Abstract
Individuals with social phobia report experiencing recurrent negative images of themselves in social situations. However, research on the role of visual imagery in social phobia has relied exclusively on self-report measures. In the first study, we used a visual image generation task with social-threat and neutral stimuli to test the hypothesis that individuals with Generalized Social Phobia (GSP, n = 32) are more efficient at generating images related to social-threat words than are non-anxious-controls (NACs, n = 28). We found that, contrary to our hypothesis, the GSP and NAC groups did not differ in speed of generating images related to social-threat words. However, the GSP group was significantly slower than the NAC group at generating images related to neutral words. To further examine the generation of neutral images, we conducted a second study using a well-validated neutral image generation task, and found that the GSP group (n = 24) was slower to generate neutral images than were the NAC (n = 21) and anxious-control (AC, n = 15) groups, which did not differ from each other. Taken together, findings from the two studies suggest that social phobia is characterized by less efficient generation of neutral images.
Keywords: Social phobia, imagery, information-processing
Introduction
Social phobia is a common and debilitating condition, associated with social and occupational impairment, and considerable comorbidity with other psychiatric conditions (e.g., Schneier, Johnson, Hornig, Liebowitz, & Weissman, 1992; Stein & Kean, 2000). Generalized Social Phobia (GSP) is a subtype of social phobia in which the fears include most social situations. Cognitive-behavioral models of GSP posit that during social situations, intrusive, negative, visual self-images at the expense of benign images may serve to maintain social anxiety (e.g., Clark & Wells, 1995; Rapee & Heimberg, 1997). A visual image is an event that possesses sensory qualities of the visual modality experienced in the absence of any corresponding sensory stimulation (Richardson, 1994, p.1). Indeed, research suggests that socially anxious individuals report spontaneous, distorted, negative visual self-images (Hackmann, Surawy, & Clark, 1998), which may elicit observable anxious behaviors as well as exaggerated negative self-appraisal of performance (Hirsch, Clark, Mathews, & Williams, 2003; Hirsch, Meynen, & Clark, 2004).
Although extant research on the nature of visual mental imagery in social anxiety has been informative, one drawback is that it has relied solely on self-report measures. The validity of self-report data may be limited because, although individuals with social phobia can report on the content of their images, they may not always be cognizant of, or be able to report accurately on, the process of image generation (MacLeod, 1993). For example, it is unlikely that individuals with social phobia can accurately report how efficient they are in forming these images. Furthermore, the studies described above have focused primarily on the content or representational characteristics of the individual s image, rather than assessing the processes the individual uses in generating the image. Finally, previous research on imagery in social phobia has examined exclusively negatively-valenced self-images, rather than imagery ability as a more general process (i.e., imagery ability for neutral and socially threatening information).
Study 1
Theoretical models of social phobia also suggest that, because negative self-images are accessed repeatedly in the context of fear-provoking social situations, they may be over-practiced, thereby leading them to be generated more efficiently in individuals with GSP compared to non-anxious controls (NACs). Thus, in Study 1, we tested the hypothesis that individuals with GSP are more efficient at generating images related to social-threat words than are NACs.
Weber and Castleman (1970) were the first to study visual imagery based on the perceptual properties of letters of the alphabet. Based on Weber and Castleman s study, Brown, Kosslyn, Breiter, Baer, and Jenike (1994) developed a paradigm that allows us to examine the generation of images related to specific words. In this task, participants are asked to compare the heights of the first and last letters of words that are presented auditorily. The mode of stimulus presentation requires participants to imagine the word in lowercase letters. In the control task, participants are asked to compare the heights of the first and last letters of words that are presented visually, in which case presumably there is no involvement of visual imagery. Brown et al. used this task to test reality monitoring (distinguishing between something that was imagined and something that was actually perceived) in patients with OCD. In the current study, we used the task to examine the ability to generate images related to social-threat words and to neutral words in individuals with GSP, compared to NAC participants. Because visual images are processed serially (Weber & Castleman, 1970), slower responses to long words compared to short words presented auditorily indicate speed of image generation. We did not expect a difference in the speed of response to long words and short words presented visually, because presumably these trials do not require the image of the words to be generated serially.
We defined image generation efficiency as the difference in speed of generating short words versus long words. We hypothesized that individuals with GSP would be more efficient than NACs at generating images related to socially threatening material.
Method
Participants
Participants comprised 32 individuals with GSP and 28 NAC individuals. All participants were recruited through posted announcements in community settings and local newspapers that described the program and provided a telephone contact number. Diagnostic assessment was based on an initial telephone screening followed by an in-person diagnostic interview using the Structured Clinical Interview for the Diagnostic and Statistical Manual (4th ed.; DSM-IV; American Psychiatric Association, 2000) Axis I Disorders (SCID-I; First, Spitzer, Gibbon, & Williams, 1994). All interviews were videotaped for reliability assessment, and a randomly selected portion of the interviews (55%) was rated by a second, independent clinician. Interrater agreement for the GSP diagnosis was good (κ = .89). Exclusionary criteria were evidence of suicidal intent, current substance abuse or dependence, current or past schizophrenia, bipolar disorder, or developmental disorders. All participants received $10 per hour as compensation for their participation.
Participants completed the self-report version of the Liebowitz Social Anxiety Scale (LSAS; Liebowitz, 1987), the Beck Depression Inventory-II (BDI-II; Beck, Steer, & Brown, 1987) and the State-Trait Anxiety Inventory (STAI-S/T; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983). The groups did not differ on age t(58) = 1.63, p > .10, education, t(58) = .70, p > .48, or sex, χ2(1) = .90, p > .34. The mean LSAS score for the GSP group was within the severe range for normed clinical samples (Fresco et al., 2001). As expected, individuals with GSP had significantly higher scores on the LSAS, t(58) = 21.35, p < .001, STAI-state, t(58) = 9.42, p < .001, STAI-trait, t(58) = 10.48, p < .001, and BDI, t(58) = 8.18, p < .001, than did individuals in the NAC group. Demographic information and means and standard deviations for the self-report measures are presented in Table 1.
Table 1.
Demographic and Self-Report Data
| Group | ||
|---|---|---|
| GSP (n = 32) | NAC (n = 28) | |
| % Female | 65.63a | 53.57a |
| Age | 30.69 (9.74)a | 35.64 (13.68)a |
| Education | 13.84 (4.74)a | 14.71 (4.83)a |
| LSAS | 82.34 (16.13)a | 12.36 (6.79)b |
| STAI-State | 49.25 (10.51)a | 27.18 (7.02)b |
| STAI-Trait | 55.91 (10.34)a | 29.32 (9.14)b |
| BDI | 22.91 (11.32)a | 3.08 (5.48)b |
GSP = Generalized Social Phobia; NAC = Non-Anxious Control; AC = Anxious Control; LSAS = Liebowitz Social Anxiety Scale; STAI = Speilberger State-Trait Anxiety Inventory-State/Trait Form; BDI = Beck Depression Inventory. Means with different subscripts differ significantly.
Materials
For this task we employed the imagery paradigm used in the study by Brown, Kosslyn, Breiter, Baer, and Jenike (1994). Practice stimuli comprised eight color words (e.g., blue) and experimental stimuli comprised a total of 48 words, 24 socially-threatening (e.g., stupid) and 24 neutral (e.g., closet). We conducted a median-split on the length of the words used as experimental stimuli and classified words with seven or fewer letters as short words and those with eight or more letters as long words. During the task, participants were required to decide whether the last letter of the word in lowercase letters was taller than the first letter of the word. For example, in the word “couch” the last letter is taller than the first letter. Half of each type of word had taller last letters than first letters (e.g., stupid, couch) and the other half comprised three subtypes of words in which the last letter was not taller than the first: These included words in which both the first and last letters were short (e.g., plumbing, worthless), both the first and last were tall (e.g., bathtub, foolish), and the first was taller than the last (e.g., lemon, failure). For the imagery task, words were presented auditorily, in a voice recording through the computer s speakers; for the perception control task, words were presented visually, as a typed word on the computer screen in black ink on a tan background in Microsoft Office Word s font style “Times New Roman,” font size 48. Therefore, on trials with visual stimuli, participants were able to discriminate letter height based on visual information, whereas on trials with auditory stimuli, participants were required to visualize the word to make the letter height discrimination. Thus, words in this condition were evaluated using mental imagery (Weber & Castleman, 1970). The 48 words were divided into two sets, matched on word length and frequency. For half of the participants, the first word set was presented visually and the second half presented auditorily. For the other half of participants, the first set was presented auditorily and the second half visually. Each participant was presented the 48 trials in a different random order.
Procedure
Participants were told that the purpose of the experiment was to assess how people with or without anxiety process information. Participants read and signed an informed consent form. They then completed a demographics questionnaire and other self-report measures. Following completion of the questionnaires, participants completed the imagery and the perception control task. Participants were debriefed at the end of the session.
Imagery and Perception Control Task
First, the experimenter read instructions for the task aloud as participants read along on the computer screen. The experimenter answered any questions and then administered the practice trials. The experimenter remained in the testing room during the practice trials to provide corrective feedback and answer questions. Once the participant completed the practice task and could reiterate the task instructions, the experimenter administered the experimental trials and left the testing room. During the task, each type of trial began with the presentation of a fixation cross in the center of the computer screen for 500ms. Following a 200ms blank screen, participants were presented with a word, either printed visually in the center of the computer screen or auditorily through a voice recording presented through the computer speakers. Participants were asked to respond “Yes” or “No” to whether the last letter of the word was taller than the first letter by clicking the corresponding mouse button. On visual trials, the printed word remained on the screen until a response was made. Participants response times were recorded for each trial.
Results
Missing Data
One participant who completed the image generation task (auditory condition) did not complete the perception control task (visual condition). As a result, N = 60 for the following analyses for the imagery task and N = 59 for analyses for the perception task.
Imagery Task
For the imagery task we examined the trials in which the words were presented auditorily. Outliers, defined as trials with response latencies greater than ± 3 standard deviations from the mean response latency for that participant, were eliminated. This resulted in the removal of 1.84% of the trials (GSP: 1.91%; NAC: 1.75%). Response latencies for trials with incorrect responses were also eliminated (12.36% of remaining trials; GSP: 13.43%; NAC: 11.08%).
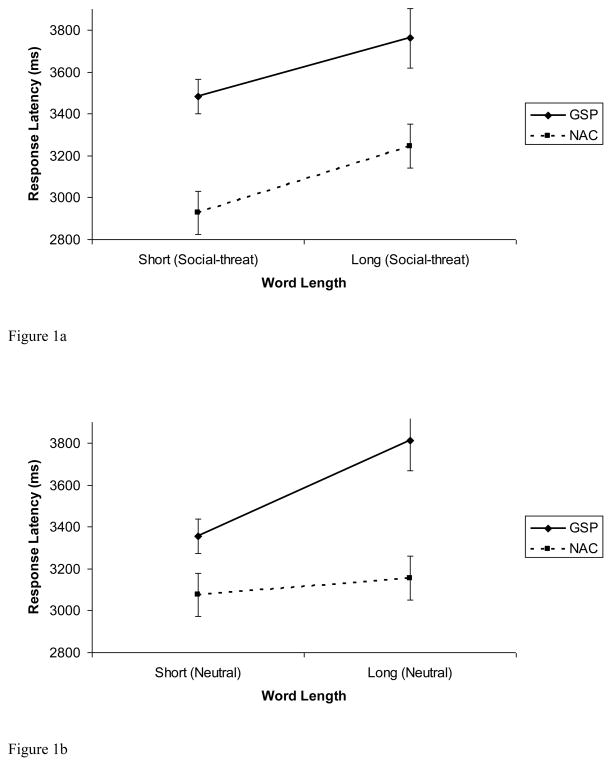
We submitted response latencies to a 2 (Group: GSP, NAC) × 2 (Word-type: Social-threat, Neutral) x 2 (Word-length: Short, Long) ANOVA with repeated measurement on the second and third factors (see Figures 1a and 1b). The main effect of Group was significant, F(1, 58) = 5.28, p < .03, η2 = .08. The main effect of Word-type was not significant, F(1, 58) = .01, p > .92, η2 = .001. There was a significant main effect of Word-length, F(1, 58) = 24.81, p < .001, η2 = .30. This was modified by a significant interaction of Group × Word-type × Word-length, F(1, 58) = 3.98, p = .05, η2 = .06.
Figure 1.
Figure 1a. Response latencies for short and long social-threat words in the image generation task for individuals with generalized social phobia (GSP) and non-anxious control (NAC) participants.
Figure 1b. Response latencies for short and long neutral words in the image generation task for individuals with generalized social phobia (GSP) and non-anxious control (NAC) participants.
To break down this 3-way interaction, we submitted response latencies to a 2 (Group: GSP, NAC) × 2 (Word-length: Short, Long) ANOVA for each Word-type. For social-threat words, there was a significant main effect of Group, F(1, 58) = 5.53, p = .02, η2 = .09, and Word-length, F(1, 58) = 19.33, p < .001, η2 = .25, but the Group × Word-length interaction was not significant, F(1, 58) = .08, p > .77, η2 = .001.
For neutral words, there was a significant main effect of Group, F(1, 58) = 4.41, p = .04, η2 = .07, and Word-length, F(1, 58) = 9.87, p = .003, η2 = .15, that was modified by a significant Group × Word-length interaction, F(1, 58) = 4.89, p = .03, η2 = .08. Analysis of simple effects revealed that the GSP group was significantly slower than the NAC group in responding to long neutral words, t(58) = 2.66, p = .01, d = .70, but there was no significant difference in the groups response to short neutral words, t(58) = 1.21, p = .23, d = .32.
These results suggest that, contrary to our hypothesis, GSP participants were not significantly faster than NAC participants at generating images related to social-threat words. Moreover, GSP participants were significantly slower than NAC participants at generating images related to neutral words.
Perception Control Task
For the perception control task we examined the trials in which the words were presented visually. Response latencies for trials with incorrect responses were eliminated. Outliers, defined as trials with response latencies greater than ± 3 standard deviations from the mean response latency for that participant, were eliminated. This resulted in the removal of 0.37% of the trials (GSP: 0.34%; NAC: 0.40%). Response latencies for trials with incorrect responses were also eliminated (9.84% of remaining trials; GSP: 9.94%; NAC: 9.72%).
We submitted response latencies to a 2 (Group: GSP, NAC) × 2 (Word-type: Social-Threat, Neutral) × 2 (Word-length: Short, Long) ANOVA with repeated measurement on the second and third factors. The main effect of Group was not significant, F(1, 57) = 3.13, p = .08, η2 = .05. The main effect of Word-type was not significant, F(1, 57) = .51, p = .48, η2 = .009, nor was the main effect of Word-length, F(1, 57) = 1.47, p = .23, η2 = .03, or the Group × Word-type × Word-length interaction, F(1, 57) = 2.70, p > .10, η2 = .05. Thus, the GSP and NAC groups did not perform differently on the perception control task.
Taken together, results from the imagery task show, as depicted in Figure 1a, for social-threat words, the slope for the GSP group (difference between response latencies for long and short words) was similar to the slope for the NAC group, indicating that participants with GSP spent the same amount of time generating images related to socially threatening material when compared to NACs. Figure 1b shows that, for neutral words, the slope for the GSP group was steeper than the slope for the NAC group, indicating that participants with GSP spent more time generating images related to neutral material than did the NAC group. Thus, in Study 1 we found no evidence for more efficient generation of images related to social-threat words in individuals with GSP, compared to NACs.
However, because we found evidence for impaired processing of images related to neutral words in individuals with GSP relative to NACs, we decided to further examine the generation of neutral images in social phobia. We conducted Study 2 using a classic neutral image generation task that has been well-validated in previous research (e.g., Appollonio, Grafman, Clark, Kosslyn, & Frattola, 1996; Dror, Kosslyn, & Waag, 1993; Emmorey, Kosslyn, & Bellugi, 1993; Kosslyn, Cave, Provost, & von Gierke, 1988; Kosslyn, Margolis, Barrett, Goldknopf, & Daly, 1990; Kosslyn et al., 1993; Kosslyn, Thompson, & Alpert, 1997; Morrison, Amir, & Taylor, 2011). Moreover, we added an anxious control group to test the specificity of our findings to social phobia.
Study 2
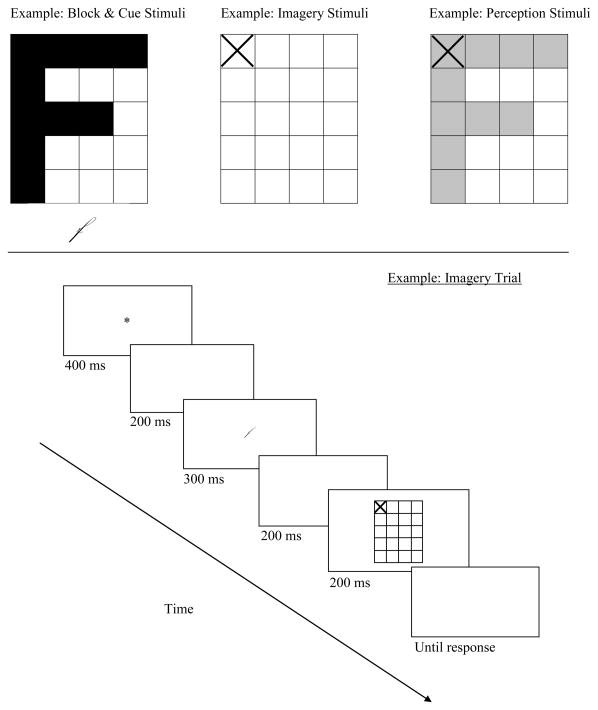
To examine the generation of neutral images in social phobia we used a well-validated image generation task first developed by Podgorny and Shepard (1978) and modified by Kosslyn et al., 1988). In this task, participants first become familiar with the appearance of uppercase block letters set in 4 × 5 grids (Figure 2). Next, they complete a computerized image generation task. In each trial, a lowercase script letter is followed by an empty grid. An X in one of the cells of the grid prompts participants to decide if the X would have been covered by the corresponding block letter if the block version of the just-cued letter were superimposed on the grid. Half of the probes fall on a segment of the letter that is typically drawn early in the letter-writing process and half fall on a segment of the letter that is typically drawn late in the letter-writing process. Previous research suggests that in the image generation task participants respond faster to early probe locations compared to late probe locations, suggesting that an image of the letter is generated segment by segment (Kosslyn et al., 1988). Responses to probes located on segments of the letter typically drawn later in the letter-writing process compared to those drawn early index speed of image generation.
Figure 2.
Example stimuli and example imagery trial.
In addition to the image generation task, participants also complete a perception task in which the gray block letters remain in the grids while participants decide if the X is placed in a cell of the grid covered by the letter (Figure 2). This task is used as a control task to show that participants do not take longer to respond to late probe locations compared to early probe locations when they can see the grayed letter displayed on the grid (i.e., when they do not need to create the image segment by segment).
Variations of this task have been used in studies of group differences in imagery in pilots (Dror et al., 1993), American Sign Language signers (Emmorey et al., 1993), children (Kosslyn et al., 1990), and patients with Parkinson s disease (Appollonio et al., 1996), and socially anxious college students (Morrison et al., 2011). Most relevant to the current study, Morrison et al. (2011) used this neutral image generation task to assess image generation ability in subclinical social anxiety. They found that socially anxious college students were slower to generate images of letters of the alphabet compared to their non-anxious counterparts. To date, only one study has used this task in individuals with emotional disorders: Zarrinpar, Deldin, and Kosslyn (2006) found that, compared to control participants, depressed individuals were not impaired in their ability to generate neutral images. Neuroimaging studies that have used this task (e.g., Kosslyn et al., 1993; Kosslyn, Thompson, & Alpert, 1997) suggest that the neural activation that underlies top-down processing during object identification of a percept also occurs when generating a visual mental image; additionally, the neural activation that underlies shifting attention to the segments of the percept also occurs when scanning the mental image. In other words, “during imagery, the process that primes the representation of an expected object or part during perception primes that representation so strongly that the shape itself is reconstructed in the visual buffer…Once the image is present in the visual buffer… it is thereafter processed the same way that perceptual input is processed” (Kosslyn et al., 1997, p. 322).
With the aim of replicating the finding from Study 1 regarding neutral image generation, in Study 2 we hypothesized that individuals with GSP would be less efficient in generating neutral images compared to NAC participants. This hypothesis was based in part on the results of Study 1 and in part due to similar findings from Morrison et al. (2011) in subclinical socially anxious college students. We also included an anxious control (AC) group comprising a mixed group of individuals with Generalized Anxiety Disorder (GAD) and Obsessive-Compulsive Disorder (OCD). This allowed us to determine whether any obtained effects were specific to GSP or characteristic of anxious individuals in general. We hypothesized that individuals with GSP would be slower in generating neutral images compared to NAC participants. We did not have a priori hypotheses regarding the AC group.
Method
Participants
Participants comprised 24 individuals with GSP, 21 NAC, and 15 AC participants. The AC group comprised a mixed anxiety group of 6 individuals with GAD and 9 individuals with OCD. Participant recruitment, diagnostic assessment, exclusionary criteria, and monetary compensation were identical to what is described above for Study 1.
Participants in the GSP and NAC groups completed the self-report version of the LSAS. Participants in all three groups completed the BDI-II and the STAI-S/T. The groups did not differ in age F(2, 56) = .15, p > .86, education, F(2, 53) = .49, p > .61, or sex, χ2(2) = .99, p > .61. The mean LSAS score for the GSP group was within the severe range for normed clinical samples (Fresco et al., 2001). As expected, individuals with GSP had significantly higher scores on the LSAS, t(43) = 22.49, p < .001, STAI-state, t(43) = 12.22, p < .001, STAI-trait, t(43) = 11.01, p < .001, and BDI, t(40) = 7.92, p < .001, than did individuals in the NAC group, but did not differ from the AC group on the STAI-state, t(36) = .50, p > .62, STAI-trait, t(38) = 1.36, p > .18, and BDI, t(36) = .90, p > .37. The AC group had higher scores on the STAI-state, t(33) = 7.42, p < .001, STAI-trait, t(33) = 5.93, p < .001, and BDI, t(30) = 4.52, p < .001, than did individuals in the NAC group. Demographic information and means and standard deviations for the self-report measures are presented in Table 2.
Table 2.
Demographic and Self-Report Data
| Group | |||
|---|---|---|---|
| GSP (n = 24) | NAC (n = 21) | AC (n = 15) | |
| % Female | 45.83a | 61.11a | 50.00a |
| Age | 32.96 (11.93)a | 33.76 (12.05)a | 35.21 (13.04)a |
| Education | 15.29 (1.92)a | 14.29 (5.54)a | 15.36 (2.46)a |
| LSAS | 80.88 (12.41)a | 10.00 (7.87)b | __ |
| STAI-State | 54.88 (9.58)a | 25.69 (5.63)b | 52.86 (15.41)a |
| STAI-Trait | 59.21 (9.94)a | 25.74 (10.44)b | 53.29 (17.10)a |
| BDI | 24.38 (11.22)a | 2.61 (3.53)b | 20.36 (16.24)a |
GSP = Generalized Social Phobia; NAC = Non-Anxious Control; AC = Anxious Control; LSAS = Liebowitz Social Anxiety Scale; STAI = Speilberger State-Trait Anxiety Inventory-State/Trait Form; BDI = Beck Depression Inventory. Means with different subscripts differ significantly.
Materials
The image generation task and the perception control task were administered on a computer with a color monitor using the Delphi programming language. Two numbers (1, 7) and 13 letters (A, B, C, E, F, G, H, J, L, O, P, S, U) served as practice and experimental stimuli, respectively. Block stimuli were created by filling in cells of a 4 × 5 grid to form segments of the numbers and uppercase letters, resulting in angular shapes with no curves (Figure 2). Script stimuli (i.e., cues) comprised the numbers and letters in lowercase form using Microsoft Word s French Script MT font style, font size 24. During the study phase, a script cue was presented just below its corresponding block stimulus. During the imagery task, the script cue indicated which block letter should be created as an image in order to complete the task. Participants were then presented with an empty 4 × 5 grid with the X probe in one of the cells. For the perception trials, the X probe was again present in one of the cells but the grid also contained a visible block stimulus in light gray (Figure 2).
Each stimulus was presented eight times, twice in each of the four possible probe locations: (1) in a cell on an early segment (i.e., true/early), (2) adjacent to a cell on an early segment (i.e., false/early), (3) in a cell on a late segment (i.e., true/late), and (4) adjacent to a cell on a late segment (i.e., false/late) (for review, see Kosslyn et al., 1988). Participants saw 16 practice trials followed by 104 experimental trials. Trials were presented in a different random order to each participant.
Procedure
We told participants that the purpose of the experiment was to assess how people with or without anxiety process information. Participants read and signed an informed consent form. They then completed a demographics questionnaire and other self-report measures. Following completion of the questionnaires, participants completed the study phase, the imagery generation task, and the perception control task. Participants were debriefed at the end of the session.
Study Phase
Participants were asked to memorize the appearance of the block and script stimuli presented simultaneously on the computer screen. Participants were given as much time as they needed to study the letter pairs. When participants reported that they had memorized the stimuli, the experimenter administered a test sheet with empty 4 × 5 grids. A script cue was presented below each of the empty grids indicating which block stimulus the participant should reproduce in that empty grid. The experimenter checked the participant s reproductions for accuracy. If participants made a mistake, they were asked to review the stimulus on the computer s study screen and redraw the stimulus on a new sheet of empty grids. This was repeated until participants were able to reproduce all block stimuli correctly. The experimenter recorded the number of attempts and total time taken to reproduce all block stimuli correctly.
Image Generation Task
Following the study phase, the experimenter read the instructions for the image generation task with the participant. Each trial consisted of a series of screens (Figure 2). First, an asterisk appeared in the center of the screen for 400ms to direct attention to the center of the screen. After 200ms of a blank screen, a script cue was presented in the center of the screen for 300ms. This was again followed by a blank screen for 200ms, after which a grid, with a single X placed in one of the cells, was presented on the screen for 300ms. Participants were instructed to respond “Yes” or “No” to whether the X would be covered if the block version of the just-cued stimulus were superimposed on the grid. Participants were asked to respond as quickly and accurately as possible by pressing a corresponding key on the mouse. Error rates and response latencies were recorded for each trial.
Perception Control Task
The procedure for this task was identical to that for the imagery task, with the exception that the grid contained the block stimulus in a visible, light gray color. Participants were asked to click “Yes” or “No” as before, but now they were deciding whether the X probe fell in a cell filled by the visible gray block stimulus. Error rates and response latencies were recorded for each trial.
Results
Missing Data
Three participants did not complete the BDI, and one participant did not complete the demographics questionnaire. These participants were excluded from the means reported in Table 2. Data for the study phase were not recorded for three participants due to experimenter error and were not included in the analyses reported in the Study Phase below. Finally, one participant who completed the image generation task did not complete the perception control task. As a result, N = 60 for the following analyses for the imagery task and N = 59 for analyses for the perception task.
Study Phase
To examine group differences in the number of attempts and total time required by the participant to reproduce all block stimuli correctly during the study phase, we conducted univariate ANOVAs for the two variables. Results showed that the groups differed both in the number of attempts to draw all block letters correctly, F(2, 54) = 3.19, p < .05, η2 = .11, and total time, F(2, 52) = 5.04, p = .01, η2 = .16. Follow-up t-tests showed that there was no significant difference between the GSP and NAC groups in number of attempts, t(40) = 1.09, p = .28, d = .35, or total time taken to draw all block letters correctly, t(38) = 1.44, p = .16, d = .47. However, the AC group required more attempts than did the GSP group, t(36) = 2.52, p < .02, d = .84, although there was no difference in total time taken by the two groups, t(35) = 1.91, p = .07, d = .65. The AC group took more time than did the NAC group, t(31) = 3.07, p < .01, d = 1.10, but did not require a significantly different number of attempts to draw all block letters correctly, t(32) = 1.44, p = .16, d = .51.
We did not have a priori hypotheses regarding the study phase in Study 2. These results suggest that the AC group was less efficient during the study phase than the GSP and NAC groups, although it is difficult to interpret this finding because it was not consistent across the two outcome measures.
Image Generation Task
For the image generation task, in order to remain consistent with previous studies (e.g., Kosslyn et al., 1988; Kosslyn et al., 1997), we examined only the trials where the X would have been covered by the block letter.
Error Rates
We submitted participants error rates to a 3 (Group: GSP, NAC, AC) × 2 (Probe Location: Early, Late) analysis of variance (ANOVA) with repeated measurement on the second factor. Replicating Kosslyn et al. (1988), the main effect of Probe Location was significant, F(1, 57) = 41.05, p < .001, η2 = .42. Examination of error rates revealed that participants made more errors for probes located on the late segments of the letters compared to probes located on the early segments of the letters. The main effect of Group was not significant, F(2, 57) = 2.63, p > .08, η2 = .08, nor was the interaction of Group × Probe Location, F(2, 57) = 1.69, p > .19, η2 = .06.
Response Latencies
Response latencies for trials with incorrect responses were eliminated. This resulted in the removal of 10.9% of the trials. Outliers, defined as trials with response latencies greater than ± 2.5 standard deviations from the mean response latency for that participant, were eliminated (3.7% of remaining trials). This proportion of trials is within the range of dropped trials in other studies using this task (6.7%–19.9%; Kosslyn et al., 1997; Zarrinpar et al., 2006). Moreover, a similar proportion of outlier trials was dropped in the GSP (3.13%), NAC (3.99%), and AC (3%) groups.
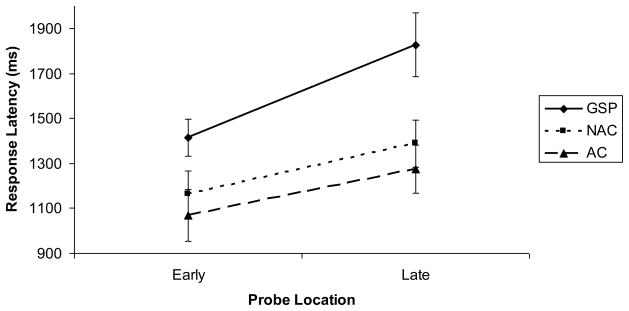
Response latencies were submitted to a 3 (Group: GSP, NAC, AC) × 2 (Probe Location: Early, Late) ANOVA with repeated measurement on the second factor (Figure 3). The main effect of Group was significant, F(2, 57) = 4.77, p < .02, η2 = .14. There was a significant main effect of Probe Location, F(1, 57) = 62.25, p < .001, η2 = .52. Thus, we replicated the behavioral indicators of image generation found in previous studies (i.e., participants responded slower and with less accuracy to probes located on segments of letters typically drawn later in the letter-writing process compared to probes located on segments of letters typically drawn earlier in the letter-writing process; Kosslyn et al., 1988). The main effect of Probe Location was modified by a significant interaction of Group × Probe Location, F(2, 57) = 3.78, p < .03, η2 = .12.
Figure 3.
Response latencies for early and late probe locations in the image generation task for individuals with generalized social phobia (GSP), non-anxious control (NAC) participants, and anxious-control (AC) participants.
To follow-up the 3 × 2 interaction, we conducted two univariate ANOVAs, the first to test whether the three groups differed in response latencies for early probe locations, and the second to test whether they differed in response latencies for late probe locations. Results of the first univariate ANOVA showed that the groups were significantly different in their response latencies for early probe locations, F(2, 57) = 3.39, p = .04, η2 = .10. Follow-up t-test showed that the GSP and NAC groups did not differ in response latencies for early probe locations, t(43) = 1.94, p = .06, d = .59. However, the GSP group responded significantly slower to early probes than did the AC group, t(37) = 2.50, p < .02, d = .82. The NAC and AC groups did not differ in response latencies for early probe locations, t(34) = .61, p > .54, d = .21. Results of the univariate ANOVA for the late probe locations showed that the groups were significantly different in their response latencies for late probe locations, F(2, 57) = 5.49, p < .01, η2 = .16. Follow-up t-test showed that individuals with GSP responded significantly slower to late probe locations than did NACs, t(43) = 2.42, p = .02, d = .74, and ACs, , t(37) = 2.77, p < .01, d = .91. The NAC and AC groups did not differ in response latencies for late probe locations, t(34) = .75, p = .46, d = .26.
Additionally, as expected, paired samples t-tests showed that all three groups were slower at responding to probes located on the late segments of the letters compared to probes located on the early segments of the letters. [GSP: t(23) = 5.53, p < .001; NAC: t(20) = 5.12, p < .001; AC: t(14) = 5.51, p < .001].
Perception Control Task
Error Rates
Error rates for the perception trials were submitted to a 3 (Group: GSP, NAC, AC) × 2 (Probe Location: Early, Late) ANOVA with repeated measurement on the second factor. The main effect of Probe Location was significant, F(1, 56) = 4.16, p < .05, η2 = .07. Neither the main effect of Group, F(2, 56) = 1.63, p > .20, η2 = .06, nor the interaction of Group × Probe Location, F(2, 56) = 1.87, p > .16, η2 = .06, was significant.
Response Latencies
For analysis of response latencies, trials with incorrect responses were eliminated. This resulted in the removal of 4.7% of the trials. Outliers, defined as trials with response latencies greater than ± 2.5 standard deviations from the mean response latency for that participant, were eliminated (2.7% of remaining trials).
Response latencies were submitted to a 3 (Group: GSP, NAC, AC) × 2 (Probe Location: Early, Late) ANOVA with repeated measurement on the second factor. Neither the main effects nor the interaction effect was significant, ps > .13. Thus, the groups did not perform differently on the perception control task.
Taken together, results of Study 2, as depicted in Figure 3, show that the slope for the GSP group (difference between response to early and late probes) was steeper than the slope for the NAC and the AC groups, indicating that participants with GSP spent more time generating neutral images than did the other two groups. Moreover, there was no significant difference in speed of image generation between the NAC and AC groups.
Discussion
The aim of the present studies was to examine the generation of visual images in clinical social anxiety. In contrast to previous research on imagery in social anxiety which has examined only the content of imagery, the use of behavioral tasks permitted an assessment of basic processes involved in imagery (in this case, image generation ability). In the present studies we did not find evidence for differences between individuals with GSP and control participants in speed of generating images related to socially threatening words. However, results of the two studies demonstrate that individuals with GSP are impaired in their ability to generate neutral images in that they are slower to generate neutral images than are control participants.
How do our findings relate to cognitive-behavioral models of social phobia?
These models purport that, within social situations, individuals with GSP are spontaneously flooded with negative self-images, and that these images interfere with performance and serve to maintain anxiety. We did not find evidence from Study 1 for more efficient generation of images related to social-threat words in individuals with GSP compared to NACs. One explanation for our findings is that the task in Study 1 was inadequate to test our hypothesis. More specifically, because the image generation task in Study 1 was not of a social nature, it is possible that presentation of the social-threat words did not activate the generation of threatening self-images. Alternatively, although there is no way for us to determine this from these data, it is also possible that presentation of the social-threat words did result in activating threatening self-images but that the speed with which these images were generated was “canceled out” by a slowing down due to selective attention to threat-related stimuli. Evidence from other information processing tasks (e.g., Mathews & MacLeod, 2005) does suggest that the threatening meaning of social-threat stimuli tends to capture attention in individuals with GSP, so that conceivably the efficiency of generating social-threat self-images was counteracted by an attentional capture by social-threat words.
Another possibility is that the tasks in the two studies did function as intended, and that individuals with GSP are indeed impaired at image generation in general, as evidenced by their slower speed of generating neutral images, compared to NACs. However, because, as cognitive-behavioral models of social anxiety posit, individuals with GSP are over-practiced at social-threat images, they are able to compensate for their image generation impairment specifically for these types of images and hence perform no differently from NACs. There is no way for us to determine this from these data; however, it is nonetheless a possible alternative explanation for our findings. Finally, it is possible that the negative self-images experienced by socially anxious individuals are maintained by impairment in the generation of neutral images to compete with the socially threatening images. Cognitive-behavioral models suggest that negative self-images maintain social anxiety, and it is reasonable to assume that socially phobic individuals, whose cognitive resources are engaged in generating negative self-images in social situations, might have difficulty with the simultaneous generation of neutral images. Thus, one might expect socially anxious individuals to generate negative images at the expense of neutral images in stressful social situations. To the extent that the tasks were experienced as stressful due to the possibility of negative evaluation, one possibility is that GSP participant had difficulty generating neutral images because their cognitive resources were engaged in generating negative ones.
What might account for the finding in Study 2 of slowed image generation in individuals with GSP with respect to the mixed anxious control group?
Given that the role of imagery is different in cognitive models of GAD and OCD, we did not have specific a priori hypotheses regarding the AC group. Results of our study showed that the GSP group had impaired ability to generate neutral images compared to the mixed GAD and OCD anxious control group. Given the groups similar response on the perception control task, it is unlikely that general concentration difficulties are sufficient to explain this effect. Moreover, the only previous study of psychopathology that has used this task (Zarrinpar et al., 2006) did not find evidence for impaired image generation ability in depressed participants, who presumably also have difficulties with concentration. Thus, the most parsimonious explanation is that individuals with GSP are indeed impaired in their ability to generate visual mental images of neutral stimuli. Finally, given the composition of our AC group, an alternative possibility is that the subgroups within this group (GAD, OCD) performed differently on the task, in essence “cancelling” each other out. In post-hoc analyses, we did not find evidence for differential performance in the GAD and OCD subgroups, although that could possibly be because of the small sample size of the subgroups.
Limitations
One limitation of our design is that the perception tasks that we used in our studies are not matched on task complexity to the corresponding imagery tasks. For example, given the greater complexity of the imagery task, it is likely associated with more stress on working memory capacity and potentially greater interference from emotional imagery. Nevertheless, our rationale for using these perception control tasks was that they require inspecting the presented stimuli and making the required decision whereas the corresponding imagery tasks involve first forming an image and then inspecting it and making the required decision (Kosslyn et al., 1988). Thus, the control tasks that we used were exactly analogous to the imagery tasks, except that they did not require the generation of an image. A limitation specific to Study 1 is that there is no way to know whether participants were generating images of the words alone or images of the words as well as the content of the words. However, our goal was to refrain from using self-report in studying the generation of neutral and threatening visual images. One way to increase the possibility of generating threatening imagery would be to place participants in a socially threatening situation (e.g., instructing participants to give a speech). That we did not do this is a limitation of our design. Another limitation of Study 1 is that this is the first study to use this imagery task to test our specific hypotheses, and hence the findings must be considered preliminary until replicated. Moreover, we did not include an anxious control group in Study 1, which is necessary to determine the specificity of our findings to social phobia. In Study 2, we aimed to rectify this limitation by including an anxious control group. A limitation of the design of Study 2 is that we cannot rule out the possibility that negative, intrusive thoughts or negative self-images (at the cost of neutral ones) interfered with the GSP group s ability to perform the task more so than they did the NAC and AC groups. For example, as mentioned above, the social nature of undertaking a performance-based task might be particularly distressing to the GSP group. Another limitation specific to Study 2 is that we had some missing data as well as unequal cell sizes for the three groups. Finally, a limitation of both studies is that the sample sizes are small and hence the findings need to be replicated in larger samples.
These limitations notwithstanding, the present studies are the first to assess image generation ability in individuals diagnosed with social phobia using behavioral rather than self-report measures. Thus, these studies mark a first step in understanding the processes underlying image generation in social anxiety. Future studies should target the distinction between spontaneously generated images and those in response to task instructions.
References
- Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: Evidence of episodic memory dysfunction. Journal of Psychiatric Research. 2005;39:207–214. doi: 10.1016/j.jpsychires.2004.06.001. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders—Text revision. 4. Washington, DC: Author; 2000. [Google Scholar]
- Amir N, Foa E, Coles ME. Implicit memory bias for threat-relevant information in individuals with generalized social phobia. Journal of Abnormal Psychology. 2000;4:713–720. doi: 10.1037//0021-843x.109.4.713. [DOI] [PubMed] [Google Scholar]
- Appollonio I, Grafman J, Clark K, Kosslyn SM, Frattola L. Image generation from long-term memory in Parkinson’s disease. In: Battistin L, Scarlato G, Caraceni T, Ruggieri S, editors. Advances in neurology, Vol. 69: Parkinson’s disease. Philadelphia, PA: Lippincott-Raven; 1996. pp. 349–359. [PubMed] [Google Scholar]
- Asmundson CJG, Stein MB, Larsen DK, Walker JR. Neurocognitive functions in panic disorder and social phobia patients. Anxiety. 1995;1:201–207. [PubMed] [Google Scholar]
- Beck J, Steer RA, Brown GK. Beck Depression Inventory manual. 2. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Borkovec TD, Inz J. The nature of worry in generalized anxiety disorder: A predominance of thought activity. Behaviour Research and Therapy. 1990;28:153–158. doi: 10.1016/0005-7967(90)90027-g. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Ray WJ, Stober J. Worry: A cognitive phenomenon intimately linked to affective, physiological, and interpersonal behavioural processes. Cognitive Therapy and Research. 1998;22:561–576. [Google Scholar]
- Brown HD, Kosslyn SM, Breiter HC, Baer L, Jenike MA. Can patients with obsessive-compulsive disorder discriminate between percepts and mental images? A signal detection analysis. Journal of Abnormal Psychology. 1994;103:445–454. [PubMed] [Google Scholar]
- Clark DM, Wells A. A cognitive model of social phobia. In: Heimberg R, Liebowitz M, Hope DA, Schneier FR, editors. Social phobia: Diagnosis, assessment and treatment. New York: Guilford Press; 1995. [Google Scholar]
- Dror IE, Kosslyn SM, Waag W. Visual-spatial abilities of pilots. Journal of Applied Psychology. 1993;78:763–773. [Google Scholar]
- Emmorey K, Kosslyn SM, Bellugi U. Visual imagery and visual-spatial language: Enhanced imagery abilities in deaf and hearing ASL signers. Cognition. 1993;46:139–181. doi: 10.1016/0010-0277(93)90017-p. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders-Patient Edition. New York: New York State Psychiatric Institute Biometrics Research Department; 1994. [Google Scholar]
- Fresco DM, Coles ME, Heimberg RG, Liebowitz MR, Hami S, Stein MB, et al. The Liebowitz Social Anxiety Scale: a comparison of the psychometric properties of self-report and clinician-administered formats. Psychological Medicine. 2001;31:1025–1035. doi: 10.1017/s0033291701004056. [DOI] [PubMed] [Google Scholar]
- Gilboa-Schechtman E, Foa EB, Amir N. Attentional biases for facial expressions in social phobia: The face-in-the-crowd paradigm. Cognition and Emotion. 1999;13:305–318. [Google Scholar]
- Hackmann A, Surawy C, Clark DM. Seeing yourself through others eyes: A study of spontaneously occurring images in social phobia. Behavioural and Cognitive Psychotherapy. 1998;26:3–12. [Google Scholar]
- Hirsch CR, Clark DM, Mathews A, Williams R. Self-images play a causal role in social phobia. Behaviour Research and Therapy. 2003;41:901–921. doi: 10.1016/s0005-7967(02)00103-1. [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Meynen T, Clark DM. Negative self-imagery in social anxiety contaminates social situations. Memory. 2004;12:496–506. doi: 10.1080/09658210444000106. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Maljkovic V, Weise SB, Chabris CF, et al. Visual mental imagery activates topographically organized visual cortex: PET investigations. Journal of Cognitive Neuroscience. 1993;5:263–287. doi: 10.1162/jocn.1993.5.3.263. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Cave C, Provost DA, von Gierke SM. Sequential processes in image generation. Cognitive Psychology. 1988;20:319–343. doi: 10.1016/0010-0285(88)90002-3. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Margolis JA, Barrett AM, Goldknopf EJ, Daly PF. Age differences in imagery abilities. Child Development. 1990;61:995–1010. [PubMed] [Google Scholar]
- Kosslyn SM, Thompson WL, Alpert NM. Neural systems shared by visual imagery and visual perception: A positron emission tomography study. Neuroimage. 1997;6:320–334. doi: 10.1006/nimg.1997.0295. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR. Social phobia. Modern Problems in Pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- MacLeod C. Cognition in clinical psychology: Measures, methods or models? Behaviour Change. 1993;10:169–195. [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Morrison AS, Amir N, Taylor CT. A behavioral index of imagery ability in social anxiety. Cognitive Therapy and Research (in press) [Google Scholar]
- Podgorny P, Shepard RN. Functional representations common to visual perception and imagination. Journal of Experimental Psychology: Human Perception and Performance. 1978;4:21–35. doi: 10.1037//0096-1523.4.1.21. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research and Therapy. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- Richardson A. Individual differences in imaging: Their measurement, origins and consequences. New York: Baywood; 1994. [Google Scholar]
- Ruane D, MacLeod A, Holmes EA. The simulation heuristic and visual imagery in pessimism for future negative events in anxiety. Clinical Psychology and Psychotherapy. 2005;12:313–325. [Google Scholar]
- Sacco G, Ruggieri V. Mental imagery and symptom patterns. Imagination, Cognition, and Personality. 1998;17:313–321. [Google Scholar]
- Schneier FR, Johnson J, Hornig CD, Liebowitz MR, Weissman MM. Social phobia: Comorbidity and morbidity in an epidemiologic sample. Archives of General Psychiatry. 1992;49:282–288. doi: 10.1001/archpsyc.1992.01820040034004. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- Stein M, Kean Y. Disability and quality of life in social phobia: epidemiologic findings. American Journal of Psychiatry. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- van den Hout M, Kindt M. Obsessive-compulsive disorder and the paradoxical effects of perseverative behavior on experienced uncertainty. Journal of Behavior Therapy and Experimental Psychiatry. 2004;35:165–181. doi: 10.1016/j.jbtep.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Weber RJ, Castleman J. The time it takes to imagine. Perception & Psychophysics. 1970;8:165–168. [Google Scholar]
- Zarrinpar A, Deldin PJ, Kosslyn S. Effects of depression on sensory/motor versus central processing in visual mental imagery. Cognition and Emotion. 2006;20(6):737–758. [Google Scholar]