Abstract
The thiol-containing tripeptide glutathione is an important cellular constituent of many eukaryotic and prokaryotic cells. In addition to its disulfide reductase activity, glutathione is known to protect cells from many forms of physiological stress. This report represents the first investigation into the role of glutathione in the Gram-positive pathogen Streptococcus pneumoniae. We demonstrate that pneumococci import extracellular glutathione using the ABC transporter substrate binding protein GshT. Mutation of gshT and the gene encoding glutathione reductase (gor) increases pneumococcal sensitivity to the superoxide generating compound paraquat, illustrating the importance of glutathione utilization in pneumococcal oxidative stress resistance. In addition, the gshT and gor mutant strains are hypersensitive to challenge with the divalent metal ions copper, cadmium, and zinc. The importance of glutathione utilization in pneumococcal colonization and invasion of the host is demonstrated by the attenuated phenotype of the gshT mutant strain in a mouse model of infection.
INTRODUCTION
The accumulation of low-molecular-weight thiol-containing compounds is a widespread feature of both eukaryotic and prokaryotic cells. The tripeptide glutathione (γ-l-glutamyl-l-cysteinyl-glycine) is the most prevalent intracellular thiol, present in almost all eukaryotes as well as aerobic proteobacteria and some Gram-positive genera (29). Glutathione biosynthesis in bacteria has been shown to be a two-step process catalyzed by ATP-dependent enzymes. Glutamic acid is first linked to cysteine by γ-glutamylcysteine synthetase (encoded by gshA), and the product of this reaction is linked to glycine by glutathione synthetase (encoded by gshB) to form GSH (29). In some Gram-positive bacteria, these two reactions are completed by a single multidomain fusion protein (10, 16). The biological roles of glutathione in bacteria are numerous and varied. It has a central role in the maintenance of normal cellular processes via prevention of the formation of aberrant disulfides. This is achieved through the action of glutathione as a protein reductant, which may be direct or via the reduction of glutaredoxin enzymes (36). A consequence of this activity is that glutathione is converted from its reduced form (GSH) to a disulfide-bonded, dimeric form (GSSG). The ratio of GSH to GSSG in the cytoplasm is tightly controlled through the action of glutathione reductase (GOR), which uses reducing equivalents from NADPH. However, the disulfide-reducing activity of GSH is not essential in some organisms due to compensation by the thioredoxin/thioredoxin reductase system (9).
GSH has also been shown to provide protection from different forms of physiological stress, including reactive oxygen and nitrogen species (ROS/RNS), toxic concentrations of metal ions, as well as osmotic and acid stress (reviewed in references 27 and 29). ROS and RNS, or the toxic by-products of the reactions of these species with cellular components, may be detoxified via direct interaction with GSH or via specific enzymes, such as glutathione peroxidase, which uses GSH to catalyze the reduction of peroxides (3). Glutathione is able to form metal complexes via nonenzymatic reactions (48) and has been associated with heavy metal tolerance in both eukaryotes (32) and prokaryotes (12, 22, 26). The concentration of GSH in Escherichia coli has been found to increase during osmotic shock, and a mutant defective in GSH synthesis shows defective growth in media with high osmolarity (31, 40). The specific mechanism behind the protection conferred by GSH in this respect is still unclear. The role of GSH in protecting bacterial cells from exposure to acidic conditions may be linked to the effect of GSH on the activity of potassium export channels (8, 35). GSH can also participate in the detoxification of specific toxic compounds, including xenobiotics and endogenous electrophiles, such as methylglyoxal, through conjugation catalyzed by glutathione S-transferase (GST) or spontaneous reactions and subsequent export or metabolism (48).
Investigations into the role of GSH in bacteria have largely been undertaken using Escherichia coli as the model organism. The role of GSH in Gram-positive species has received significantly less attention, a result of the fact that GSH utilization is significantly less common in members of this group, compared with their Gram-negative counterparts (7). The Gram-positive bacterium Streptococcus pneumoniae is a human pathogen of major significance, causing approximately 1 million deaths in children under 5 years old annually (33). The pneumococcus is carried asymptomatically in the nasopharynx of a large proportion of the human population but is capable of invading deeper sites within the body, resulting in diseases such as otitis media, pneumonia, sepsis, and meningitis (6). Analysis of published S. pneumoniae genome sequences (21) reveals that the genes required for glutathione biosynthesis are not present. However, pneumococci have been shown to take up exogenous glutathione through an as-yet-unidentified transport system (19, 39). Furthermore, the pneumococcal genome encodes proteins with significant homology to glutathione-dependent proteins (21). The work presented here represents an investigation into the role of glutathione in pneumococcal biology.
MATERIALS AND METHODS
Strains and growth conditions.
S. pneumoniae virulent serotype 2 strain D39 was routinely cultured on Columbia blood agar base supplemented with 5% (vol/vol) horse blood at 37°C/5% CO2. Blood agar plates were supplemented with 0.2 μg/ml erythromycin for selection of mutant strains. Growth experiments of pneumococci in liquid culture were performed using a casein-based semisynthetic medium (C+Y) (20) or a chemically defined medium (11) at 37°C under static conditions. For animal challenge experiments, pneumococci were grown in nutrient broth supplemented with 10% (vol/vol) horse serum.
Construction of mutant strains.
The gor and gshT genes were deleted from S. pneumoniae D39 and replaced with an erythromycin resistance cassette by transformation with a linear DNA fragment constructed by overlap extension PCR (14) by using the primers listed in Table 1. Generation of competent S. pneumoniae cells and subsequent transformation were performed as previously described (34).
Table 1.
List of primers used in this study
| Primer name | Nucleotide sequence (5′→3′) |
|---|---|
| JM214 | GAAGGAGTGATTACATGAACAA |
| JM215 | CTCATAGAATTATTTCCTCCCG |
| gor-F | CCTTATCGCCCACAGAAACA |
| gor-R | CTTAACAACACCACTCTTGC |
| gor-ERY-F | CGGGAGGAAATAATTCTATGAGTTGTAACCATGCGTTAATCG |
| gor-ERY-R | TTGTTCATGTAATCACTCCTTCATTCTCTCATTGAAAACTCC |
| gshT-F | GGCAGCTATTGCTATGACGG |
| gshT-R | ACAGCGATTTCTCCGTTTGC |
| gshT-ERY-F | CGGGAGGAAATAATTCTATGAGTTATCTACCGGCAGAAGCTG |
| gshT-ERY-R | TTGTTCATGTAATCACTCCTTCCTAGGGCAGCAAGAGATGAG |
Measurement of total intracellular glutathione.
The total intracellular glutathione concentration of S. pneumoniae strains was determined using the glutathione assay kit from Sigma. Strains were grown in C+Y medium which contains GSH from yeast extract. The GSH concentration of LB medium (which contains an equivalent concentration of yeast extract to C+Y) has been measured at approximately 26 μM (12). Strains were grown to an optical density at 600 nm (OD600) of 0.4, washed twice with phosphate-buffered saline (PBS), and resuspended in a 5% 5-sulfosalicylic acid solution. Cell suspensions were disrupted by sonication and clarified by centrifugation at 13,000 × g for 10 min. The total glutathione concentration of the extracts was then measured according to the kit manufacturer's instructions.
Glutathione reductase assays.
Glutathione reductase activity of S. pneumoniae cell extracts was determined using the colorimetric assay described by Smith et al. (41). Pneumococcal strains were grown to an OD600 of 0.4 in C+Y medium, washed once with PBS, and resuspended in assay buffer (0.1 M potassium phosphate buffer [pH 7.5], 1 mM EDTA). Cell suspensions were disrupted by sonication and clarified by centrifugation at 13,000 × g for 10 min. Extracts were added to assay buffer with 0.1 mM NADPH, 0.2 mM DTNB, and 1 mM GSSG, and the increase in absorbance at 412 nm was measured using a Spectromax spectrophotometer (Molecular Devices). Total cellular protein was measured spectrophotometrically as described by Layne (23).
Growth inhibition assays.
Pneumococci were grown in C+Y medium to an OD600 of 0.4 before being diluted 100-fold into fresh C+Y medium containing the specified stress reagent in a final volume of 200 μl in a 96-well microtiter plate. Plates were incubated at 37°C, and OD600 readings were recorded at 30-min intervals using a Spectromax spectrophotometer (Molecular Devices). All stress reagents were purchased from Sigma. Statistical analyses were performed using a Mann-Whitney U test, and a P value of <0.05 was taken as statistically significant.
Murine infection model.
Animal experiments were approved by the University of Adelaide Animal Ethics Committee. Female outbred 4- to 6-week-old CD-1 (Swiss) mice were inoculated intranasally with 5 × 106 CFU of S. pneumoniae, as described previously (46). Groups of 15 mice were inoculated for each strain, and five randomly selected mice from each group were euthanized by CO2 asphyxiation at 24, 48, and 72 h postinfection. Nasal wash, nasopharyngeal tissue, and lung and blood samples were collected and processed as previously described (24). Samples were serially diluted and plated onto blood agar plates for enumeration of viable pneumococci. Statistical analyses of log-transformed data were performed using a two-tailed Student t test; a P value of <0.05 was taken as statistically significant.
RESULTS
Identification of the S. pneumoniae glutathione transporter.
The transport mechanisms responsible for acquisition of extracellular sources of glutathione have been characterized in E. coli and Haemophilus influenzae. Despite the presence of a functional GSH biosynthetic pathway, E. coli expresses an oligopeptide ABC transporter (encoded by the yliABCD operon) to import GSH (45). The obligate human pathogen H. influenzae, on the other hand, is auxotrophic for GSH, and uptake is mediated by the dipeptide ABC transporter DppBCDF primed with the periplasmic-binding protein GbpA (47). The genome of S. pneumoniae encodes numerous ABC transporters, and we hypothesized that one of these is responsible for the acquisition of GSH that has been previously observed (19, 39). AliA, AliB, and AmiA are substrate binding proteins of ABC transporters known to transport oligopeptides in S. pneumoniae (2), and these share significant homology with YliA from E. coli and GbpA from H. influenzae. However, we found that mutation of the genes encoding these proteins did not affect the ability of S. pneumoniae strain D39 to accumulate intracellular GSH (data not shown). An ABC transporter substrate binding protein from Streptococcus mutans has recently been shown to be required for the ability of this bacterium to use GSH as a sole source of sulfur amino acids (43). These authors designated this protein GshT and demonstrated that gshT transcription was activated by the cysteine synthesis regulator CysR. S. pneumoniae possesses a homologue of gshT (SPD_0150), which we inactivated in strain D39 to investigate its potential role in GSH acquisition. Measurement of intracellular GSH levels revealed that mutation of this gene completely abolishes the accumulation of GSH in pneumococci (Fig. 1A). Pneumococci have been shown to be auxotrophic for cysteine when grown in chemically defined medium (CDM) (11). To determine if pneumococci are able to use GSH as a source of cysteine, and if this is dependent on GSH uptake via GshT as is the case in S. mutans, we conducted growth experiments in CDM. Omitting cysteine from CDM was found to completely abolish growth of S. pneumoniae D39 and gshT strains as expected; however, growth of both strains could be completely restored by supplementation with GSH (Fig. 1B). Thus, in contrast to S. mutans, transport of GSH into the cell is not required for pneumococci to use the tripeptide as a source of cysteine.
Fig 1.
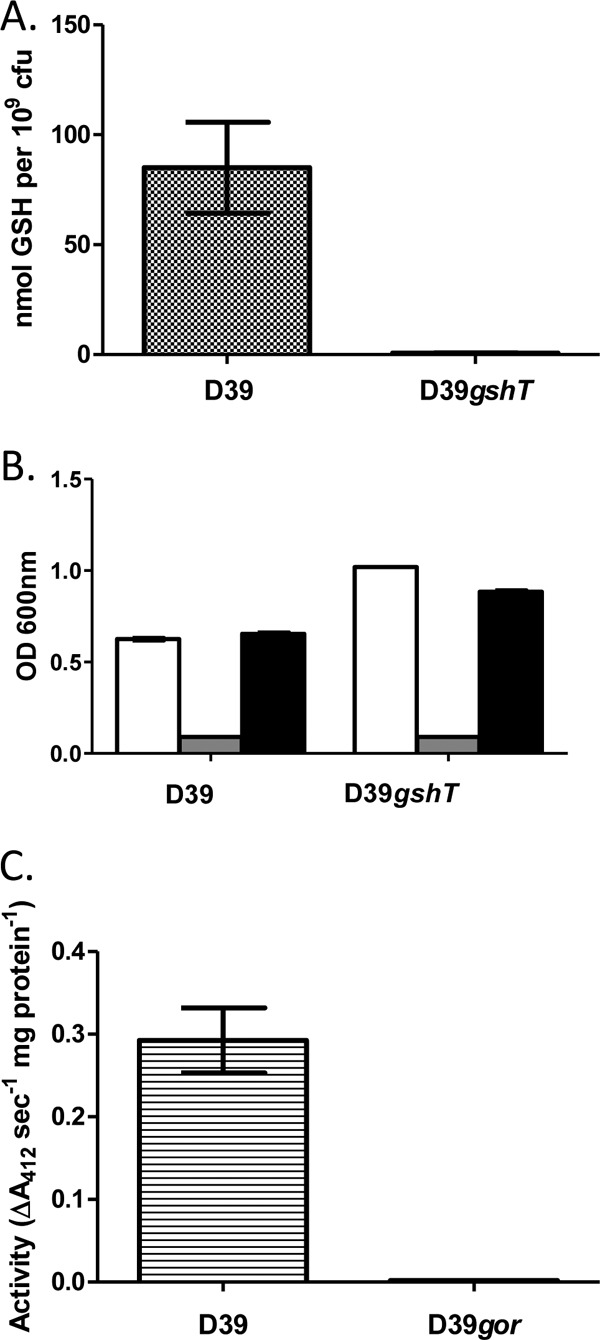
Characterization of S. pneumoniae D39gshT and D39gor mutant strains. (A) Total intracellular glutathione levels of D39 and D39gshT strains; (B) growth of D39 and D39gshT strains after 12 h in a chemically defined medium (CDM) (white bars), in CDM with cysteine omitted (gray bars), and in CDM lacking cysteine supplemented with 3.2 mM glutathione (black bars); (C) glutathione reductase activity of cell extracts of D39 and D39gor strains. Bars represent the mean values from triplicate cultures, and error bars represent the standard deviations from the means.
S. pneumoniae expresses a functional glutathione reductase.
Glutathione reductase (GOR) is a key enzyme employed by cells that utilize glutathione to recycle the dimeric oxidized form of the tripeptide (GSSG) to the reduced form (GSH). S. pneumoniae encodes a gene annotated as a glutathione reductase (gor; SPD_0685). To confirm that this gene encodes a functional glutathione reductase, the gene was deleted in strain D39, and the NADPH-dependent GSSG-reducing activity of cell extracts was measured. Mutation of gor was found to completely abolish the glutathione reductase activity of pneumococci (Fig. 1C).
Glutathione metabolism is required for pneumococci to defend against oxidative stress.
To investigate the physiological role of glutathione utilization in pneumococci, we initially examined the in vitro growth kinetics of the gshT and gor mutant strains. Given the established importance of glutathione in redox biochemistry and oxidative stress tolerance in many cell types, we conducted growth experiments under aerobic conditions. Under these conditions, the gshT mutant strain exhibited a markedly slower growth phenotype than the wild-type strain D39 (P = 0.01 during exponential phase) but was able to reach a culture density equivalent to that of wild-type pneumococci (Fig. 2A). The gor mutant strain exhibited growth kinetics nearly identical to those of wild-type D39 under the same conditions (Fig. 2B). The well-characterized disulfide reductase activity of glutathione suggested that GSH utilization may be required for pneumococcal resistance to the thiol-specific oxidant diamide. However, neither the gshT strain nor the gor strain was found to exhibit a growth phenotype significantly different from wild-type pneumococci in the presence of diamide (data not shown). Pneumococci are known to produce significant quantities of the reactive oxygen intermediate hydrogen peroxide (H2O2) through the action of pyruvate oxidase during aerobic growth (42). Interestingly, pneumococci do not express catalase, the enzyme typically involved in peroxide detoxification by bacteria, but they do encode other putative peroxidases, including a thiol peroxidase (PsaD) (30) and a glutathione peroxidase (SPD_0286). The aerobic growth properties of the gshT and gor mutant strains suggest that GSH may not play a large role in pneumococcal resistance to peroxide. This was confirmed by the fact that growth of these mutants in the presence of exogenous H2O2, or the organic peroxide tert-butyl hydroperoxide, was not significantly different from that of wild-type pneumococci (data not shown). However, the spectrum of reactive molecules encountered by a cell during conditions of oxidative stress is vast. In addition to peroxides, superoxide (O2−), produced via reduction of molecular oxygen, is a well-characterized reactive oxygen intermediate. To determine if GSH utilization by pneumococci is required for resistance to superoxide, strains were grown in the presence of the superoxide-generating compound paraquat (PQ). While both the gshT and gor mutant strains were able to grow in the presence of 0.5 mM paraquat, the growth rate of these strains was slower (P = 0.047 for both gshT and gor strains with PQ compared to no stress at exponential phase), and the maximum culture density reached was lower than that of wild-type cells under the same conditions (P = 0.004 for both gshT and gor at stationary phase) (Fig. 2A and B).
Fig 2.
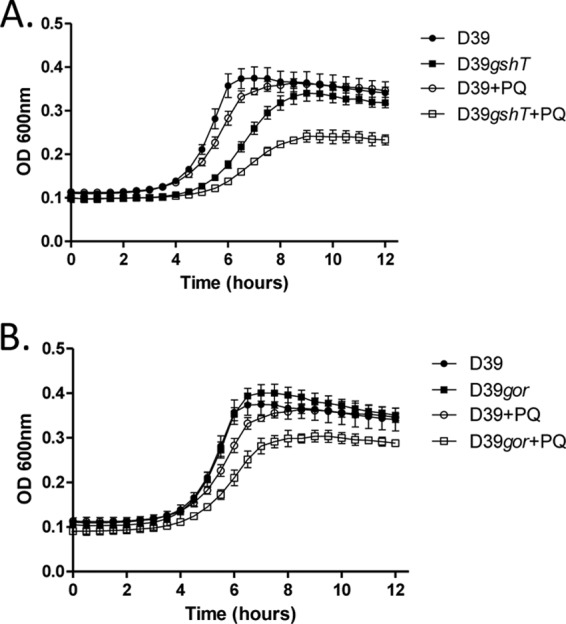
Growth of S. pneumoniae D39, and either D39gshT (A) or D39gor (B), with or without the addition of 0.5 mM paraquat (PQ). Curves represent the means from triplicate cultures, and error bars represent the standard deviations from the means.
S. pneumoniae uses glutathione to defend against toxic concentrations of divalent metal ions.
E. coli strains deficient in GSH synthesis have been shown to be less tolerant to challenge with chromium, cadmium, copper, zinc (12), mercury, and arsenite (22). The importance of metal ion resistance for pneumococcal physiology and pathogenesis has been well established (17, 37, 38). To determine if glutathione utilization is required for pneumococcal resistance to metal ions, we tested the ability of both the gshT and gor mutant strains to grow in the presence of a variety of metal ions. Inhibition of glutathione uptake was found to severely inhibit pneumococcal growth in the presence of copper, cadmium, and zinc (Fig. 3A, B, and C) (P < 0.01 for gshT compared to D39 in the presence of each metal ion at stationary phase). Mutation of glutathione reductase also resulted in severe perturbation of pneumococcal growth in the presence of copper and cadmium (Fig. 4A and B), while perturbation by zinc was less severe although still significant (Fig. 4C) (P < 0.01 for gor compared to D39 in the presence of each metal ion at stationary phase). Deletion of either gshT or gor did not significantly alter the resistance of pneumococci to cobalt, nickel, or chromium (data not shown).
Fig 3.
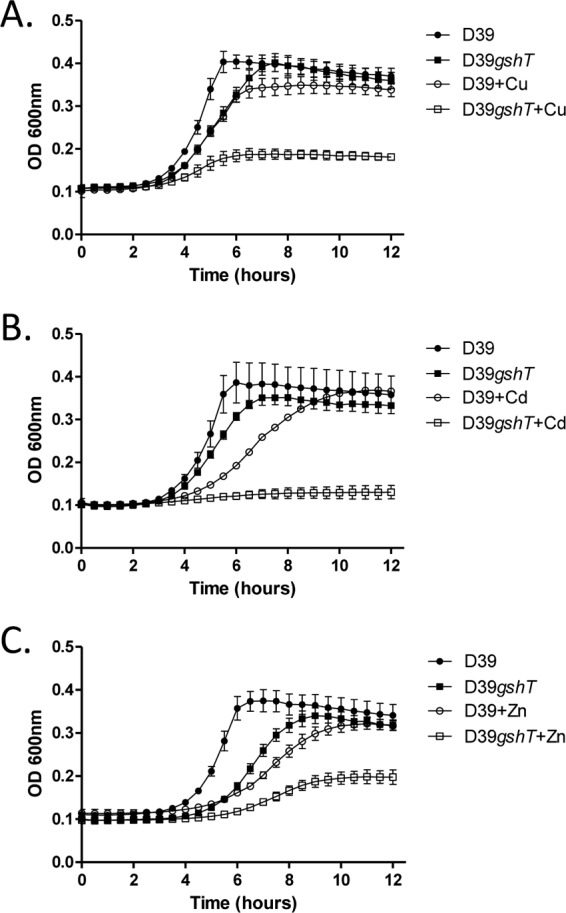
Growth of S. pneumoniae D39 and D39gshT with or without the addition of 250 μM copper sulfate (Cu) (A), 10 μM cadmium chloride (Cd) (B), and 100 μM zinc sulfate (Zn) (C). Curves represent the means from triplicate cultures, and error bars represent the standard deviations from the means.
Fig 4.
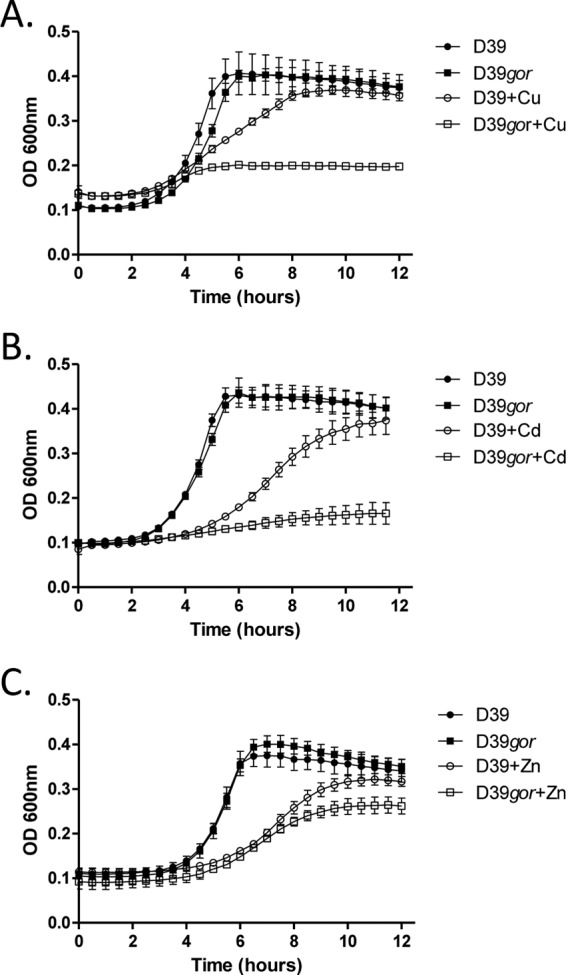
Growth of S. pneumoniae D39 and D39gor with or without the addition of 250 μM copper sulfate (Cu) (A), 10 μM cadmium chloride (Cd) (B), and 100 μM zinc sulfate (Zn) (C). Curves represent the means from triplicate cultures, and error bars represent the standard deviations from the means.
GshT is important for nasopharyngeal colonization and invasion in a mouse model of pneumococcal infection.
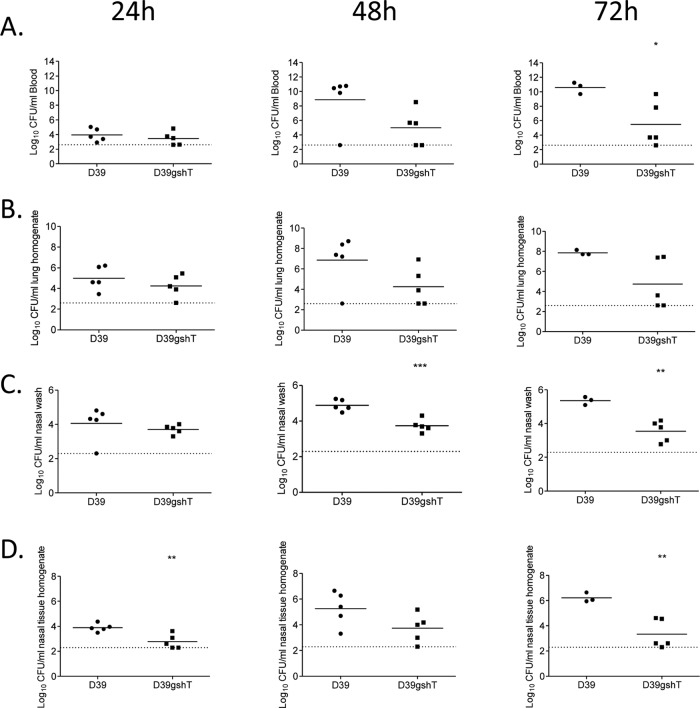
To examine the role of glutathione utilization during pneumococcal infection, the phenotypes of S. pneumoniae D39, gshT, and gor strains in a mouse model of infection were determined. Groups of mice were challenged intranasally with each strain, and the numbers of pneumococci present in various niches (nasal wash, nasal tissue, lungs, and blood) were determined at 24, 48, and 72 h postchallenge. The number of gor mutant cells recovered from these niches was found to not differ significantly from the number of WT cells recovered at any time point (data not shown). However, the gshT mutant strain exhibited a significantly attenuated phenotype (Fig. 5). The attenuation was particularly prevalent in the nasopharyngeal niche, with lower numbers of gshT mutant cells recovered from the nasal wash at 48 and 72 h (Fig. 5C) and from the nasal tissue at 24 and 72 h (Fig. 5D). The data also illustrate the importance of GshT for pneumococcal proliferation in the blood. There was no difference between the number of mutant and wild-type pneumococci present in the blood of mice at 24 h, but the gshT strain was unable to proliferate in this niche as well as wild-type D39, and a statistically significant difference in numbers recovered at 72 h was observed (Fig. 5A). A similar trend was observed in the lungs of mice, although the difference in bacterial numbers recovered from this niche did not reach statistical significance (Fig. 5B).
Fig 5.
The number of S. pneumoniae D39 and D39gshT cells recovered from blood (A), lungs (B), nasal wash (C), and nasal tissue of mice (D) 24, 48, and 72 h following intranasal challenge. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to WT. The horizontal dotted line denotes the limit of detection. NB, two mice challenged with WT succumbed to infection prior to 72 h, and so only three mice from this group could be processed at this time point.
DISCUSSION
Although the ability of S. pneumoniae to import extracellular GSH has been known for many years, the role of glutathione in pneumococcal physiology has not been previously investigated. In the present study, we have shown that the ABC transporter substrate binding protein GshT is essential for intracellular GSH accumulation in S. pneumoniae. GshT from S. pneumoniae strain D39 shares 53% identity with GshT of S. mutans, previously shown to be required for the ability of this organism to use GSH as the sole source of sulfur amino acids (43). These authors demonstrated that gshT transcription is regulated by the cysteine synthesis regulator (CysR) in S. mutans and identified putative CysR recognition sequences in the promoter regions of gshT orthologs in other streptococci, including S. pneumoniae. Interestingly, we found that although pneumococci are able to use GSH as a source of cysteine, this occurs independently of GshT. It should be noted that neither the genome of S. mutans nor of S. pneumoniae appears to encode the enzyme gamma-glutamyl transpeptidase (GGT). The hydrolysis of GSH is thought to be restricted to the activity of GGT due to the fact that the glutamate and cysteine residues are linked via the γ-carboxyl group of glutamate, as opposed to the α-carboxyl peptide linkage common in proteins. This particular peptide linkage prevents GSH from being degraded by most cellular peptidases. GGT has been shown to be essential for the ability of E. coli and Francisella tularensis to use glutathione as a source of cysteine (1, 44). The mechanism by which pneumococci obtain free cysteine from GSH remains to be elucidated, although our results indicate that it occurs in the extracellular environment.
The importance of glutathione uptake and recycling of reduced GSH from GSSG in protecting pneumococci from oxidative stress is evident from the impaired growth rate displayed by the gor and gshT mutants in the presence of the superoxide generator paraquat. Oxidative stress is an unavoidable consequence of growth in an aerobic environment, and pneumococci need to detoxify reactive species that may arise from a variety of endogenous reactions or through interactions with host cells. Pneumococci express a manganese-containing superoxide dismutase (SodA) which confers resistance to paraquat and contributes to virulence in mice (51). Superoxide is able to oxidize solvent-exposed iron-sulfur clusters, resulting in loss of enzyme activity (15). Superoxide may also oxidize compounds produced during central metabolism, such as an intermediate of the transketolase reaction (4), as well as short-chain sugars (15). Superoxide stress results in an auxotrophy for sulfur amino acids in E. coli, although the specific mechanism by which cysteine synthesis is inhibited is not clear (5). The protective effect of GSH on pneumococci under superoxide stress may either be due to a direct interaction with superoxide itself or a by-product of its reaction with cellular components. The importance of glutathione utilization in protection of other lactic acid bacteria from oxidative stress has also been established; however, the mechanism by which GSH confers this resistance appears to be different to that observed in pneumococci. Glutathione metabolism protects S. mutans from challenge with the thiol-specific oxidant diamide (39, 50), while GSH accumulation in Lactococcus lactis confers resistance of this organism to H2O2 (25). These phenotypes are most likely a result of glutathione's role in disulfide reductase and glutathione peroxidase activity.
In addition to a role in oxidative stress resistance, we also illustrated the importance of glutathione utilization in pneumococcal resistance to divalent metal ions, specifically cadmium, copper, and zinc. The importance of dedicated metal efflux transporters to pneumococcal resistance to the latter two metals has been established. Pneumococci encode a copper-responsive regulon which includes a P1-type ATPase, copA, required for copper resistance and virulence in mice (38). Zinc resistance has been demonstrated to be conferred by the cation efflux system protein CzcD (17). The importance of GSH to pneumococcal metal ion resistance likely occurs at concentrations that overwhelm the dedicated metal efflux systems. The basis of this protection is likely a result of the fact that GSH is able to form metal complexes via nonenzymatic reactions. The sulfhydryl group of the cysteine moiety of GSH, in particular, has a high affinity for metals (48). While the pneumococcal gor and gshT mutant strains exhibited similar sensitivities to copper and cadmium stress, we observed that the strain lacking GOR activity was significantly less sensitive to zinc stress than the strain deficient in GSH uptake. This may be explained by the fact that Zn(II)-GSSG complexes have been shown to occur, albeit under in vitro conditions (18). These authors demonstrate that the affinity of GSSG for zinc is lower than that of GSH, thus our observation that the pneumococcal gor mutant is still more sensitive to zinc than wild-type pneumococci might be expected.
It is interesting to note that there is considerable overlap in the mechanisms by which metal ions and superoxide intoxicate cells. Divalent metal ions can displace iron from iron-sulfur clusters, inhibiting enzyme activity (28, 49), and also interfere with cysteine synthesis (13). Thus, it might be expected that the effects of GSH on pneumococcal biology outlined in this report are achieved via protection of similar cellular processes. The attenuation of the gshT mutant observed in a murine model of infection suggests that this protection is important for both pneumococcal colonization of a host and development of invasive disease. The fact that GOR appears to be dispensable in this respect may reflect the fact that reduced GSH is likely to be the predominant form of the tripeptide in a host environment, thus pneumococci may be able to bypass the need for GOR by importing GSH and exporting GSSG and glutathione-metal conjugates. We cannot discount the possibility, however, that glutathione has roles in pneumococci in addition to those characterized here. The pneumococcal gor mutant exhibits growth kinetics similar to those of the WT under standard culture conditions, while the gshT mutant exhibits a slower growth phenotype. Thus, glutathione might carry out roles in pneumococci that are independent of GOR.
ACKNOWLEDGMENTS
This work was supported by program grant no. 565526 and project grant no. 627142 from the National Health and Medical Research Council of Australia (NHMRC). J.C.P. is an NHMRC Australia Fellow. C.T. is a Garnett Passe and Rodney Williams Memorial Foundation Fellow.
Footnotes
Published ahead of print 14 September 2012
REFERENCES
- 1. Alkhuder K, Meibom KL, Dubail I, Dupuis M, Charbit A. 2009. Glutathione provides a source of cysteine essential for intracellular multiplication of Francisella tularensis. PLoS Pathogens 5:e1000284 doi:10.1371/journal.ppat.1000284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alloing G, Dephilip P, Claverys JP. 1994. Three highly homologous membrane-bound lipoproteins participate in oligopeptide transport by the Ami system of the Gram-positive Streptococcus pneumoniae. J. Mol. Biol. 241:44–58 [DOI] [PubMed] [Google Scholar]
- 3. Arenas FA, et al. 2010. The Escherichia coli btuE gene encodes a glutathione peroxidase that is induced under oxidative stress conditions. Biochem. Biophys. Res. Commun. 398:690–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Benov L, Fridovich I. 1999. Why superoxide imposes an aromatic amino acid auxotrophy on Escherichia coli—the transketolase connection. J. Biol. Chem. 274:4202–4206 [DOI] [PubMed] [Google Scholar]
- 5. Benov L, Kredich NM, Fridovich I. 1996. The mechanism of the auxotrophy for sulfur-containing amino acids imposed upon Escherichia coli by superoxide. J. Biol. Chem. 271:21037–21040 [DOI] [PubMed] [Google Scholar]
- 6. Bogaert D, de Groot R, Hermans PWM. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144–154 [DOI] [PubMed] [Google Scholar]
- 7. Fahey RC, Brown WC, Adams WB, Worsham MB. 1978. Occurrence of glutathione in bacteria. J. Bacteriol. 133:1126–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ferguson GP, Booth IR. 1998. Importance of glutathione for growth and survival of Escherichia coli cells: detoxification of methylglyoxal and maintenance of intracellular K+. J. Bacteriol. 180:4314–4318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fernandes AP, et al. 2005. A novel monothiol glutaredoxin (Grx4) from Escherichia coli can serve as a substrate for thioredoxin reductase. J. Biol. Chem. 280:24544–24552 [DOI] [PubMed] [Google Scholar]
- 10. Gopal S, et al. 2005. A multidomain fusion protein in Listeria monocytogenes catalyzes the two primary activities for glutathione biosynthesis. J. Bacteriol. 187:3839–3847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hartel T, et al. 2012. Characterization of central carbon metabolism of Streptococcus pneumoniae by isotopologue profiling. J. Biol. Chem. 287:4260–4274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Helbig K, Bleuel C, Krauss GJ, Nies DH. 2008. Glutathione and transition-metal homeostasis in Escherichia coli. J. Bacteriol. 190:5431–5438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helbig K, Grosse C, Nies DH. 2008. Cadmium toxicity in glutathione mutants of Escherichia coli. J. Bacteriol. 190:5439–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horton RM, et al. 1993. Gene-splicing by overlap extension. Methods Enzymol. 217:270–279 [DOI] [PubMed] [Google Scholar]
- 15. Imlay JA. 2003. Pathways of oxidative damage. Annu. Rev. Microbiol. 57:395–418 [DOI] [PubMed] [Google Scholar]
- 16. Janowiak BE, Griffith OW. 2005. Glutathione synthesis in Streptococcus agalactiae—one protein accounts for gamma-glutamylcysteine synthetase and glutathione synthetase activities. J. Biol. Chem. 280:11829–11839 [DOI] [PubMed] [Google Scholar]
- 17. Kloosterman TG, van der Kooi-Pol MM, Bijlsma JJE, Kuipers OP. 2007. The novel transcriptional regulator SczA mediates protection against Zn2+ stress by activation of the Zn2+-resistance gene czcD in Streptococcus pneumoniae. Mol. Microbiol. 65:1049–1063 [DOI] [PubMed] [Google Scholar]
- 18. Krezel A, Wojcik J, Maciejczyk M, Bal W. 2011. Zn(II) complexes of glutathione disulfide: structural basis of elevated stabilities. Inorg. Chem. 50:72–85 [DOI] [PubMed] [Google Scholar]
- 19. Kumaresan KR, Springhorn SS, Lacks SA. 1995. Lethal and mutagenic actions of N-methyl-N′-nitro-N-nitrosoguanidine potentiated by oxidized glutathione, a seemingly harmless substance in the cellular environment. J. Bacteriol. 177:3641–3646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lacks S, Hotchkiss RD. 1960. A study of the genetic material determining an enzyme activity in pneumococcus. Biochim. Biophys. Acta 39:508–518 [DOI] [PubMed] [Google Scholar]
- 21. Lanie JA, et al. 2007. Genome sequence of Avery's virulent serotype 2 strain D39 of Streptococcus pneumoniae and comparison with that of unencapsulated laboratory strain R6. J. Bacteriol. 189:38–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Latinwo LM, Donald C, Ikediobi C, Silver S. 1998. Effects of intracellular glutathione on sensitivity of Escherichia coli to mercury and arsenite. Biochem. Biophys. Res. Commun. 242:67–70 [DOI] [PubMed] [Google Scholar]
- 23. Layne E. 1957. Spectrophotometric and turbidimetric methods for measuring proteins. Methods Enzymol. 3:447–454 [Google Scholar]
- 24. LeMessurier KS, Ogunniyi AD, Paton JC. 2006. Differential expression of key pneumococcal virulence genes in vivo. Microbiology 152:305–311 [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Hugenholtz J, Abee T, Molenaar D. 2003. Glutathione protects Lactococcus lactis against oxidative stress. Appl. Environ. Microbiol. 69:5739–5745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lima AIG, Corticeiro SC, Figueira EMDP. 2006. Glutathione-mediated cadmium sequestration in Rhizobium leguminosarum. Enzyme. Microb. Technol. 39:763–769 [Google Scholar]
- 27. Lushchak VI. 2012. Glutathione homeostasis and functions: potential targets for medical interventions. J. Amino Acids 2012:736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Macomber L, Imlay JA. 2009. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. U. S. A. 106:8344–8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Masip L, Veeravalli K, Georgiou G. 2006. The many faces of glutathione in bacteria. Antioxid. Redox Signal. 8:753–762 [DOI] [PubMed] [Google Scholar]
- 30. McAllister LJ, et al. 2004. Molecular analysis of the psa permease complex of Streptococcus pneumoniae. Mol. Microbiol. 53:889–901 [DOI] [PubMed] [Google Scholar]
- 31. McLaggan D, Logan TM, Lynn DG, Epstein W. 1990. Involvement of gamma-glutamyl peptides in osmoadaptation of Escherichia coli. J. Bacteriol. 172:3631–3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mendoza-Cozatl D, Loza-Tavera H, Hernandez-Navarro A, Moreno-Sanchez R. 2005. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 29:653–671 [DOI] [PubMed] [Google Scholar]
- 33. O'Brien KL, et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374:893–902 [DOI] [PubMed] [Google Scholar]
- 34. Pozzi G, et al. 1996. Competence for genetic transformation in encapsulated strains of Streptococcus pneumoniae: two allelic variants of the peptide pheromone. J. Bacteriol. 178:6087–6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riccillo PM, et al. 2000. Glutathione is involved in environmental stress responses in Rhizobium tropici, including acid tolerance. J. Bacteriol. 182:1748–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ritz D, Beckwith J. 2001. Roles of thiol-redox pathways in bacteria. Annu. Rev. Microbiol. 55:21–48 [DOI] [PubMed] [Google Scholar]
- 37. Rosch JW, Gao G, Ridout G, Wang YD, Tuomanen EI. 2009. Role of the manganese efflux system mntE for signalling and pathogenesis in Streptococcus pneumoniae. Mol. Microbiol. 72:12–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shafeeq S, et al. 2011. The cop operon is required for copper homeostasis and contributes to virulence in Streptococcus pneumoniae. Mol. Microbiol. 81:1255–1270 [DOI] [PubMed] [Google Scholar]
- 39. Sherrill C, Fahey RC. 1998. Import and metabolism of glutathione by Streptococcus mutans. J. Bacteriol. 180:1454–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smirnova GV, Krasnykh TA, Oktyabrsky ON. 2001. Role of glutathione in the response of Escherichia coli to osmotic stress. Biochemistry 66:973–978 [DOI] [PubMed] [Google Scholar]
- 41. Smith IK, Vierheller TL, Thorne CA. 1988. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 175:408–413 [DOI] [PubMed] [Google Scholar]
- 42. Spellerberg B, et al. 1996. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 19:803–813 [DOI] [PubMed] [Google Scholar]
- 43. Sperandio B, et al. 2010. Three paralogous LysR-type transcriptional regulators control sulfur amino acid supply in Streptococcus mutans. J. Bacteriol. 192:3464–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Suzuki H, Hashimoto W, Kumagai H. 1993. Escherichia coli K-12 can utilize an exogenous gamma-glutamyl peptide as an amino acid source, for which gamma-glutamyl transpeptidase is essential. J. Bacteriol. 175:6038–6040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suzuki H, Koyanagi T, Izuka S, Onishi A, Kumagai H. 2005. The yliA, -B, -C, and -D genes of Escherichia coli K-12 encode a novel glutathione importer with an ATP-binding cassette. J. Bacteriol. 187:5861–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Trappetti C, Ogunniyi AD, Oggioni MR, Paton JC. 2011. Extracellular matrix formation enhances the ability of Streptococcus pneumoniae to cause invasive disease. PLoS One 6:e19844 doi:10.1371/journal.pone.0019844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vergauwen B, Elegheert J, Dansercoer A, Devreese B, Savvides SN. 2010. Glutathione import in Haemophilus influenzae Rd is primed by the periplasmic heme-binding protein HbpA. Proc. Natl. Acad. Sci. U. S. A. 107:13270–13275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang W, Ballatori N. 1998. Endogenous glutathione conjugates: occurrence and biological functions. Pharmacol. Rev. 50:335–355 [PubMed] [Google Scholar]
- 49. Xu FF, Imlay JA. 2012. Silver(I), mercury(II), cadmium(II), and zinc(II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl. Environ. Microbiol. 78:3614–3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yamamoto Y, Kamio Y, Higuchi M. 1999. Cloning, nucleotide sequence, and disruption of Streptococcus mutans glutathione reductase gene (gor). Biosci. Biotechnol. Biochem. 63:1056–1062 [DOI] [PubMed] [Google Scholar]
- 51. Yesilkaya H, et al. 2000. Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae. Infect. Immun. 68:2819–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]