Abstract
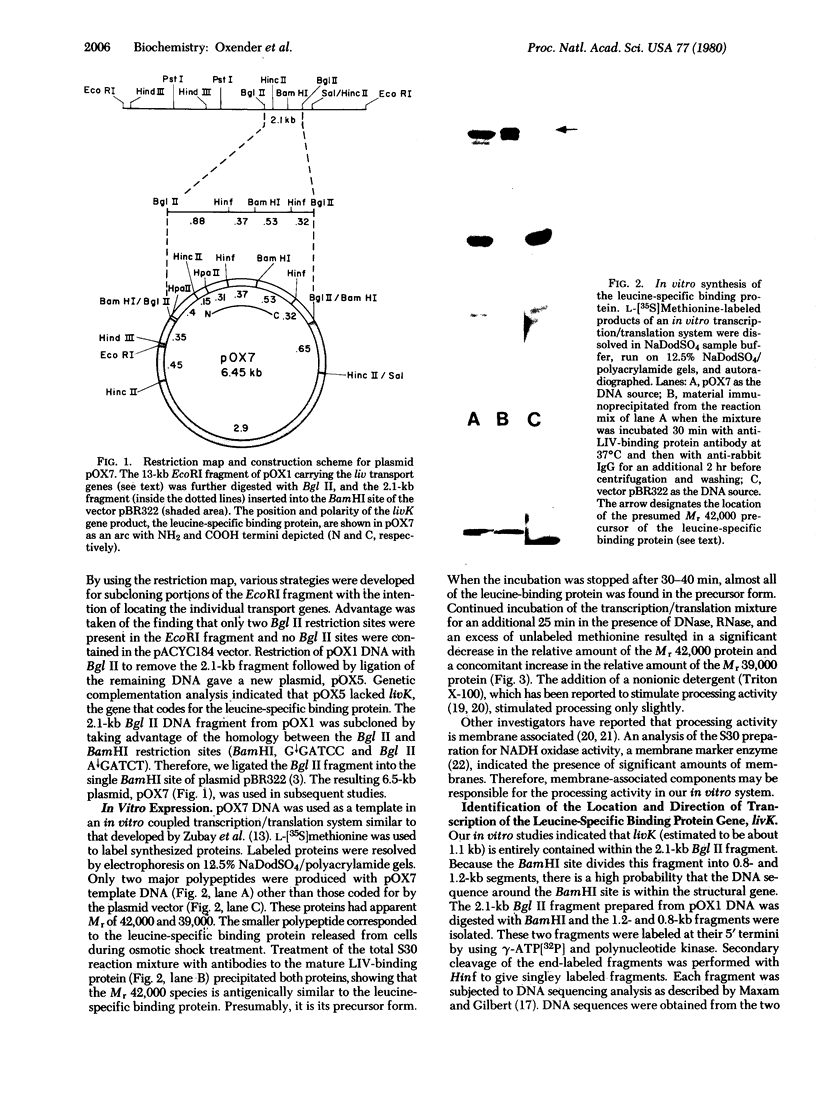
A 2.1-kilobase Bgl II DNA fragment from Escherichia coli containing livK, the gene coding for the leucine-specific binding protein, has been cloned into the BamHI site of the plasmid vector pBR322. The DNA sequence of segments of the resulting plasmid, pOX7, established the location of the livK gene and the direction of its transcription. In vitro protein synthesis directed by pOX7DNA yielded the Mr 42,000 precursor of the leucine-specific binding protein and a small amount of the Mr 39,000 mature protein. Continued incubation of the in vitro reaction mixture after DNase and RNase treatment resulted in additional processing. The DNA sequence of the beginning of livK suggested that 23 additional amino acid residues are present as an extension of the NH2 terminus of the mature protein. Amino acid sequence analysis established that the precursor has the predicted 23-residue extension. Proteolytic digestion studies with the precursor and mature forms of the leucine-specific binding protein indicate that there are conformational differences between the two. This suggests a possible role for the signal sequence in determining the conformation of the binding protein precursor that is recognized by the membrane.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Oxender D. L. Genetic separation of high- and low-affinity transport systems for branched-chain amino acids in Escherichia coli K-12. J Bacteriol. 1978 Oct;136(1):168–174. doi: 10.1128/jb.136.1.168-174.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl C. P., Marians K. J., Wu R. A general method for inserting specific DNA sequences into cloning vehicles. Gene. 1976;1(1):81–92. doi: 10.1016/0378-1119(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Bickle T. A., Pirrotta V., Imber R. A simple, general procedure for purifying restriction endonucleases. Nucleic Acids Res. 1977 Aug;4(8):2561–2572. doi: 10.1093/nar/4.8.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang C. N., Blobel G., Model P. Detection of prokaryotic signal peptidase in an Escherichia coli membrane fraction: endoproteolytic cleavage of nascent f1 pre-coat protein. Proc Natl Acad Sci U S A. 1978 Jan;75(1):361–365. doi: 10.1073/pnas.75.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice L. C., Weintraub B. D. Evidence for conformational differences between precursor and processed forms of thyroid-stimulating hormone beta subunit. J Biol Chem. 1979 Dec 25;254(24):12679–12683. [PubMed] [Google Scholar]
- Greene P. J., Heyneker H. L., Bolivar F., Rodriguez R. L., Betlach M. C., Covarrubias A. A., Backman K., Russel D. J., Tait R., Boyer H. W. A general method for the purification of restriction enzymes. Nucleic Acids Res. 1978 Jul;5(7):2373–2380. doi: 10.1093/nar/5.7.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus R. P., Zurawski G., Yanofsky C. Structural and functional analysis of cloned deoxyribonucleic acid containing the trpR-thr region of the Escherichia coli chromosome. J Bacteriol. 1979 Oct;140(1):106–113. doi: 10.1128/jb.140.1.106-113.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mandel G., Wickner W. Translational and post-translational cleavage of M13 procoat protein: extracts of both the cytoplasmic and outer membranes of Escherichia coli contain leader peptidase activity. Proc Natl Acad Sci U S A. 1979 Jan;76(1):236–240. doi: 10.1073/pnas.76.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Daniels C. J., Landick R., Gunsalus R. P., Zurawski G., Selker E., Yanofsky C. Structural and functional analysis of cloned DNA containing genes responsible for branched-chain amino acid transport in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1412–1416. doi: 10.1073/pnas.77.3.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L., Anderson J. J., Mayo M. M., Quay S. C. Leucine binding protein and regulation of transport in E. coli. J Supramol Struct. 1977;6(3):419–431. doi: 10.1002/jss.400060315. [DOI] [PubMed] [Google Scholar]
- Oxender D. L. Membrane transport proteins. Biomembranes. 1974;5:25–79. doi: 10.1007/978-1-4684-7389-6_2. [DOI] [PubMed] [Google Scholar]
- Penrose W. R., Zand R., Oxender D. L. Reversible conformational changes in a leucine-binding protein from Escherichia coli. J Biol Chem. 1970 Mar 25;245(6):1432–1437. [PubMed] [Google Scholar]
- Randall L. L., Josefsson L. G., Hardy S. J. Processing in vitro of precursor periplasmic proteins from Escherichia coli. Eur J Biochem. 1978 Dec;92(2):411–415. doi: 10.1111/j.1432-1033.1978.tb12761.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Selker E., Brown K., Yanofsky C. Mitomycin C-induced expression of trpA of Salmonella typhimurium inserted into the plasmid ColE1. J Bacteriol. 1977 Jan;129(1):388–394. doi: 10.1128/jb.129.1.388-394.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer G. V., Zurawski G., Yanofsky C. Single base-pair alterations in the Escherichia coli trp operon leader region that relieve transcription termination at the trp attenuator. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4833–4837. doi: 10.1073/pnas.75.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau S. N., Palmiter R. D., Walsh K. A. Precursor of egg white ovomucoid. Amino acid sequence of an NH2-terminal extension. J Biol Chem. 1978 Dec 25;253(24):9018–9023. [PubMed] [Google Scholar]
- Wickner W. The assembly of proteins into biological membranes: The membrane trigger hypothesis. Annu Rev Biochem. 1979;48:23–45. doi: 10.1146/annurev.bi.48.070179.000323. [DOI] [PubMed] [Google Scholar]









