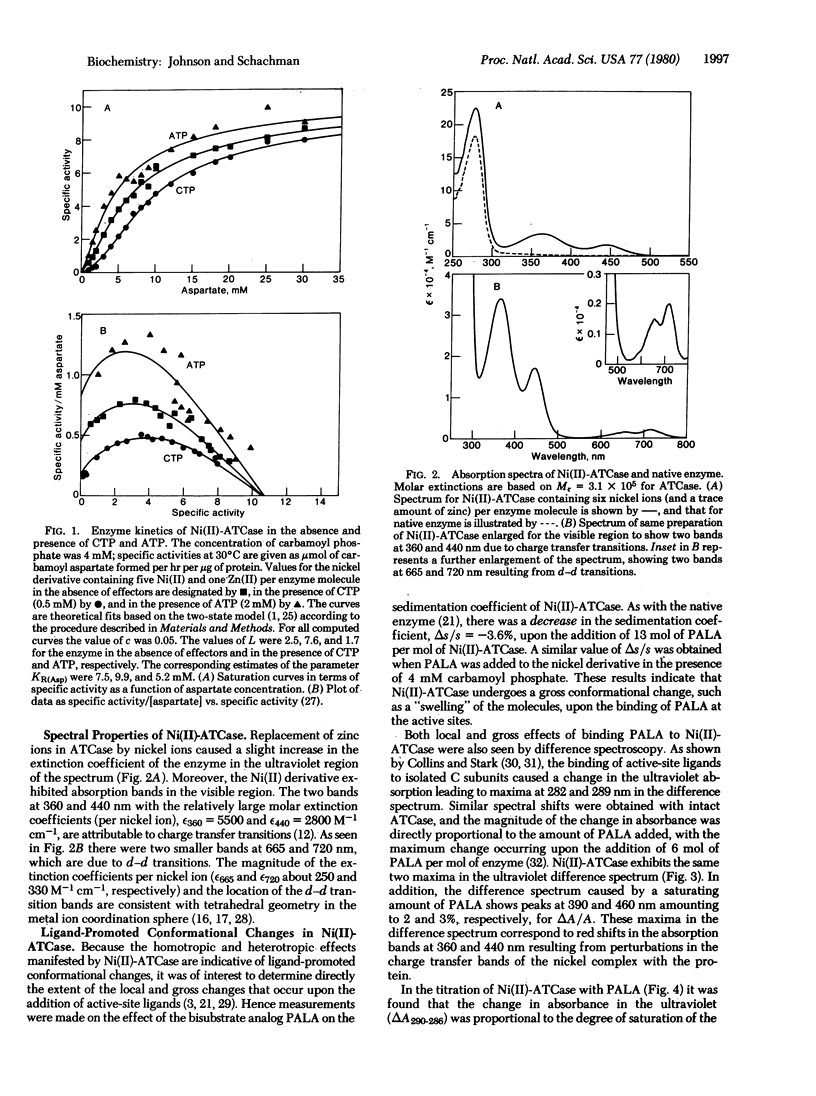
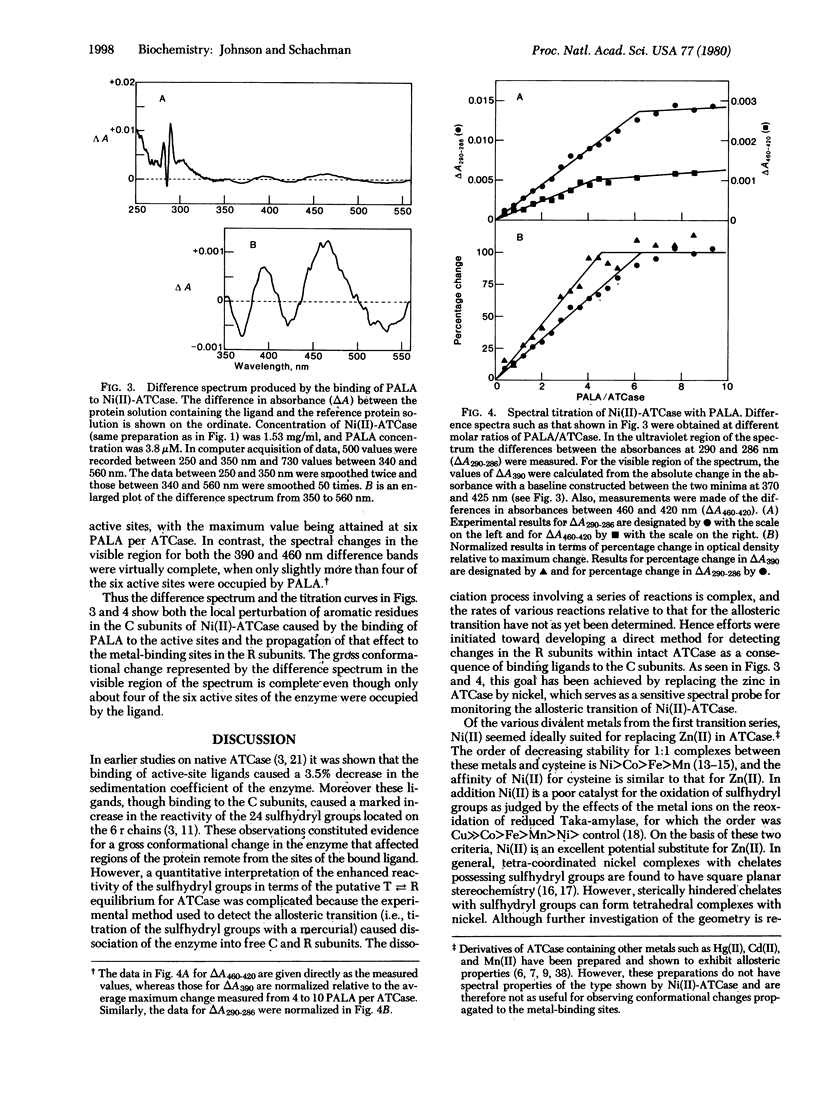
Although the importance of ligand-promoted conformational changes in allosteric enzymes has been recognized, it often has been difficult to determine whether the effects of binding are propagated to remote positions in different chains. Efforts were made, therefore, to demonstrate that changes due to ligand binding to the catalytic chains of aspartate transcarbamoylase (carbamoylphosphate:L-aspartate carbamoyltransferase, EC 2.1.3.2) of Escherichia coli are “communicated” to the regulatory chains. For these studies the endogenous zinc in the latter chains was replaced by nickel, which served as a discriminating spectral probe. The Ni(II)-enzyme was constructed by dissociating the native enzyme, separating the catalytic and regulatory subunits, removing Zn(II) from the latter, replacing it with Ni(II), and reconstituting the enzyme from native catalytic and Ni(II)-containing regulatory subunits. Ni(II) derivatives containing either six Ni(II) or five Ni(II) and one Zn(II) possess the allosteric properties of the native enzyme and exhibit absorption bands at 360 and 440 nm due to charge transfer transitions. Smaller bands were also observed at 665 and 720 nm from d-d transitions, which are consistent with tetrahedral geometry in the coordination sphere of nickel. Binding of the bisubstrate ligand N-(phosphonacetyl)-L-aspartate to the catalytic subunit of Ni(II)-aspartate transcarbamoylase perturbed the Ni(II) chromophore, giving rise to two difference spectral bands (at 390 and 465 nm). Spectral titrations showed that the conformational changes at the metal-ion-binding sites were complete even though about one-third of the active sites were unoccupied. This propagation of conformational changes is in accord with other evidence indicating that the allosteric transition in aspartate transcarbamoylase is concerted.
Keywords: allosteric enzymes, communication between subunits, cooperativity, spectral probes, metalloproteins
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBERT A. Quantitative studies of the avidity of naturally occurring substances for trace metals. II. Amino-acids having three ionizing groups. Biochem J. 1952 Mar;50(5):690–697. doi: 10.1042/bj0500690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn M. N., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Effect of active site ligands on the reactivity of sulfhydryl groups of the regulatory subunits. Biochemistry. 1977 Nov 15;16(23):5084–5091. doi: 10.1021/bi00642a022. [DOI] [PubMed] [Google Scholar]
- Cohlberg J. A., Pigiet V. P., Jr, Schachman H. K. Structure and arrangement of the regulatory subunits in aspartate transcarbamylase. Biochemistry. 1972 Aug 29;11(18):3396–3411. doi: 10.1021/bi00768a013. [DOI] [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Interaction with the transition state analogue N-(phosphonacetyl)-L-aspartate. J Biol Chem. 1971 Nov;246(21):6599–6605. [PubMed] [Google Scholar]
- Collins K. D., Stark G. R. Aspartate transcarbamylase. Studies of the catalytic subunit by ultraviolet difference spectroscopy. J Biol Chem. 1969 Apr 10;244(7):1869–1877. [PubMed] [Google Scholar]
- Davies G. E., Vanaman T. C., Stark G. R. Aspartate transcarbamylase. Stereospecific restrictions on the binding site for L-aspartate. J Biol Chem. 1970 Mar 10;245(5):1175–1179. [PubMed] [Google Scholar]
- Fan S., Harrison L. W., Hammes G. G. Nuclear magnetic resonance study of ligand binding to Mn-aspartate transcarbamylase. Biochemistry. 1975 May 20;14(10):2219–2224. doi: 10.1021/bi00681a027. [DOI] [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. The enzymology of control by feedback inhibition. J Biol Chem. 1962 Mar;237:891–896. [PubMed] [Google Scholar]
- Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. II. Evidence for different conformational states of the protein in the presence and absence of specific ligands. Biochemistry. 1968 Feb;7(2):538–552. doi: 10.1021/bi00842a600. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Ritchey J. M., Schachman H. K. Concerted allosteric transition in hybrids of aspartate transcarbamoylase containing different arrangements of active and inactive sites. Biochemistry. 1976 Mar 23;15(6):1324–1330. doi: 10.1021/bi00651a024. [DOI] [PubMed] [Google Scholar]
- Griffin J. H., Rosenbusch J. P., Blout E. R., Weber K. K. Conformational changes in aspartate transcarbamylase. II. Circular dichroism evidence for the involvement of metal ions in allosteric interactions. J Biol Chem. 1973 Jul 25;248(14):5057–5062. [PubMed] [Google Scholar]
- Hensley P., Schachman H. K. Communication between dissimilar subunits in aspartate transcarbamoylase: effect of inhibitor and activator on the conformation of the catalytic polypeptide chains. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3732–3736. doi: 10.1073/pnas.76.8.3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett G. J., Blackburn M. N., Compton J. G., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Analysis of the structural and functional behavior in terms of a two-state model. Biochemistry. 1977 Nov 15;16(23):5091–5100. doi: 10.1021/bi00642a023. [DOI] [PubMed] [Google Scholar]
- Howlett G. J., Schachman H. K. Allosteric regulation of aspartate transcarbamoylase. Changes in the sedimentation coefficient promoted by the bisubstrate analogue N-(phosphonacetyl)-L-aspartate. Biochemistry. 1977 Nov 15;16(23):5077–5083. doi: 10.1021/bi00642a021. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Stark G. R. Aspartate transcarbamylase. A study of possible roles for the sulfhydryl group at the active site. J Biol Chem. 1973 Dec 10;248(23):8003–8014. [PubMed] [Google Scholar]
- LENZ G. R., MARTELL A. E. METAL CHELATES OF SOME SULFUR-CONTAINING AMINO ACIDS. Biochemistry. 1964 Jun;3:745–750. doi: 10.1021/bi00894a001. [DOI] [PubMed] [Google Scholar]
- LIBBY R. A., MARGERUM D. W. A KINETIC STUDY OF NICKER (II). COMPLEXES OF SULFUR-CONTAINING AMINO ACIDS. Biochemistry. 1965 Apr;4:619–625. doi: 10.1021/bi00880a002. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Monaco H. L., Crawford J. L., Lipscomb W. N. Three-dimensional structures of aspartate carbamoyltransferase from Escherichia coli and of its complex with cytidine triphosphate. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5276–5280. doi: 10.1073/pnas.75.11.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelbach M. E., Pigiet V. P., Jr, Gerhart J. C., Schachman H. K. A role for zinc in the quaternary structure of aspartate transcarbamylase from Escherichia coli. Biochemistry. 1972 Feb 1;11(3):315–327. doi: 10.1021/bi00753a002. [DOI] [PubMed] [Google Scholar]
- Rosenbusch J. P., Weber K. Localization of the zinc binding site of aspartate transcarbamoylase in the regulatory subunit. Proc Natl Acad Sci U S A. 1971 May;68(5):1019–1023. doi: 10.1073/pnas.68.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachman H. K. Anatomy and physiology of a regulatory enzyme-aspartate transcarbamylase. Harvey Lect. 1974;68:67–113. [PubMed] [Google Scholar]
- TAKAGI T., ISEMURA T. ACCELERATING EFFECT OF COPPER ION ON THE REACTIVATION OF REDUCED TAKA-AMYLASE A THROUGH CATALYSIS OF THE OXIDATION OF SULFHYDRYL GROUPS. J Biochem. 1964 Oct;56:344–350. doi: 10.1093/oxfordjournals.jbchem.a127999. [DOI] [PubMed] [Google Scholar]
- Yang Y. R., Kirschner M. W., Schachman H. K. Aspartate transcarbamoylase (Escherichia coli): preparation of subunits. Methods Enzymol. 1978;51:35–41. doi: 10.1016/s0076-6879(78)51007-0. [DOI] [PubMed] [Google Scholar]
- Yang Y. R., Syvanen J. M., Nagel G. M., Schachman H. K. Aspartate transcarbamoylase molecules lacking one regulatory subunit. Proc Natl Acad Sci U S A. 1974 Mar;71(3):918–922. doi: 10.1073/pnas.71.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]



