Abstract
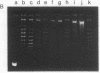
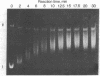
Superhelical and partially relaxed DNAs of simian virus 40 were allowed to react in vitro with (+/-)-7 beta,8 alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10-tetrahydrobenzo[a]pyrene (BaP diol epoxide). The modified DNA contained N2 guanine and N6 adenine hydrocarbon adducts in the ratio 86:14. Superhelical simian virus 40 DNA was approximately 6% more susceptible to modification than was partially relaxed viral DNA. Counterions inhibited DNA alkylation by up to 90%, Mg2+ being 50-fold more effective than Na+. The sensitivity of covalent binding to helix stability is consistent with a reaction complex in which BaP diol epoxide is intercalated. The superhelical density of the modified DNA substrates was determined electrophoretically relative to partially relaxed standards, and an unwinding angle for the hydrocarbon adducts was calculated. The angle was dependent upon the superhelicity of the DNA molecule and ranged from 330 degrees to 30 degrees. These data indicate that the modified base pairs are disrupted and, in the presence of torsional strain, act as centers for the further denaturation of up to eight adjacent base pairs. In the absence of such strain the alkylation sites have an ordered structure, with the attached hydrocarbon probably oriented in the minor or major groove of the helix.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Bauer W. Supercoiling in closed circular DNA: dependence upon ion type and concentration. Biochemistry. 1978 Feb 21;17(4):594–601. doi: 10.1021/bi00597a006. [DOI] [PubMed] [Google Scholar]
- BROOKES P., LAWLEY P. D. EVIDENCE FOR THE BINDING OF POLYNUCLEAR AROMATIC HYDROCARBONS TO THE NUCLEIC ACIDS OF MOUSE SKIN: RELATION BETWEEN CARCINOGENIC POWER OF HYDROCARBONS AND THEIR BINDING TO DEOXYRIBONUCLEIC ACID. Nature. 1964 May 23;202:781–784. doi: 10.1038/202781a0. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. Interaction of closed circular DNA with intercalative dyes. II. The free energy of superhelix formation in SV40 DNA. J Mol Biol. 1970 Feb 14;47(3):419–435. doi: 10.1016/0022-2836(70)90312-8. [DOI] [PubMed] [Google Scholar]
- Beard P., Morrow J. F., Berg P. Cleavage of circular, superhelical simian virus 40 DNA to a linear duplex by S1 nuclease. J Virol. 1973 Dec;12(6):1303–1313. doi: 10.1128/jvi.12.6.1303-1313.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beland F. A. Computer-generated graphic models of the N2-substituted deoxyguanosine adducts of 2-acetylaminofluorene and benzo[a]pyrene and the O6-substituted deoxyguanosine adduct of 1-naphthylamine in the DNA double helix. Chem Biol Interact. 1978 Sep;22(2-3):329–339. doi: 10.1016/0009-2797(78)90136-9. [DOI] [PubMed] [Google Scholar]
- Cohen G. L., Bauer W. R., Barton J. K., Lippard S. J. Binding of cis- and trans-dichlorodiammineplatinum(II) to DNA: evidence for unwinding and shortening of the double helix. Science. 1979 Mar 9;203(4384):1014–1016. doi: 10.1126/science.370979. [DOI] [PubMed] [Google Scholar]
- Drinkwater N. R., Miller J. A., Miller E. C., Yang N. C. Covalent intercalative binding to DNA in relation to the mutagenicity of hydrocarbon epoxides and N-acetoxy-2-acetylaminofluorene. Cancer Res. 1978 Oct;38(10):3247–3255. [PubMed] [Google Scholar]
- Gamper H. B., Tung A. S., Straub K., Bartholomew J. C., Calvin M. DNA strand scission by benzo[a]pyrene diol epoxides. Science. 1977 Aug 12;197(4304):671–674. doi: 10.1126/science.877583. [DOI] [PubMed] [Google Scholar]
- Geacintov N. E., Gagliano A., Ivanovic V., Weinstein I. B. Electric linear dichroism study on the orientation of benzo[alpha]pyrene-7,8-dihydrodiol 9,10-oxide covalently bound to DNA. Biochemistry. 1978 Nov 28;17(24):5256–5262. doi: 10.1021/bi00617a027. [DOI] [PubMed] [Google Scholar]
- Hallick L. M., Yokota H. A., Bartholomew J. C., Hearst J. E. Photochemical addition of the cross-linking reagent 4,5', 8-trimethylpsoralen (trioxaslen) to intracellular and viral simian virus 40 DNA-histone complexes. J Virol. 1978 Jul;27(1):127–135. doi: 10.1128/jvi.27.1.127-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E. Mammalian cell transformation and cell-mediated mutagenesis by carcinogenic polycyclic hydrocarbons. Mutat Res. 1975 Aug;29(2):285–291. doi: 10.1016/0027-5107(75)90181-5. [DOI] [PubMed] [Google Scholar]
- Hyde J. E., Hearst J. E. Binding of psoralen derivatives to DNA and chromatin: influence of the ionic environment on dark binding and photoreactivity. Biochemistry. 1978 Apr 4;17(7):1251–1257. doi: 10.1021/bi00600a019. [DOI] [PubMed] [Google Scholar]
- Jeffrey A. M., Weinstein I. B., Jennette K. W., Grzeskowiak K., Nakanishi K., Harvey R. G., Autrup H., Harris C. Structures of benzo(a)pyrene--nucleic acid adducts formed in human and bovine bronchial explants. Nature. 1977 Sep 22;269(5626):348–350. doi: 10.1038/269348a0. [DOI] [PubMed] [Google Scholar]
- Keller W. Determination of the number of superhelical turns in simian virus 40 DNA by gel electrophoresis. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4876–4880. doi: 10.1073/pnas.72.12.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King H. W., Osborne M. R., Brookes P. The in vitro and in vivo reaction at the N7-position of guanine of the ultimate carcinogen derived from benzolalpyrene. Chem Biol Interact. 1979 Mar;24(3):345–353. doi: 10.1016/0009-2797(79)90082-6. [DOI] [PubMed] [Google Scholar]
- Koreeda M., Moore P. D., Wislocki P. G., Levin W., Yagi H., Jerina D. M. Binding of benzo[a]pyrene 7,8-diol-9,10-epoxides to DNA, RNA, and protein of mouse skin occurs with high stereoselectivity. Science. 1978 Feb 17;199(4330):778–781. doi: 10.1126/science.622566. [DOI] [PubMed] [Google Scholar]
- Levine A. F., Fink L. M., Weinstein I. B., Grunberger D. Effect of N-2-acetylaminofluorene modification on the conformation of nucleic acids. Cancer Res. 1974 Feb;34(2):319–327. [PubMed] [Google Scholar]
- McCann J., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals: discussion. Proc Natl Acad Sci U S A. 1976 Mar;73(3):950–954. doi: 10.1073/pnas.73.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan T., Straub K. Double-stranded DNA steroselectively binds benzo(a)pyrene diol epoxides. Nature. 1979 Feb 1;277(5695):410–412. doi: 10.1038/277410a0. [DOI] [PubMed] [Google Scholar]
- Mengle L., Gamper H., Bartholomew J. Base specificity in the binding of benzo[a]pyrene diol epoxide to simian virus 40 DNA. Cancer Lett. 1978 Sep;5(3):131–137. doi: 10.1016/s0304-3835(78)80029-9. [DOI] [PubMed] [Google Scholar]
- Osborne M. R., Beland F. A., Harvey R. G., Brookes P. The reaction of (+/-)-7alpha, 8beta-dihydroxy-9beta, 10beta-epoxy-7,8,9,10-tetrahydrobenzo(a)pyrene with DNA. Int J Cancer. 1976 Sep 15;18(3):362–368. doi: 10.1002/ijc.2910180315. [DOI] [PubMed] [Google Scholar]
- Povirk L. F., Hogan M., Dattagupta N. Binding of bleomycin to DNA: intercalation of the bithiazole rings. Biochemistry. 1979 Jan 9;18(1):96–101. doi: 10.1021/bi00568a015. [DOI] [PubMed] [Google Scholar]
- Prusik T., Geacintov N. E., Tobiasz C., Ivanovic V., Weinstein I. B. Fluorescence study of the physico-chemical properties of a benzo(a)pyrene 7,8-dihydrodiol 9,10-oxide derivative bound covalently to DNA. Photochem Photobiol. 1979 Feb;29(2):223–232. doi: 10.1111/j.1751-1097.1979.tb07043.x. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Shooter K. V., Osborne M. R., Harvey R. G. The interaction of the 7,8-dihydrodiol-9, 10-oxides of benzo(a) pyrene with bacteriophages R17 and T7. Chem Biol Interact. 1977 Nov;19(2):215–223. doi: 10.1016/0009-2797(77)90033-3. [DOI] [PubMed] [Google Scholar]
- Shure M., Vinograd J. The number of superhelical turns in native virion SV40 DNA and minicol DNA determined by the band counting method. Cell. 1976 Jun;8(2):215–226. doi: 10.1016/0092-8674(76)90005-2. [DOI] [PubMed] [Google Scholar]
- Straub K. M., Meehan T., Burlingame A. L., Calvin M. Identification of the major adducts formed by reaction of benzo(a)pyrene diol epoxide with DNA in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5285–5289. doi: 10.1073/pnas.74.12.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vähäkangas K., Nebert D. W., Pelkonen O. The DNA binding of benzo[alpha]pyrene metabolites catalysed by rat lung microsomes in vitro and in isolated perfused rat lung. Chem Biol Interact. 1979 Feb;24(2):167–176. doi: 10.1016/0009-2797(79)90005-x. [DOI] [PubMed] [Google Scholar]
- Wiesehahn G., Hearst J. E. DNA unwinding induced by photoaddition of psoralen derivatives and determination of dark-binding equilibrium constants by gel electrophoresis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2703–2707. doi: 10.1073/pnas.75.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]