Abstract
Matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) imaging mass spectrometry (IMS) applied directly to microbes on agar-based medium captures global information about microbial molecules, allowing for direct correlation of chemotypes to phenotypes. This tool was developed to investigate metabolic exchange factors of intraspecies, interspecies, and polymicrobial interactions. Based on our experience of the thousands of images we have generated in the laboratory, we present five steps of microbial IMS: culturing, matrix application, dehydration of the sample, data acquisition, and data analysis/interpretation. We also address the common challenges encountered during sample preparation, matrix selection and application, and sample adherence to the MALDI target plate. With the practical guidelines described herein, microbial IMS use can be extended to bio-based agricultural, biofuel, diagnostic, and therapeutic discovery applications.
INTRODUCTION
Microbial metabolites are chemical messages that act as indicators of population density (4, 41, 61) and as cues for cellular differentiation (9, 34, 35, 51, 57) and that commonly have protective roles (6, 36, 44, 47). Their functional diversity is reflected in their structural diversity, ranging from small molecules and iron scavengers to peptides and lipids. Traditionally, individual microbial metabolites have been targeted using bioactivity-guided fractionation. More recent “omics” technologies, such as metabolomics, begin to capture molecules on a global scale, but current mass spectrometry-based approaches do not distinguish between molecules which are always present and molecules with changing spatial distributions, disregarding the spatial localization of the molecules within a phenotype and the multifaceted chemical exchange within and between microbial cell populations (18, 19, 60).
The development of imaging mass spectrometry (IMS) techniques complements metabolomic approaches by enabling the preservation of molecular localization, yielding insight into the underlying biology. Matrix-assisted laser desorption–ionization time of flight (MALDI-TOF) imaging mass spectrometry (IMS) technology has been applied to molecular pathology for the past 17 years (11, 50, 57) to visualize fundamental disease signatures from biological samples by detecting proteins, peptides (14, 58), and lipids (43) directly from tissue sections. Most MALDI mass spectrometers are capable of imaging experiments with vendor-specific software and freeware to automate data acquisition and assist in analysis (20, 28), and other IMS methods are rapidly emerging (56, 64, 71), thus reaffirming the need for these technologies.
In 2009, we applied MALDI-TOF IMS to study various molecules in the context of interacting microbial colonies (70), hereafter referred to as microbial IMS. Theoretically, any culturable microbe can be subjected to microbial IMS. To date, our lab has been able to capture chemical information from a wide range of microbes, including terrestrial (31, 34), freshwater, and marine bacteria (69) as well as amoebae, fungi, and yeast. By providing a spatial snapshot for each observed mass signal, it is possible to directly visualize the metabolic exchange within and among microbial species on various types of agar media, as shown in Fig. 1.
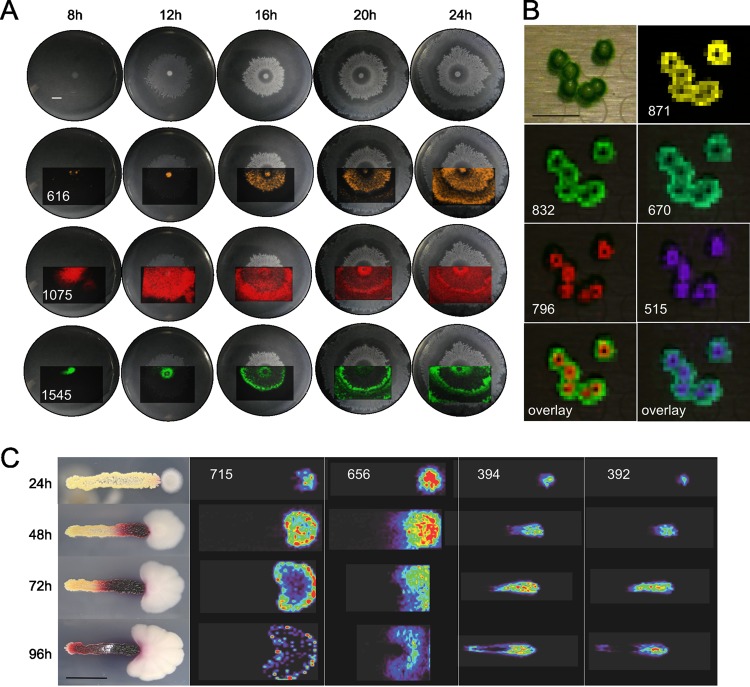
Fig 1.
Molecular snapshots from microbial MALDI-TOF imaging mass spectrometry, obtained on different agar surfaces. (A) Representative ions of swarming Bacillus subtilis 3610 (29, 33, 34) on ISP2 (0.7% agar) medium at 30°C over time. m/z 616, 1,075, and 1,545 were assigned to an uncharacterized ion, surfactin-C15 [M+K]+, and plipastatin-C17-Val [M+K]+, respectively (70). Due to the size of the MALDI target plate, only the displayed regions were imaged. (B) We observed the development of previously unreported macrostructures in the growth of freshwater Synechococcus elongatus PCC 7942 colonies, a model photosynthetic organism for the study of prokaryotic circadian biology (37). A confluent culture was diluted 1:50,000 with BG-11 M and then spotted and grown on BG-11 M (1.5% agar) medium (10) at 30°C inside a moisture chamber under a constant light intensity of 350 μE/m2 · s for 16 days prior to IMS. The [M+H]+ of pheophytin a, chlorophyll a without Mg2+, is at m/z 871, while m/z 796 and 515 putatively correspond to the observed macrostructures. (C) Interaction between Bacillus subtilis PY79 and Streptomyces coelicolor A3 (2) on ISP2 (1.5% agar) medium at 30°C over time. The color gradient is from least-intense ions (purple) to most-intense ions (red). The prodiginines streptorubin B and undecylprodigiosin [M+H]+ are at m/z 392 and 394, respectively. m/z 656 is an uncharacterized bacillus ion, and 715 is a partially characterized polyglutamate compound (70). Scale bars, 1 cm.
FIVE STEPS OF MICROBIAL IMS
Culturing—use thin agar.
Microbial IMS sample preparation workflow (Fig. 2) begins with culturing one or more microbes on agar-based medium directly on top of the MALDI target plate (70) or in petri dishes (18, 34). The agar is 1 to 1.5 mm thick, equivalent to 10 to 11 ml medium in a standard 10-cm petri dish, and typically contains 1 to 2% agar. The petri dishes are either sealed with Parafilm or placed in a humidity-controlled chamber to minimize dehydration of the thin agar during incubation. Although various media can be used, a medium that we frequently use is yeast extract/malt extract ISP2 (4 g/liter yeast extract, 10 g/liter malt extract, 4 g/liter dextrose, and 1.5 to 2% agar).
Fig 2.
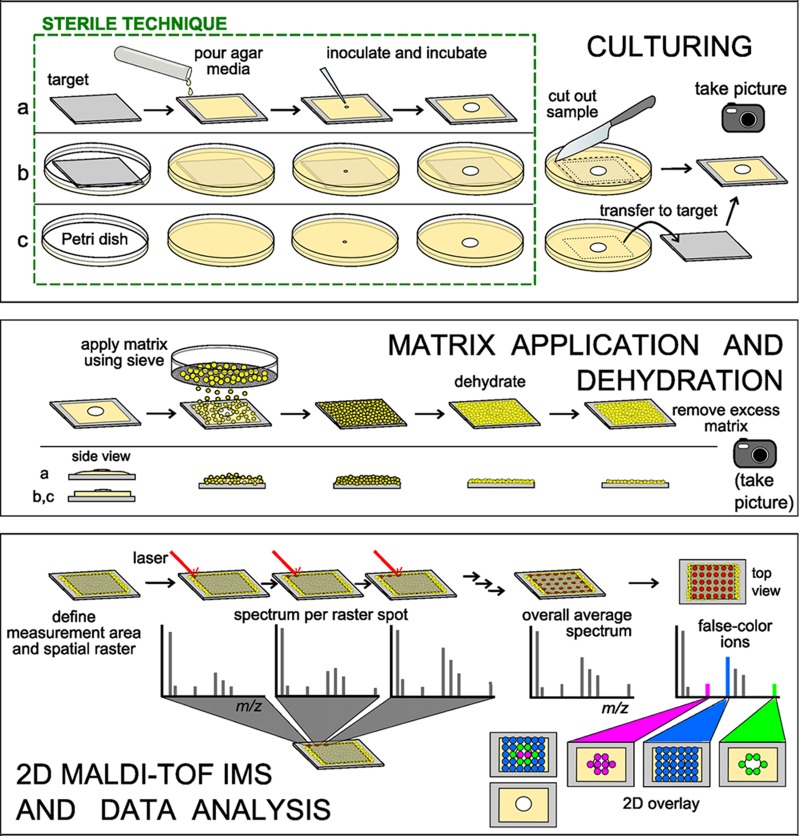
The 5 major steps to obtain a microbial chemotype image are culturing, matrix application, dehydration of the samples, imaging mass spectrometry, and data analysis. Culturing. Three approaches to grow microbial samples for IMS are (a) pouring agar and inoculating directly on the target, (b) embedding the target in agar prior to inoculation and incubation, and (c) culturing the microbe in a petri dish, excising the region of interest, and then transferring it to the target. Matrix application. The sample edges from culturing approach a are tapered toward the target plate, compared to approaches b and c. This will affect the matrix coverage at the edges of the sample; matrix application is depicted for samples prepared via approaches b and c. Universal matrix is applied to the sample as a dry powder using a 20- or 53-μm test sieve. The hydration of the agar is sufficient for surface absorption of matrix. Sample dehydration. The sample is dehydrated at an elevated temperature (37°C), and the excess matrix is removed prior to microbial IMS. The sample height should be less than 1.0 mm. Also, the sample is examined for sufficient adherence to the target. Imaging mass spectrometry. User-defined spatial resolution and measurement area generate the x-y raster grid for the imaging run. A MALDI-TOF mass spectrum is collected per raster spot; spectra from the entire measurement area are averaged. Data analysis. False colors are assigned to ions in the overall average mass spectrum and displayed over a photograph of the sample. The interpretation of the ion distributions is the responsibility of the experimenter.
The three approaches used to prepare the microbial samples for IMS are (i) pouring agar directly onto the MALDI target plate, (ii) embedding the target plate in agar medium prior to inoculation, and (iii) cutting the microbial colony and surrounding agar directly from the petri dish and transferring it to the target plate (see Video S1 in the supplemental material). Approaches one and two require vigilant sterility, are generally used to study one colony or one interaction per target plate, and occupy the target plate for days or weeks at a time. We currently use approach three, as this approach does not occupy the MALDI plates for long periods of time, increasing sample throughput and allowing numerous samples to be placed onto a single MALDI target for analysis. It is important to photograph the microbes before the matrix is applied in order to correlate the phenotype to the observed chemistry.
Matrix application—saturate the sample.
MALDI-TOF mass spectrometry requires matrix, small organic acids that cocrystallize with the sample and absorb the laser energy to assist in desorption and ionization of molecules from the sample surface (21). The spatial resolution of imaging mass spectrometry is dependent on two main factors, the diameter of the laser beam and the size of the matrix crystals. We have found that using either a 20- or 53-μm stainless steel test sieve (Hogentogler & Co., Inc.) (43, 70) to apply matrix is an efficient, low-cost, and robust method to uniformly dry coat and saturate microbial samples (see Video S2 in the supplemental material) with no discernible differences in the quality of IMS data at a spatial resolution of 100 μm by 100 μm or greater. Microbial IMS is currently unable to resolve single cells.
A 1:1 mixture of 2,5-dihydroxybenzoic acid (DHB) and α-cyano-4-hydroxycinnamic acid (CHCA) matrices, sold as Universal MALDI Matrix (Sigma-Aldrich), is used for both positive- and negative-mode microbial IMS. We hypothesize that saturation of the sample with this matrix mixture is crucial for sample adherence and for yielding matrix crystals of sufficient quality to ionize molecules from the sample (see Fig. S2 and the supplemental discussion in the supplemental material). Although CHCA is commonly used for small proteins and peptides and DHB is typically used for carbohydrates and glycopeptides, the mixture has been reliable for the detection of peptides, carbohydrates, lipids, and other metabolic exchange factors from microbial samples.
Dehydration—check for flaking, sample height of about 1 mm.
A critical step in microbial IMS is dehydration of the sample at 37°C, which may be followed by storage in a vacuum desiccator. This step ensures that the MALDI mass spectrometer components (the source, mass analyzer, and detector) achieve the vacuum pressure necessary for operation and that the thickness of the dehydrated microbial sample is compatible with the allowed sample height of 0.4 to 1.0 mm. In general, dehydrated microbial samples account for most of the sample height; our desiccated agar is about 100 to 200 μm thick, and the contribution of matrix to sample height is at most double the thickness for many of the organisms we have tested.
Variable sample height impacts the mass calibration of a TOF instrument. We typically observe 0.1- to 0.5-Da mass shifts, in agreement with the 100- to 200-μm thickness of the sample. Mass measurement in a MALDI-TOF mass spectrometer relies on accurate timing of an analyte molecule traveling from the target plate through the flight tube to the detector, with longer travel paths, such as reflectron mode and longer flight tubes, resulting in larger mass deviances. When high mass accuracy is critical, an internal calibration is possible by spotting microgram amounts of calibrant on the dried agar surface.
During the drying process, sample flaking may occur. The term flaking is used to describe air bubbles, fissures, and partial or complete detachment of the dehydrated sample from the MALDI target plate (see Fig. S1 in the supplemental material), and ways to minimize flaking are discussed later and in the supplemental discussion in the supplemental material. When sample adherence is questionable, the sample should not be inserted into the mass spectrometer.
2D MALDI-TOF IMS—data acquisition.
The fundamentals of data acquisition for microbial IMS are identical to those for tissue IMS (11). The user defines the desired spatial resolution and measurement area using a reference photograph of the bacterial sample on the target, determining the two-dimensional (2D) raster grid. Mass spectra generated at each raster spot are associated with coordinates.
Data analysis and interpretation.
After MALDI-TOF spectra are acquired, the signal intensity of a discrete mass across the sample surface can be visualized using a false color superimposed onto a photograph of the microbial sample. These IMS images are composed of pixels, similar to images on a computer or TV screen, where each pixel contains molecular information. By correlating spatial distributions of each molecule with observed phenotypes, putative functions of observed molecules can be assigned and phenotypes undetectable by eye may be revealed.
CHALLENGES IN MICROBIAL IMS
Sample adherence.
The primary limitation of microbial IMS is flaking. Flaking occurs (see Fig. S1 in the supplemental material) as a result of the inability of the dried and brittle agar to remain adhered to the target plate, a result which can be due to trapped air underneath the growth medium, insufficient matrix saturation of the sample, use of smooth or polished MALDI target plates, certain medium components (e.g., high salt), and microbial molecules (1, 5, 17, 18, 22, 26, 27, 34, 38, 39, 59, 70). In some cases, sample flaking does not interfere with instrument operation (Fig. 3B, number 5); however, care must be taken to minimize flaking inside the mass spectrometer. While sample adherence is a major obstacle in the microbial IMS workflow, we have found that optimization of the sample preparation can overcome such issues. In Fig. S1 and the supplemental discussion in the supplemental material, we present guidelines and a flowchart with checkpoints to obtain quality microbial IMS images and decrease the risk of sample flaking.
Fig 3.
Data interpretation challenges—flaking, black holes, and gradients. (A) Various data interpretation challenges. (1) Uniform molecule distribution and (2) nonuniform molecule distribution. The observed signal reflects the true amount of molecule in the sample. (3) Presence of an intense competing ion. With even matrix coverage, the observed signal does not reflect the true amount of molecule in the sample due to signal suppression by a competing ion. (4) Nonuniform matrix coverage. This is common with microbes that produce lipopolysaccharide (LPS) or surfactant, sporulate, or form aerial structures that physically inhibit matrix saturation. (5) Incomplete contact between sample and target. Incomplete contact between the sample and the target, such as a bubble, a crack, or flaking, ultimately results in decreased detection of ions. The sample has (6) decreased conductivity and (7) charge buildup. The thickness of the microbial sample may act as an insulator, decreasing the conductivity and, in turn, ionization of molecules. Similarly, charge can build up on the MALDI target over time, manifesting in a gradient of higher intensity in the beginning of acquisition and little to no intensity at the end of acquisition. Charge buildup is more common in tissue IMS, which uses indium tin oxide (ITO)-coated slides. (B) Real examples of the challenges described in panel A. From left to right, Escherichia coli, Staphylococcus aureus, Pantoea agglomerans (Lab Environmental Strain Collection), B. subtilis, and Serratia marcescens (Lab Environmental Strain Collection) interacting with B. subtilis 3610. Select ions from IMS data are displayed over photographs depicting matrix coverage of the dehydrated sample. Photographs of the sample on the target prior to matrix coverage and dehydration are to the left of the matrix coverage photographs. The colors in the gradient example are from most-intense (red) to least-intense (purple) ions.
Data interpretation.
The challenges in data interpretation for microbial IMS are common to all MALDI-TOF-based IMS. Black holes or areas of no signal may be due to nonuniform matrix coverage or ion suppression, whereas false gradients or signals of regular increasing or decreasing intensities may be due to decreased conductivity, charge buildup or charging (25, 66), or a combination of factors (Fig. 3). Charge buildup is recognizable by an optimal signal early in the IMS run and a decreased signal over time (Fig. 3B). Analysis of multiple microbial samples in a randomized fashion will provide insight into the significance of the gradient signals. Data interpretation is the responsibility of the experimenter who evaluates the causes of patterns and whether they are real or artifacts.
Determining the origin of observed molecules.
IMS of dual-species interactions yields many ions, some of which are detected only upon interaction. Oftentimes, the distribution of the ion of interest is interfacial and the producer is ambiguous. By employing analytical and biological approaches, such as microbial IMS, IMS of multiple time points (67), traditional solvent extraction, purification, and structural determination methods, such as tandem MS and nuclear magnetic resonance (NMR) (18, 30, 33, 67, 68), genetics and microbiology, 16S rRNA sequencing (54), established MALDI-TOF protocols (12, 49, 53), genome mining approaches (7, 13, 15, 45, 63) and predictive programs (2, 3, 16, 33, 40, 48, 55, 62, 65, 68), peptidogenomics (31), and literature and database searches (23, 52) (AntiMarin database, Dictionary of Natural Products, the National Institute of Standards and Technology [NIST] databases, and the SciFinder database), one can typically annotate microbial IMS data. Two main strategies to confirm the producing microbe and the resultant phenotype are genetic knockout and complementation studies or assays with purified compound, in combination with more IMS.
FINAL REMARKS
The microbial metabolic maps from 2D MALDI-TOF IMS serve as a starting point for the identification of microbial metabolites for virtually any culturable microbe. A single metabolite of low abundance can now be detected using IMS analysis due to high, localized concentrations within the preserved 2D phenotype, but annotating the molecules is still a challenge. The microbial interactions can be scaled up and extracted to isolate enough material for further mass spectrometry-guided isolation and subsequent structural characterization with mass spectrometry and NMR (69). However, this process is inefficient and costly. The challenge of annotating mass spectrometry data also applies to proteomics, metabolomics, and lipidomics. These advanced “omics” tools, even when combined with the power of genome mining, peptidogenomics, and search algorithms, annotate only a small percentage of what are presumably some of the most abundant ions, as observed in an imaged sample (32), indicating that there are still many opportunities in mass spectrometry to develop novel approaches to identify (ID) molecules (8, 24, 42, 46). We encourage the scientific community to develop systematic, integrated workflows for handling unknowns in IMS data that may capture more than 50% of the IMS signals. Finally, we hope that this article provides a starting point for researchers and that it encourages other labs to apply microbial IMS to different systems and generate tools to complement this approach to push the boundaries of spatial systems microbiology.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the support of the U.S. National Institutes of Health grants AI095125 and S10RR029121, the Interfaces Training Grant—a Ruth L. Kirschstein National Research Service Award, NIH 1 T32 EB009380-01, grant GM097509, and the DFG Research Fellowship (WE 4754/1-1). The freshwater cyanobacteria research was supported by DOE grant DE-EE0003373.
We acknowledge the Bruker Therapeutic Discovery Mass Spectrometry Center at the UCSD Skaggs School of Pharmacy.
Footnotes
Published ahead of print 20 July 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Alaupovic P, Olson AC, Tsang J. 1966. Studies on the characterization of lipopolysaccharides from two strains of Serratia marcescens. Ann. N. Y. Acad. Sci. 133:546–565 [DOI] [PubMed] [Google Scholar]
- 2. Anand S, et al. 2010. SBSPKS: structure based sequence analysis of polyketide synthases. Nucleic Acids Res. 38:W487–W496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ansari MZ, Yadav G, Gokhale RS, Mohanty D. 2004. NRPS-PKS: a knowledge-based resource for analysis of NRPS/PKS megasythases. Nucleic Acids Res. 32:W405–W413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Antunes LC, Ferreira RB, Buckner MC, Finlay BB. 2010. Quorum sensing in bacterial virulence. Microbiology 156:2271–2282 [DOI] [PubMed] [Google Scholar]
- 5. Arima K, Kakinuma A, Tamura G. 1968. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 31:488–494 [DOI] [PubMed] [Google Scholar]
- 6. Bassler BL, Losick R. 2006. Bacterially speaking. Cell 125:237–246 [DOI] [PubMed] [Google Scholar]
- 7. Begley M, Cotter PD, Hill C, Ross RP. 2009. Identification of a novel two-peptide lantibiotic, lichenicidin, following rational genome mining for LanM proteins. Appl. Environ. Microbiol. 75:5451–5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowen BP, Northen T. 2010. Dealing with the unknown: metabolomics and metabolite atlases. J. Am. Soc. Mass Spectrom. 21:1471–1476 [DOI] [PubMed] [Google Scholar]
- 9. Branda SS, Gonzalez-Pastor JE, Ben-Yehuda S, Losick R, Kolter R. 2001. Fruiting body formation by Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 98:11621–11626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bustos SA, Golden SS. 1991. Expression of the psbDII gene in Synechococcus sp. strain PCC 7942 requires sequences downstream of the transcription start site. J. Bacteriol. 173:7525–7533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caprioli RM, Farmer TB, Gile J. 1997. Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal. Chem. 69:4751–4760 [DOI] [PubMed] [Google Scholar]
- 12. Carbonnelle E, et al. 2012. Robustness of two MALDI-TOF mass spectrometry systems for bacterial identification. J. Microbiol. Methods 89:133–136 [DOI] [PubMed] [Google Scholar]
- 13. Challis GL. 2008. Genome mining for novel natural product discovery. J. Med. Chem. 51:2618–2628 [DOI] [PubMed] [Google Scholar]
- 14. Chaurand P, Stoeckli M, Caprioli RM. 1999. Direct profiling of proteins in biological tissue sections by MALDI mass spectrometry. Anal. Chem. 71:5263–5270 [DOI] [PubMed] [Google Scholar]
- 15. Claesen J, Bibb M. 2010. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides. Proc. Natl. Acad. Sci. U. S. A. 107:16297–16302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. de Jong A, van Heel AJ, Kok J, Kuipers OP. 2010. BAGEL2: mining for bacteriocins in genomic data. Nucleic Acids Res. 38:W647–W651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edwards JR, Hayashi JA. 1965. Structure of a rhamnolipid from Pseudomonas aeruginosa. Arch. Biochem. Biophys. 111:415–421 [DOI] [PubMed] [Google Scholar]
- 18. Gonzalez DJ, et al. 2011. Microbial competition between Bacillus subtilis and Staphylococcus aureus monitored by imaging mass spectrometry. Microbiology 157:2485–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goulitquer S, Potin P, Tonon T. 2012. Mass spectrometry-based metabolomics to elucidate functions in marine organisms and ecosystems. Mar. Drugs 10:849–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gustafsson JO, Oehler MK, Ruszkiewicz A, McColl SR, Hoffmann P. 2011. MALDI imaging mass spectrometry (MALDI-IMS)—application of spatial proteomics for ovarian cancer classification and diagnosis. Int. J. Mol. Sci. 12:773–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hankin JA, Barkley RM, Murphy RC. 2007. Sublimation as a method of matrix application for mass spectrometric imaging. J. Am. Soc. Mass Spectrom. 18:1646–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hisatsuka K, Nakahara T, Minoda T, Yamada K. 1971. Formation of rhamnolipid by Pseudomonas aeruginosa and its function in hydrocarbon fermentation. Agric. Biol. Chem. 35:686–692 [Google Scholar]
- 23. Horai H, et al. 2010. MassBank: a public repository for sharing mass spectral data for life sciences. J. Mass. Spectrom. 45:703–714 [DOI] [PubMed] [Google Scholar]
- 24. Hufsky F, Rempt M, Rasche F, Pohnert G, Böcker S. 2012. De novo analysis of electron impact mass spectra using fragmentation trees. Anal. Chim. Acta 739:67–76 [DOI] [PubMed] [Google Scholar]
- 25. Ibanez AJ, Muck A, Svatos A. 2007. Dissipation of charge on MALDI-TOF polymeric chips using an electron-acceptor: analysis of proteins. J. Mass Spectrom. 42:634–640 [DOI] [PubMed] [Google Scholar]
- 26. Inagawa H, et al. 1992. Homeostasis as regulated by activated macrophage. II. LPS of plant origin other than wheat flour and their concomitant bacteria. Chem. Pharm. Bull. (Tokyo) 40:994–997 [DOI] [PubMed] [Google Scholar]
- 27. Itoh S, Honda H, Tomita F, Suzuki T. 1971. Rhamnolipid produced by Pseudomonas aeruginosa grown on n-paraffin. J. Antibiot. 24:855–859 [DOI] [PubMed] [Google Scholar]
- 28. Jardin-Mathe O, et al. 2008. MITICS (MALDI Imaging Team Imaging Computing System): a new open source mass spectrometry imaging software. J. Proteomics 71:332–345 [DOI] [PubMed] [Google Scholar]
- 29. Jeffries CD, Rogers HE. 1968. Enhancing effect of agar on swarming by Proteus. J. Bacteriol. 95:732–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kearns DB. 2010. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 8:634–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kersten RD, et al. 2011. A mass spectrometry-guided genome mining approach for natural product peptidogenomics. Nat. Chem. Biol. 7:794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim S, Bandiera N, Pevzner PA. 2009. Spectral profiles, a novel representation of tandem mass spectra and their applications for de novo peptide sequencing and identification. Mol. Cell. Proteomics 8:1391–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li MH, Ung PM, Zajkowski J, Garneau-Tsodikova S, Sherman DH. 2009. Automated genome mining for natural products. BMC Bioinformatics 10:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu W-T, et al. 2010. Imaging mass spectrometry of intraspecies metabolic exchange revealed the cannibalistic factors of Bacillus subtilis. Proc. Natl. Acad. Sci. U. S. A. 107:16286–16290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopez D, Kolter R. 2010. Extracellular signals that define distinct and coexisting cell fates in Bacillus subtilis. FEMS Microbiol. Rev. 34:134–149 [DOI] [PubMed] [Google Scholar]
- 36. Lowery CA, Dickerson TJ, Janda KD. 2008. Interspecies and interkingdom communication mediated by bacterial quorum sensing. Chem. Soc. Rev. 37:1337–1346 [DOI] [PubMed] [Google Scholar]
- 37. Mackey SR, Golden SS, Ditty JL. 2011. The itty-bitty time machine genetics of the cyanobacterial circadian clock. Adv. Genet. 74:13–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Matsuyama T, Sogawa M, Nakagawa Y. 1989. Fractal spreading growth of Serratia marcescens which produces surface active exolipids. FEMS Microbiol. Lett. 52:243–246 [DOI] [PubMed] [Google Scholar]
- 39. Matsuyama T, et al. 1992. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J. Bacteriol. 174:1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Medema MH, et al. 2011. antiSMASH: rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 39:W339–W346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miller MB, Bassler BL. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165–199 [DOI] [PubMed] [Google Scholar]
- 42. Padliya ND, Wood TD. 2004. A strategy to improve peptide mass fingerprinting matches through the optimization of matrix-assisted laser desorption/ionization matrix selection and formulation. Proteomics 4:466–473 [DOI] [PubMed] [Google Scholar]
- 43. Puolitaival SM, Burnum KE, Cornett DS, Caprioli RM. 2008. Solvent-free matrix dry-coating for MALDI imaging of phospholipids. J. Am. Soc. Mass Spectrom. 19:882–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ratcliff WC, Denison RF. 2011. Alternative actions for antibiotics. Science 332:547–548 [DOI] [PubMed] [Google Scholar]
- 45. Rausch C, Weber T, Kohlbacher O, Wohlleben W, Huson DH. 2005. Specificity prediction of adenylation domains in nonribosomal peptide synthetases (NRPS) using transductive support vector machines (TSVMs). Nucleic Acids Res. 33:5799–5808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rojas-Cherto M, et al. 2012. Metabolite identification using automated comparison of high-resolution multistage mass spectral trees. Anal. Chem. 84:5524–5534 [DOI] [PubMed] [Google Scholar]
- 47. Romero D, Traxler MF, Lopez D, Kolter R. 2011. Antibiotics as signal molecules. Chem. Rev. 111:5492–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Röttig M, et al. 2011. NRPSpredictor2—a web server for predicting NRPS adenylation domain specificity. Nucleic Acids Res. 39:W362–W367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saffert RT, et al. 2011. Comparison of Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometer to BD Phoenix automated microbiology system for identification of gram-negative bacilli. J. Clin. Microbiol. 49:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seeley EH, Caprioli RM. 2011. MALDI imaging mass spectrometry of human tissue: method challenges and clinical perspectives. Trends Biotechnol. 29:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shank EA, Kolter R. 2011. Extracellular signaling and multicellularity in Bacillus subtilis. Curr. Opin. Microbiol. 14:741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Smith CA, et al. 2005. METLIN: a metabolite mass spectral database. Ther. Drug Monit. 27:747–751 [DOI] [PubMed] [Google Scholar]
- 53. Sogawa K, et al. 2011. Use of the MALDI BioTyper system with MALDI-TOF mass spectrometry for rapid identification of microorganisms. Anal. Bioanal. Chem. 400:1905–1911 [DOI] [PubMed] [Google Scholar]
- 54. Stackebrandt E, Goebel BM. 1994. Taxonomic note: a place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 44:846–849 [Google Scholar]
- 55. Starcevic A, et al. 2008. ClustScan: an integrated program package for the semi-automatic annotation of modular biosynthetic gene clusters and in silico prediction of novel chemical structures. Nucleic Acids Res. 36:6882–6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Steinhauser ML, et al. 2012. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature 481:516–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stoeckli M, Farmer TB, Caprioli RM. 1999. Automated mass spectrometry imaging with a matrix-assisted laser desorption ionization time-of-flight instrument. J. Am. Soc. Mass Spectrom. 10:67–71 [DOI] [PubMed] [Google Scholar]
- 58. Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM. 2001. Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat. Med. 7:493–496 [DOI] [PubMed] [Google Scholar]
- 59. Straight PD, Willey JM, Kolter R. 2006. Interactions between Streptomyces coelicolor and Bacillus subtilis: role of surfactants in raising aerial structures. J. Bacteriol. 188:4918–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Straight PD, Kolter R. 2009. Interspecies chemical communication in bacterial development. Annu. Rev. Microbiol. 63:99–118 [DOI] [PubMed] [Google Scholar]
- 61. Swift S, et al. 2001. Quorum sensing as a population-density-dependent determinant of bacterial physiology. Adv. Microb. Physiol. 45:199–270 [DOI] [PubMed] [Google Scholar]
- 62. Tae H, Kong EB, Park K. 2007. ASMPKS: an analysis system for modular polyketide synthases. BMC Bioinformatics 8:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Velasquez JE, van der Donk WA. 2011. Genome mining for ribosomally synthesized natural products. Curr. Opin. Chem. Biol. 15:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Watrous JD, Dorrestein PC. 2011. Imaging mass spectrometry in microbiology. Nat. Rev. Microbiol. 9:683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Weber T, et al. 2009. CLUSEAN: a computer-based framework for the automated analysis of bacterial secondary metabolite biosynthetic gene clusters. J. Biotechnol. 140:13–17 [DOI] [PubMed] [Google Scholar]
- 66. Werner HW, Morgan AE. 1976. Charging of insulators by ion bombardment and its minimization for secondary ion mass spectrometry (SIMS) measurement. J. Appl. Phys. 47:1232–1242 [Google Scholar]
- 67. Xu Y, et al. 2012. Bacterial biosynthesis and maturation of the didemnin anti-cancer agents. J. Am. Chem. Soc. 134:8625–8632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yadav G, Gokhale RS, Mohanty D. 2003. SEARCHPKS: a program for detection and analysis of polyketide synthase domains. Nucleic Acid Res. 31:3654–3658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang Y-L, et al. 2011. Connecting chemotypes and phenotypes of cultured marine microbial assemblages by imaging mass spectrometry. Angew. Chem. Int. Ed. Engl. 50:5839–5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yang Y-L, Xu Y, Straight PD, Dorrestein PC. 2009. Translating metabolic exchange with imaging mass spectrometry. Nat. Chem. Biol. 5:885–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang D-S, et al. 2012. Multi-isotope imaging mass spectrometry reveals slow protein turnover in hair-cell stereocilia. Nature 481:520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.