Abstract
Introduction
Duchenne muscular dystrophy (DMD) is the most common, severe childhood form of muscular dystrophy. Treatment is limited to glucocorticoids that have the benefit of prolonging ambulation by approximately 2 years and preventing scoliosis. Finding a more satisfactory treatment should focus on maintaining long-term efficacy with a minimal side effect profile.
Areas covered
Authors discuss different therapeutic strategies that have been used in pre-clinical and clinical settings.
Expert opinion
Multiple treatment approaches have emerged. Most attractive are molecular-based therapies that can express the missing dystrophin protein (exon skipping or mutation suppression) or a surrogate gene product (utrophin). Other approaches include increasing the strength of muscles (myostatin inhibitors), reducing muscle fibrosis, and decreasing oxidative stress. Additional targets include inhibiting NF-κB to reduce inflammation, or promoting skeletal muscle blood flow and muscle contractility using phosphodiesterase inhibitors or nitric oxide (NO) donors. The potential for each of these treatment strategies to enter clinical trials is a central theme of discussion. The review emphasizes that the goal of treatment should be to find a product at least as good as glucocorticoids with a lower side effect profile or with a significant glucocorticoid sparing effect.
Keywords: Duchenne muscular dystrophy, exon skipping, mutation suppression, stop codon readthrough, utrophin, myostatin inhibition, nitric oxide, phosphodiesterase inhibitors, NF-κB inhibition
1. Background
Duchenne muscular dystrophy (DMD) is the most common, severe childhood form of muscular dystrophy. Inheritance follows an X-linked recessive pattern. Birth prevalence estimated at 1 in 3500 (2.9 per 10,000) live male birth [1] may be slightly lower [2]. Approximately one-third of cases represent new mutations of the DMD gene. Questions usually begin to surface between ages 3 to 5 regarding reduced motor skills that alert a need for diagnostic evaluation. DMD is relentlessly progressive with loss of ambulation by age 12 [3]. Historically patients died from respiratory complications. Now, a variety of factors protect the respiratory system related to improved supportive equipment, antibiotics, vaccines, and other ancillary methods [4]. Prolonging life unmasks a decline in cardiac function with complications of dilated cardiomyopathy. This poses further clinical challenges and a need for recognition and medical intervention that did not previously exist.
Becker muscular dystrophy (BMD) is a milder variant of dystrophin deficiency. Some BMD patients lose the ability to walk as early as late teen-age years, while others ambulate until after age 60 [5]. BMD patients typically die in the fourth and fifth decades. Dystrophin deficiency can also cause a clinical condition with predominant cardiac manifestations (X-linked cardiomyopathy) [6]. Non-progressive cognitive dysfunction is well characterized in DMD and BMD. The average IQ for DMD is one standard deviation below the mean.
More than 20 years ago the DMD gene was cloned defining the molecular basis for the disease [7]. The identification of dystrophin as the deficient protein followed closely on the heels of this discovery [8]. Dystrophin is a 427kDa cytoskeletal protein required for muscle fiber stability. Loss of this protein results in susceptibility to repeated cycles of necrosis and regeneration with satellite cell depletion, diminished regenerative capacity of the muscle, ending in fat and connective tissue replacement (fibrosis).
The mutation spectrum within the DMD gene reveals that deletions of one or more exons are found in ~65% of cases clustered in two hotspot regions [9]. Originally multiplex PCR kits were developed that were able to detect 95%-98% of all deletions [10, 11]. Detection of duplications, representing about 6% of the DMD mutations, initially required Southern blots. Overtime the demand for more rapid, less expensive detection methods have encouraged the introduction of additional tools to identify the full spectrum of mutations (deletions, duplications, splice-site and point mutations). Multiplex ligation-dependent probe amplification (MLPA) [12] or multiplex amplifiable probe hybridization (MAPH) [13] will screen all exons providing detection of most deletions and duplications. If MLPA or MAPH are negative, the gene should be scanned for subexonic rearrangements or point mutations using DNA sequence analysis [14]. This has become more than an academic exercise because of treatment paradigms that depend on the full characterization of the mutation endpoints that help establish if patients are candidates for molecular therapies that will be discussed in this review.
2. Medical Need
The medical need for treatment of this devastating disease is compelling. As indicated above, patients are living longer because of improved medical therapy. DMD patients are protected from catastrophic death related to pulmonary infections with the advent of second and third generation antibiotics and non-invasive respiratory support using bilevel positive airway pressure (BiPAP). Life of the DMD patient is also extended related to meticulous and painstaking cardiac care. While these are important successes that represent life-altering results with long-term impact, the short-term effect on quality of life is less apparent. More and more time is spent in a wheelchair with limited prospects for employment; socialization rarely extends beyond the immediate family. Quality of life is also diminished by the common, yet under-recognized occurrence of pain in children with DMD [15]. The need for improved medical intervention extends to the parents and siblings of DMD patients. There is an enormous life-time financial burden, and family resources are often drained by the chronicity of disease. In addition, parents report poorer health status encumbered by anxiety and depression [16]. There is also added stress on parental relations with increased divorce because of accusations of blame thrust upon maternal inheritance [17].
3. Current Treatment
Despite virtually hundreds of clinical trials in DMD, only one treatment has consistently demonstrated efficacy. Unequivocal evidence for glucocorticoid-induced improvement was established through a double-blind, randomized controlled trial in a large cohort of subjects (n=103) [18]. Patients received 0.75 mg /kg/day or 1.5 mg/kg/day versus placebo. At six months there was approximately equal efficacy for the high and low dose prednisone groups compared to placebo in muscle strength (manual muscle testing score), the time needed to rise from supine to a standing (prednisone 3.4 vs. placebo 6.2 seconds), to walk 9 m (prednisone 7.0 vs. placebo 9.7 seconds), to climb four stairs (prednisone 4.0 vs. placebo 7.1 seconds), and in forced vital capacity (prednisone 1.7 vs. placebo 1.5 liters) (P<0.001 for all comparisons). Similar results were later reported with deflazacort (0.9 mg/kg/day), an alternative, sodium-sparing corticosteroid that was also shown to prolong ambulation (untreated DMD patients stopped walking at 9.8 ± 1.8 years compared to deflazacort-treated at 12.3 ± 2.7 years, P<.005) [19]. A dose-related effect was observed in two follow up studies that demonstrated lesser but similar benefits using prednisone 0.30 and 0.35 mg/kg/day [20, 21]. A weekend dosing regimen (10 mg/kg/wk: half on Saturday and half on Sunday) showed equal efficacy to daily steroids [22]. Additional follow up studies reported that the number of boys having scoliosis surgery in treated groups was significantly less than untreated boys (P < 0.05) [23, 24] and treatment extended independent ambulation by 3.3 years compared to untreated (9.2 ±1.48 years vs 12.5± 3.02 (p < 0.0001) [24].
The side-effect profile of glucocorticoids consistently demonstrates weight gain with a cushingoid appearance. DMD boys on steroids are also at risk for hypertension, cataract formation, loss of bone density, vertebral compression fractures, and long bone fractures. Long-term administration may be limited in some cases by steroid-induced behavioral problems frequently observed with this class of drugs.
4. Current Research Goals
The current goal for DMD is to find a treatment that exceeds or matches the efficacy of glucocorticoids with a significantly lower side effect profile; or acts as steroid-sparing agent enabling equal or enhanced efficacy at lower glucocorticoid doses. These objectives may appear modest but are practical, feasible and realistic for a disease with the challenges of DMD.
Conceptually there are competing strategies to accomplish these aims. On the one hand molecular-based pharmacologic therapies are designed to modify the DMD gene product using exon skipping [25, 26] or readthrough of stop codons (mutation suppression) [27, 28], or by upregulation of utrophin, a dystrophin-analog with potential functional homology [29].
Alternatively, strategies are evolving that attempt to pharmacologically block more directly the consequences of the dystrophic process. These include preventing oxidative stress related to free radical toxicity generated by influx of calcium through dystrophin-deficient permeable membranes [30] or thwarting the chronic activation of the NF-κB signaling concomitant with muscle fiber breakdown and inflammation [31]. Additional strategies attempt to overcome the weakness accompanying the dystrophic process by building size and strength of muscle fibers through myostatin inhibition [32, 33], or increasing nutrients to exercising muscle by enhancing the vascular supply to muscle through the increased supply of nitric oxide [34]. Another critical need for DMD is the successful treatment of fibrosis. Muscle biopsies done at an early age show prominent increase in endomysial connective tissue [35]. Irrespective of the success of any line of attack, muscle scarring must be reduced to achieve full efficacy. The evolving strategies to be discussed in this review are based on sound principles and all the approaches will be discussed with regard to premise, pre-clinical data providing proof-of-principle, and an appraisal of the current readiness for clinical trials.
5. Scientific Rationale and Competitive Environment
Molecular-based therapies that include gene or cell therapies that replace or repair the underlying DMD gene defect have intuitive appeal. However, these approaches have limitations that will require further pre-clinical and clinical testing. Obstacles for gene therapy include an immune response to viral capsid antigens or to the expression of dystrophin epitopes that are novel to the host [36]. A major constraint of DMD gene therapy is the limited packaging capacity of adeno-associated virus (AAV) that compromises delivery of the full dystrophin cDNA necessitating the use of small or mini-dystrophins that have never been functionally tested in humans. How much phenotypic correction will be achieved is an ongoing question. A potential advantage of gene replacement is the possibility for long-lasting gene expression based upon gene delivery at a single point in time. With regard to stem and progenitor cell therapies, these approaches have shown promising pre-clinical results but translation to the clinic requires additional studies, particularly related to targeting of skeletal and cardiac muscle. Neither gene therapy or stem cell therapy will be the focus for this review (fully discussed in the literature elsewhere [37])
Alternatives to direct gene replacement/repair are three molecular pharmacologic approaches that have demonstrated promising findings and represent well-defined current research aspirations. These include exon skipping, readthrough of stop codon mutations, and replacement of dystrophin using utrophin, a dystrophin-like cytoskeletal protein. The origin for the first two potential translational approaches is the presence of revertant muscle fibers in DMD muscle [38]. Revertants represent small clusters of dystrophin expressing fibers thought to be the result of second-site mutations, alternative-splicing or mutation suppression of stop codons [39, 40]. The presence of revertants has encouraged scientists to develop a pharmacologic path to reproduce and expand this spontaneous natural phenomenon in order to generate dystrophin expression levels that would result in clinically meaningful outcomes.
Exon skipping is well underway in clinical trials and the results of these initial clinical studies will be reviewed. Mutation suppression or readthrough of stop codons requires pharmacologic tools that act at the messenger RNA level, including aminoglycoside antibiotics or Ataluren, that have now been used in clinical trials [41, 42]. Utrophin upregulation naturally occurs in dystrophin-deficient muscle fibers and can be enhanced through pharmacologic therapy [29].
5.1 Molecular Based Therapies
5.1.1. Exon Skipping
Exon skipping is targeted at the pre-mRNA level allowing one or more exons to be omitted to restore the dystrophin reading frame. This is accomplished with splice-switching oligomers, typically 20–30 nucleotides in length and complementary in sequence to regions of the pre-mRNA transcript. Pre-clinical efficacy has been demonstrated in the mdx mouse, dystrophin/utrophin knock-out mouse, and CXMD dog [43–45] using both 2’O-methyl-ribo-oligonucleoside-phoshophorothioate (2OMe) and phosphorodiamidate morpholino oligomers (PMOs). Two proof-of-principle clinical trials in DMD used a 2OMe oligomer (PRO051/GSK2402968 ProsensaTherapeutics) or a PMO (AVI-4658/(Eteplirsen®); AVI BioPharma Inc.) delivered directly to muscle targeting exon 51 [25, 26]. Evidence favoring exon skipping was validated by RT-PCR and a newly synthesized dystrophin protein correctly localized to the sarcolemma. Phase I/II extension studies were performed with both oligomers following systemic delivery. In the PRO051 trial, four subcutaneous doses were tested in the initial 5 week phase (0.5, 2.0, 4.0, and 6.0 mg/kg) prior to an open-label 12-week extension phase [46]. Dose-related efficacy was achieved with evidence of new dystrophin expression in approximately 60–100% of muscle fibers in 10 of 12 patients that increased in a dose-dependent manner up to 15.6% on normal muscle. Modest improvement was seen in the 6-minute walk test at the highest dose. In the AVI phase II open-label study with AVI-4658 conducted in the UK, 7 of 19 patients showed a modest response with a mean increase of sarcolemmal dystrophin from 8.9% to 16.4% [47]. The results were variable with one patient responding following treatment with 2 mg/kg, while all other responders received the higher dosing levels of 10 and 20 mg/kg. AVI’s Eteplirsen and PRO051/GSK2402968 are now undergoing further clinical testing in randomized, double-blind, placebo-controlled, trials with the intention to extend skipping trials to include additional exons (http://www.clinicaltrials.gov). To date, reported exon skipping trials have focused on ambulatory boys (i.e., 6 minute walk test as functional outcome), but ongoing and planned trials will include non-ambulatory DMD boys.
5.1.2. Mutation Suppression
A second molecular approach involves suppression of stop codon mutations of the DMD gene that comprise approximately 15% of cases. Two pharmacologic tactics have shown pre-clinical efficacy. In mdx mice, mutation suppression was shown with the aminoglycoside antibiotic, gentamicin [48]. In a follow up clinical study, treatment of four DMD/BMD subjects failed to show a benefit [49]. A subsequent clinical trial, also in a small cohort, challenged the findings suggesting that readthrough had occurred, demonstrating full-length dystrophin as the product of gentamicin treatment [50]. In a more definitive trial, DMD patients (n = 16) with stop codons, treated weekly or twice weekly for six months (7.5 mg kg IV), showed a significant increase in dystrophin levels with the highest levels reaching 13 and 15% of normal [41]. Muscle strength was stabilized and a modest increase in forced vital capacity was achieved. Although this study demonstrated the therapeutic potential of gentamicin, higher doses might be necessary to improve functional outcomes. The known renal toxicity of aminoglycoside antibiotics and the challenges of intravenous administration pushed the field in the direction of identifying an orally administered agent.
Ataluren, formerly referred to as PTC124 (PTC therapeutics), fulfilled the requirement of an orally administered pharmacologic read-through product for stop codon mutations [51]. Pre-clinical studies in the mdx mouse demonstrated dystrophin expression in skeletal, cardiac, and diaphragm muscle and protected skeletal muscle from eccentric contraction-induced injury. A phase I study in healthy volunteers established safety and tolerability at doses exceeding what was required for pre-clinical efficacy [42]. In a phase IIa proof-of-concept, 28-day study in DMD/BMD patients, dystrophin appeared to increase post treatment in primary muscle cells obtained from muscle biopsies [52]. A randomized, double-blind, placebo-controlled phase IIb trial followed, evaluating safety and efficacy over a 48 week treatment period. High and low dose cohorts were included. PTC Therapeutics released preliminary results indicating a very strong safety profile; however, the primary endpoint of the 6 minute walk test did not reach statistical significance [52]. Additional problems in the interpretation of trial results included no data provided on dystrophin levels and the fact that a secondary analysis suggested that only the low dose group improved on the 6 minute walk test. The differential efficacy of high and low dose was ascribed to dose-related saturation of ribosomal binding sites [53]. Continued subgroup analysis to look at efficacy is underway. Influence for further pursuit of treatment in DMD may come from on-going clinical trials in cystic fibrosis [54] and hemophilia (clinicaltrials.gov NCT00947193), presuming results are favorable.
5.1.3 Utrophin Upregulation
Upregulation of utrophin is another therapeutic strategy for DMD that has shown promise in the mdx mouse. Utrophin shares 80% sequence homology with dystrophin and has been shown to partially restore function as a dystrophin surrogate in pre-clinical transgenic mice [55] or gene replacement studies [56]. In normal muscle utrophin expression is limited to the neuromuscular and myotendinous junctions but in mdx mice and DMD patients it is overexpressed throughout the sarcolemma of all muscle fibers, putatively compensating for the absence of dystrophin. Upregulation of utrophin holds particular advantage because of the unlikely occurrence of an immune response as seen following mini-dystrophin gene replacement [36].
Pharmacologic agents with potential to upregulate utrophin are summarized in Table 1. Several small molecules demonstrate upregulation of the utrophin gene through the activation of utrophin-A promoter. SMT C1100 is a novel small molecule that was identified through an exhaustive high-throughput small molecule screen. When used in the mdx mouse, utrophin staining is increased at the sarcolemma and dystrophic muscle pathology is reversed [57]. SMT C1100 was licensed to Biomarin Pharmaceuticals and tested under the name BMN 195 and used in a phase I safety trial in healthy volunteers. Disappointingly BMN 195 failed to achieve adequate blood levels. Intellectual properties were later transferred back to Summit, an Oxford, UK-based drug discovery company. Summit believes that formulation problems have been resolved and anticipate a repeat Phase I clinical trial in the near future.
Table 1.
Drugs/Approaches used for utrophin upregulation
| Drug/compound | Company | Status | Mode of Action |
|---|---|---|---|
| *SMT C1100 | Summit PLC. | Preclinical | Upregulation of utrophin promoter |
| *BMN-195 | Biomarin Pharmaceuticals | Phase I Clinical Trial | Upregulation of utrophin promoter |
| Nabumetone | GlaxoSmithKline | Preclinical | Upregulation of utrophin promoter |
| Heregulin | Sigma | Preclinical | Activation of utrophin promoter |
| Biglycan | Tivorsan Pharmaceuticals | Preclinical | Post-transcriptional upregulation of utrophin |
SMT C1100 and BMN-195 are the same compound
The non-steroidal anti-inflammatory drug (NSAID) produced by GlaxoSmithKline, nabumetone [58], (also known as Relafen®) was shown to upregulate endogenous utrophin mRNA and protein in C2C12 cells [58]; it remains a candidate agent considering its current use in the treatment of juvenile rheumatoid arthritis. However, the side effect profile of nabumetone includes heart attack or stroke and may be a limiting factor as life-long therapy for muscular dystrophy.
Heregulin represents an additional molecule capable of transactivating the utrophin-A promoter. When delivered to the mdx mouse for 3 months by intraperitoneal injections of a small peptide encoding the epidermal growth factor-like region of heregulin ectodomain, utrophin was upregulated, accompanied by increased resistance to eccentric contraction, and a reduction of muscle pathology [59]. The amelioration of dystrophic phenotype by heregulin-mediated utrophin up-regulation offers a pharmacological therapeutic modality that deserves consideration in future planning to alleviate DMD.
Another approach to upregulate utrophin employs a recombinant biglycan (rhBGN) that can be delivered systemically in the mdx mouse and ameliorate pathology and improve diaphragm function [60]. There is clear evidence that biglycan can recruit utrophin to the sarcolemma. rhBGN has shown promise when used in cell culture and in vivo without changing mRNA levels of utrophin indicating that functional benefits arise from posttranscriptional effects. rhBGN is being evaluated for clinical trials in DMD.
5.2. Muscle Growth Products
5.2.1 Myostatin Inhibition
Strategies to increase muscle size and strength through inhibition of the myostatin pathway show promise for clinical application. Myostatin is a member of the transforming growth factor-beta (TGF-β) family and is a potent regulator of muscle growth. Myostatin knock-out mice demonstrate dramatic muscle hypertrophy and hyperplasia [61]. The function of myostatin as a negative regulator of muscle mass is highly conserved across species. A report of a splice-site mutation of the myostatin gene leading to the loss of myostatin protein and a hypermuscular outcome in a young boy supports species conservation [62]. Given these observations, myostatin is an attractive therapeutic target for clinical trials in muscular dystrophy. MYO-029 is a recombinant human antibody that binds with a high affinity to myostatin and inhibits its activity [63]. This myostatin neutralizing antibody was shown to increase muscle mass in immunodeficient mice by approximately 30% over 3 months, similar to the biological response demonstrated for other myostatin neutralizing antibodies [64]. When MYO-029 was studied in a double blind randomized clinical trial in Becker, limb-girdle (including multiple subtypes), and facioscapulohumeral muscular dystrophies, safety but not efficacy was the outcome [63]. Additional strategies have been employed to inhibit myostatin. ACE-031, a soluble activin type IIB receptor was found to be promising in the mdx mouse as mediator to increase muscle mass and whole body pulling tension [65]. However, a placebo-controlled safety-tolerability study of ACE-031 in DMD was prematurely terminated (Acceleron Pharma, Inc.) because of minor nosebleeds, gum bleeding, and/or small dilated blood vessels within the skin. These events all resolved fully upon discontinuation of treatment.
Despite these stalled clinical attempts to increase muscle size and strength through myostatin inhibition, there are likely to be other clinical trials based on this principle. A potentially more potent agent is follistatin, a myostatin-binding protein that can inhibit myostatin activity and promote muscle growth. The potency of this agent is significantly greater than myostatin blockade alone based on studies in mice demonstrating retained activity despite a myostatin-null background [66]. Delivery of the follistatin gene by AAV in mice or non-human primates shows dramatic increases in muscle size and strength and this approach is poised for gene therapy (http://www.Clinicaltrials.gov). A caution for clinical trials remains that increase muscle mass may not translate to improved muscle strength [67].
5.2.2 Insulin Growth Factor-1
Insulin Growth Factor-1 (IGF-1) is another treatment strategy under investigation for DMD potentially increasing muscle mass and strength. IGF-1 is a growth factor and key mediator of an anabolic muscle building pathway[68]. Preclinical studies assessing the potential therapeutic benefit of IGF-1 demonstrated that transgenic overexpression of IGF-1 increased extensor digitorum longus and diaphragm muscle mass, enhanced force generation and reduced both myonecrosis and fibrosis in mdx mice [69, 70]. In a subsequent study, subcutaneous infusion of IGF-1 increased muscle mass and force output in mdx mice and in129ReJ dy/dy mice with laminin 211 deficiency [71, 72]. IGF-1 is currently FDA approved for severe primary IGF deficiency. A prospective, randomized, open labeled, controlled phase II clinical trial of recombinant IGF-1 (INCRELEX™) has been initiated in glucocorticoid (GC)-treated DMD patients to test its ability to preserve muscle function over six months (http://www.clinicaltrials.gov NCT01207908).
5.3 Drugs for Fibrosis Prevention
In DMD uncontrolled cycles of tissue injury with incomplete repair result in muscle fiber loss replaced by fibrotic scar tissue. Endomysial fibrosis in DMD muscle is a major contributor to muscle weakness and correlates significantly with clinical outcome [35]. This emphasizes the need to develop therapies to reduce fibrosis in muscle. Transforming growth factor-beta (TGFβ) has emerged as a therapeutic target because of its importance in the deposition of connective tissue in patients with DMD [73, 74] and in in dystrophin-deficient animal models, including the mdx mouse and the golden retriever dog [75, 76].
Multiple pre-clinical anti-fibrotic therapies blocking cytokine signaling through inhibition of the TGFβ pathway have been tested in mdx mice and are itemized in Table 2. Of the drugs listed, losartan, an angiotensin II receptor antagonist, is particularly attractive because it is widely used as an antihypertensive drug with an excellent safety record. Initial studies in mdx mice with treatment beginning at 6 weeks of age and continuing for 6–9 months reported reduced fibrosis in the diaphragm and gastrocnemius muscles and increased forelimb and hind limb grip strength compared to untreated controls [77]. Unfortunately, the enthusiasm that followed the initial favorable reports in mdx has waned because later studies showed minimal [78] or no [79] functional benefit to skeletal muscle, although cardiac muscle appeared to improve. These findings have dampened enthusiasm for bringing losartan forward as a therapeutic agent for skeletal muscle.
Table 2.
Drugs/Approaches with Antifibrotic Potential
| Drug/compound | Company | Status | Mode of Action |
|---|---|---|---|
| Losartan | Merck & Co. Inc | Preclinical | Angiotensin II receptor antagonist |
| Halofuginone | Collgard Biopharmaceuticals, Ltd | Preclinical | Inhibits Smad3 binding to DNA |
| *PirfenidoneIntermune, Inc | Preclinical | Inhibits fibroblast growth | |
| **Suramin | Parke-Davis | Preclinical | Anti-parasitic drug and reverse transcriptase inhibitor |
| ***Imatinib mesylate | Novartis | Preclinical | Anti-neoplastic drug inhibitor of tyrosine kinase |
Has been in clinical trials for idiopathic pulmonary fibrosis and has received orphan drug status for treatment of scleroderma by FDA (see text)
Used clinically as chemotherapeutic and anti-parasitic agent
Also called Gleevec ® approved for use in chronic myelogenous leukemia and gastrointestinal stromal tumor
Pre-clinical studies have also been done using other pharmacologic agents to block TGFβ. Most of these pharmacologic agents will require further study before they are ready for clinical testing. Of this group, perhaps halofuginone has the most potential. It is an analog of a low-molecular weight plant alkaloid isolated from Dichroa febrifuga that inhibits TGFβ-mediated collagen synthesis by impeding Smad3 binding to DNA [80]. Its potential as an antifibrotic was discovered incidentally when skin tearing was noted as a side effect of treating coccidiosis in poultry. This led to observations of reduced collagen-1 synthesis in animal models of human disease including pulmonary fibrosis [81]. In mdx mice, halofuginone decreases fibrosis in diaphragm and heart accompanied by improved physiologic parameters [82, 83]. Additional pre-clinical testing is required before clinical trials could be implemented.
A TGFβ inhibitor called pirfenidone is a small molecule that inhibits fibroblast growth and is designed as an antifibrotic agent for treatment of idiopathic pulmonary fibrosis (IPF). Pirfenidone has been approved for clinical use for IPF in Europe based on placebo-controlled clinical trials inclusive of more than 1,100 patients that showed slowing of disease [84]. This agent has also received orphan drug status from the FDA for treatment of scleroderma. However, when used in pre-clinical studies in the mdx mouse, the results were modest at best. There was a small reduction in the level of muscle hydroxyproline concentration but without impact on the level of type I collagen or TGFβ mRNA [85]. Further pre-clinical studies would be required to bring this agent to market for treatment of DMD.
Two antineoplastic drugs with targeted antagonistic effects on TGFβ have been used in pre-clinical testing in mdx mice. Suramin decreased serum creatine kinase (CK), and attenuated fibrosis in diaphragm and limb muscles and prevented exercise-induced functional loss during late stages of disease [86]. Imatinib mesylate, a 2-phenylaminopyridine derivative that inhibits tyrosine kinase and TGFβ pro-fibrogenic activity [87] reduced CK levels, and improved muscle strength. Favorable outcomes were accompanied by reduction of pro-inflammatory cytokines and TGFβ and associated with an increase in IL-10 [88]. The potential use of these anti-cancer agents for DMD will require study before entrée to the clinic.
MicroRNA (miRNA) composed of single-stranded RNAs of ~22 nucleotides can potentially be targeted to muscle to reduce fibrosis. miRNAs mediate gene silencing at the post-transcriptional level by pairing with bases within the 3’ UTR of mRNA, inhibiting translation or promoting mRNA degradation. A growing body of evidence suggests that miRNAs are involved in the fibrotic process in many organs including heart, liver, kidney, and lung. Reduced expression of miR-29 has been linked to DMD patient muscle tissue [89]. Electroporation of a miR-29 plasmid into mdx mouse muscle reduced the expression levels of ECM components, collagen and elastin, and strongly decreased collagen deposition in muscle sections within 25 days post-treatment [90]. miR-29 can also be delivered by AAV and is currently under investigation to diminish fibrosis in DMD.
5.4 Drugs Targeting Oxidative stress
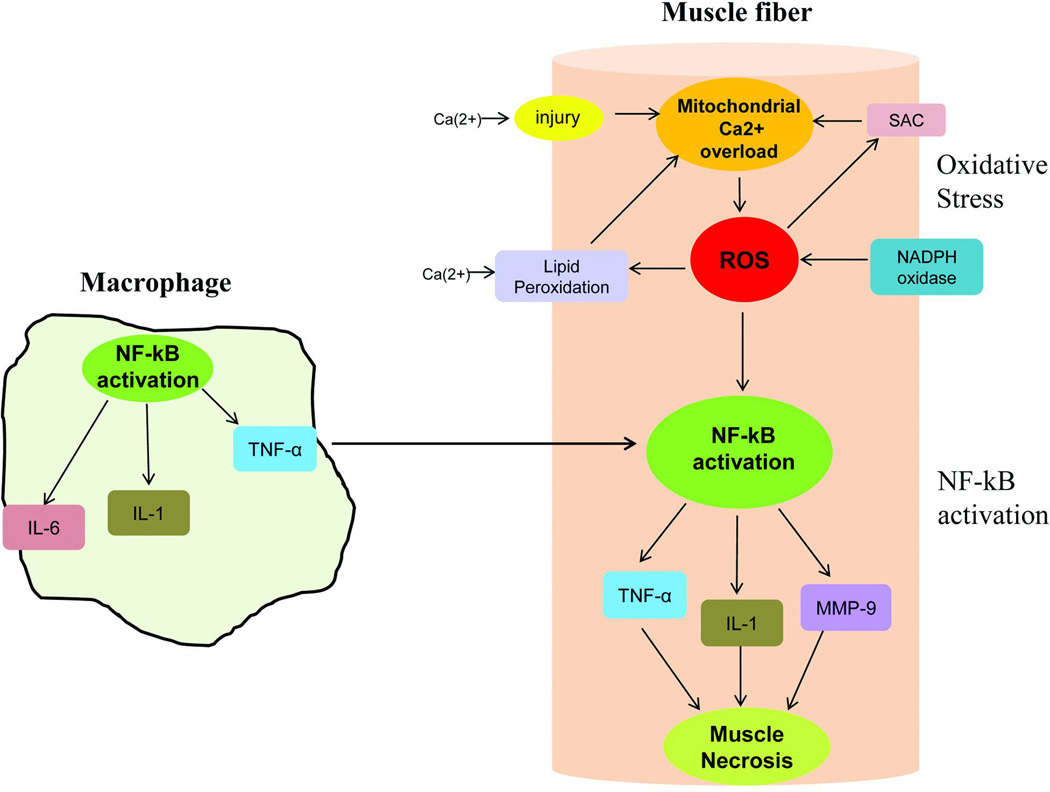
In developing therapeutic strategies to treat an exuberant inflammatory process in dystrophic muscle, it is important to appreciate the pathway resulting in this process has multiple interactive participants. The major players include reactive oxygen species (ROS) and nuclear factor-κ B (NF-κB), activated by immunomodulatory agents [such as tumor necrosis factor-α (TNF-α), and interleukins, particularly IL-1 and IL-6]. Figure 1 illustrates the components of this complex inflammatory pathway. While it is well known that deficiency of dystrophin leads to increased membrane permeability and high levels of serum CK escaping from muscle, it must also be appreciated that calcium (Ca2+) enters the cells at this sarcolemmal interface. This results in Ca2+ overload in mitochondria [30] directly responsible for increased reactive oxygen species, which in turn further contributes to membrane permeability through peroxidation of sarcolemmal lipids (lipid bilayer) [91]. An additional source of ROS is membrane-bound NADPH oxidase [92], known to accompany dystrophin deficient muscle [93]. ROS generation stimulates stretch-activated channels (SAC) leading to (Ca2+) entry into the muscle fiber further contributing to mitochondrial dysfunction. Downstream of oxidative stress, ROS induce activation of NF-κB [94], a transcription factor that plays a central role in mediating inflammation (further expanded on in the next section).
Figure 1.
Oxidative and NF-kB pathways are highly interactive in DMD muscle collaborating to produce muscle fiber necrosis. Macrophages generate inflammatory cytokines and reactive oxidative species (ROS) are the direct consequence of dystrophin deficiency and membrane instability. The illustration shows a macrophage with activated NF-κB leading to secretion of pro-inflammatory molecules, TNF-α, IL-1 & IL-6. A dystrophic muscle fiber shows reactive oxygen species generated by multiple insults to the muscle fiber: 1) Ca2+ influx through muscle membrane related to membrane fragility leads to Ca2+ overload in the mitochondria and energy deficiency; 2) increased NADPH oxidase activity known to accompany dystrophin deficiency adds to ROS generation which in turn stimulates stretch-activated channels (SAC) resulting in Ca2+ entry into muscle fiber further contributing to mitochondrial dysfunction; 3) ROS exacerbates the dystrophic pathology by peroxidation of the lipid bilayer of the muscle membrane. ROS provokes the NF-κB signaling pathway leading to production of TNF-α and other cytokines. Moreover, TNF-α produced by activated macrophages enhances NF-κB activation in the muscle.
Many antioxidant therapies have been investigated for their ameliorating effect in reducing signs of muscle damage (Table 3). One of the most promising is N-acetylcysteine (NAC) reported to prevent increased membrane permeability and promote force generation following stretch-induced muscle damage in mdx mice [95]. Moreover, NAC increased utrophin and β-dystroglycan gene expression and decreased both caveolin-3 and NF-κB. NAC has an established safety record and is available as an over-the-counter product. Properly conducted clinical trials are the missing piece.
Table 3.
Anti-oxidative and NF-kB inhibitory drugs used in preclinical/clinical studies
| Drug/compound | Company | Status | Mode of Action |
|---|---|---|---|
| Antioxidants | |||
| N-acetylcysteine (NAC) | *Twinlabs | Preclinical | Antioxidant |
| Green tea extract (Epigallocatechin-Gallate; EGCg) | *Nature’s Way | Clinical trials | Antioxidant |
| Melatonin | *GNC | Clinical trials | Antioxidant |
| Idebenone | Santhera Pharmaceuticals | Clinical trials | Antioxidant |
| NF-kB inhibition | |||
| Remicade | Merck/Schering-Plough | Preclinical | Antibody to human TNF-α |
| Etanercept | Wyeth | Preclinical | Blocks soluble receptor to TNF-α |
| NBD peptide | **Enzo Life Science | Preclinical | Inhibitor of the NF-κB signaling pathway |
Available over the counter from several sources (e.g., NOW Foods, FoodScience of Vermont)
Clinical grade product not available
Green tea is another pharmacological agent tested for inhibition of oxidative stress. It is a natural source of polyphenols and flavonoids (Latin word flavus = yellow, their color in nature). Pre-clinical studies with green tea extract and its active ingredient epigallocatechin gallate (EGCg) show histological and functional improvement in mdx mice [96, 97]. Furthermore, early treatment with green tea extract decreases dystrophic muscle pathology, potentially by regulating NF-κB activity in regenerating fibers [98]. Clinical trials are underway using green tea extract or EGCg in diseases other than DMD (Alzheimer’s disease, Huntington’s disease, multiple sclerosis, HIV, small cell carcinoma; http://www.Clinicaltrials.gov). Currently DMD patients are being recruited to a clinical trial outside the USA for safety, tolerability and efficacy (6 min walk test) of epigallocatechin gallate (EGCg).
Another product showing promise in reducing oxidative stress is melatonin, a secreted product of the pineal gland. A 10-day treatment regimen in mdx5cv mice not only improved the redox status of the muscle by lowering oxidized/reduced glutathione levels but also showed functional improvement [99]. On a clinical level, 3 to 9 months after starting therapy in DMD, melatonin reduced levels of lipid peroxidation, IL-1, IL-2, IL-6, and TNF-α, and reduced serum CK by 50%, [100]. Considering the low side effect profile of this agent, it might be justified in considering melatonin as a candidate for a more extensive clinical trial [101].
Idebenone, a short-chain benzoquinone structurally related to coenzyme Q10, is another strong anti-oxidant reported to improve mitochondrial respiratory chain function and cellular energy production [102]. In mdx mice, a long-term treatment protocol (10 months) with idebenone significantly corrected cardiac diastolic dysfunction, reduced inflammation and fibrosis and significantly improved voluntary running [103]. Respiratory functional improvement and a trend towards cardiac functional improvement were subsequently reported in a Phase II tolerability and efficacy clinical trial in DMD boys [104]. A phase III randomized controlled study is currently ongoing to further investigate the potential therapeutic role of idebenone in DMD (http://www.Clinicaltrials.gov).
5.5 NF-κB inhibition
A key feature of DMD is the widespread inflammation followed by myofiber necrosis. This is reported to be due to chronic activation of the NF-κB signaling pathway in DMD [31], also reported in the mdx mouse [94]. NF-κB is activated both in muscle fibers and in macrophages in response to ROS and many inflammatory molecules such as interleukin-1β (IL-1β) and TNF-α that result in muscle fiber necrosis (Fig 1) [31, 105]. Once activated, NF-κB regulates a plethora of genes involved in inflammatory and immune responses [106] (Fig 1, Fig 2). This pathway can be viewed as a master regulator of inflammation, usually important in the body’s defense, but when chronically activated, deleterious consequences can arise including a role in muscle fiber damage.
Figure 2.
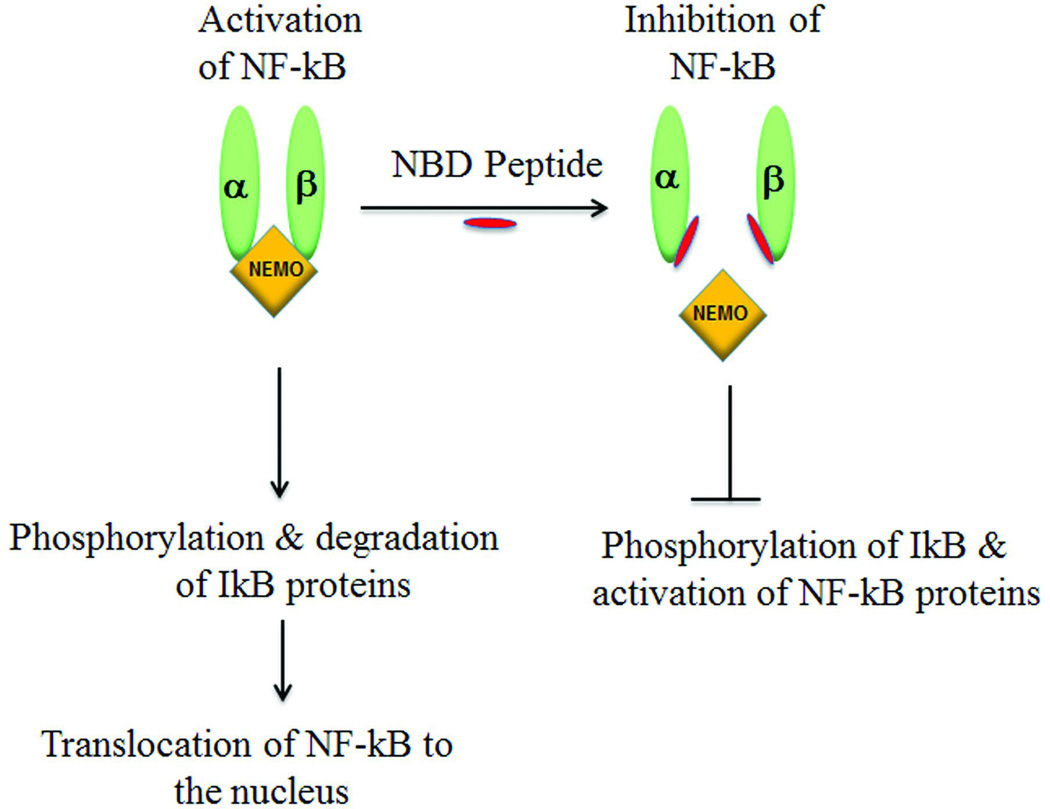
The left side of the figure depicts activation of NF-κB occuring only in the presence of a fully assembled IKK complex composed of 3 catalytic subunits: IKKα and IKKβ that bind to the regulatory subunit IKKγ, also known as NEMO. Following a stimulus for activation of NF-κB by inflammatory cytokines like TNF-α the intact IKK complex promotes phosphorylation and degradation of IkB proteins, which in turn unmasks a nuclear localization signal permitting NF-κB translocation to the nucleus and subsequent transcription of cytokine and chemokine genes that enhance the inflammatory process. The consequences of adding NEMO binding domain (NBD) peptide (red) are depicted. NBD is an 11 amino acid peptide that binds to the c-terminal region of both IKKα & IKKβ inhibiting the formation of active IKK complex. Consequently, downstream signaling events are blocked.
Understanding the sequential steps of the NF-κB pathway is important in considering potential pharmacological targets for treatment. Activation of NF-κB relies on the assembly of the IKK complex (Fig 2), composed of catalytic subunits IKKα and IKKβ that bind to the regulatory subunit IKKγ, also known as NEMO. Following a stimulus (e.g. TNF-α), the IKK complex promotes phosphorylation of IκB (inhibitory kappa B) proteins, which leads to polyubiquination and subsequent proteasome degradation. This in turn unmasks the nuclear localization signal resulting in NF-κB translocation to the nucleus and subsequent transcription of cytokine and chemokine genes, as an integral part of an inflammatory process.
Non-specific anti-inflammatory drugs like glucocorticoids, prednisone and deflazacort, used for treatment of DMD, exert their effect partially by inhibiting NF-κB [107]. These drugs are non-specific inhibitors and have multiple side effects. Pre-clinical studies in mdx mice have been reported to improve dystrophic pathology using more specific pharmacological inhibitors of the NF-κB signaling pathway (Table 3). Notable among them are the inhibitors of TNF-α, a chemokine that induces NF-κB. Remicade (antibody to human TNF-α, also known as Infliximab) and etanercept (blocks soluble receptor to TNF-α) are two candidates that are used clinically to treat inflammatory disorders. Remicade delayed and reduced inflammation and the breakdown of dystrophic muscle without adverse effects in mdx mice [108]. Etanercept counteracted exercise-induced force reduction in skeletal muscle [109] and protected against exercise-induced myofiber necrosis in mdx mice [110]. In addition, a murine specific TNF-α antibody, cV1q (not for clinical use) significantly reduced contractile dysfunction and myofiber necrosis [111]. Past studies using these TNF-α inhibitors have usually been limited to 12 weeks duration. While they suffice as demonstration of proof-of-principle, longer-term studies would be preferable to satisfy FDA guidelines for clinical trials in boys with DMD. Both Etanercept and remicade have been used in children to treat psoriasis [112] and juvenile idiopathic arthritis [113] paving a path for use in DMD boys.
Another molecule of interest is the NEMO Binding domain (NBD) peptide that functions as a specific inhibitor of the NF-κB signaling pathway. NBD is an 11 amino acid peptide that blocks activation of NF-κB by preventing assembly of the IKK complex (Fig 2). As indicated above, evidence points to the chronic activation of NF-κB in the dystrophin-deficient mdx mouse and in muscle biopsies of DMD patients [31, 106]. Administration of NBD peptide has been shown to reduce skeletal muscle damage and enhance muscle function in the mdx mouse [94, 114, 115]. NBD peptide is currently poised to move forward for clinical trials in DMD patients after additional pre-clinical testing assures safety and efficacy in mouse and dog models of DMD.
5.6 Drugs for Phosphodiesterase 5A Inhibition
More than 3 decades ago, functional ischemia was proposed as a pathogenic mechanism of muscle fiber damage in DMD based on experimental findings reproducing the histopathological pattern of muscle necrosis and regeneration [116]. Recent studies reinforce the potential role of ischemia through molecular-based findings demonstrating deficiency of nitric oxide synthase (NOS) in skeletal muscle. Understanding changes in the NOS pathway provides insight for implementation of therapeutic strategies helping to circumvent ischemia as a contributory factor in DMD muscle damage. New studies have also emphasize that NOS derivatives can have a direct effect on skeletal muscle contractile elements that modulate muscle fatigue and post-exercise force output [117].
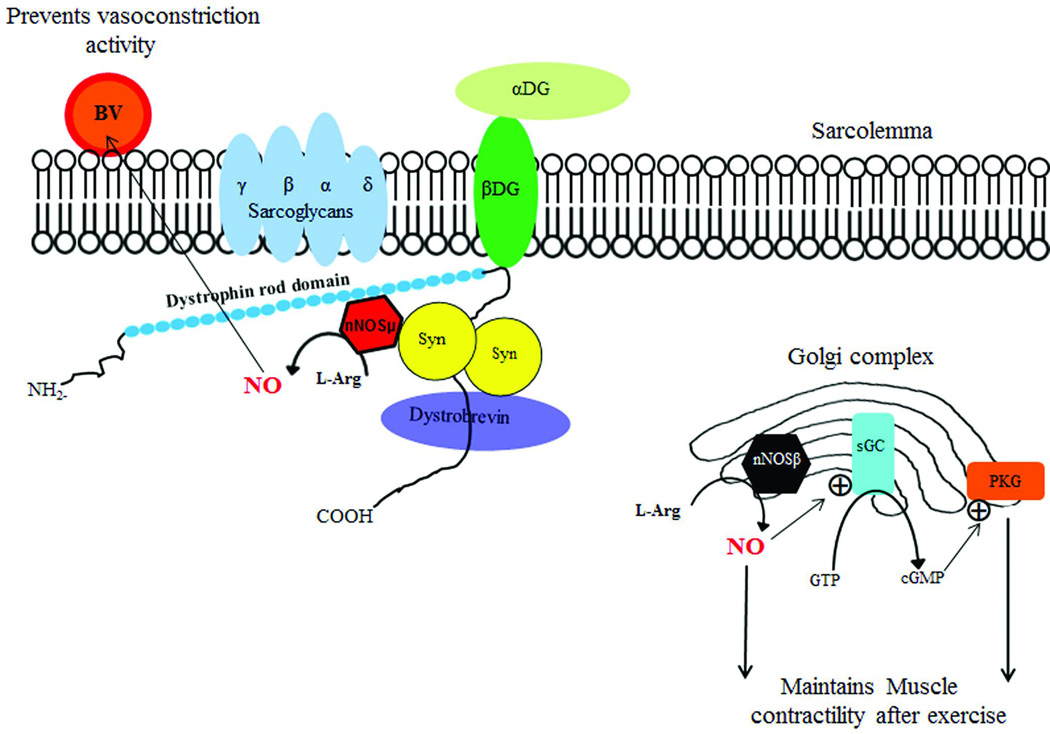
In skeletal and smooth muscle, neuronal NOS (nNOS) is the predominant source of nitric oxide (NO) (Fig 3). Two functionally distinct nNOS micro-domains are the result of alternatively spliced gene products, one localized to the sarcolemma and cytoplasm, nNOSµ and the other found in Golgi, nNOSβ. NO is the enzymatic product of both isoforms of NOS. Spliced variants of nNOS signaling have a predominant influence on two distinct targets. nNOSµ-derived NO attenuates vasoconstriction helping to balance the demands of skeletal muscle activity with required delivery of blood and oxygen. nNOSβ-derived NO maintains the ability of contracting muscle to generate force during and after exercise. The NO generated through the nNOSβ pathway binds to soluble guanylyl cyclase (sGC) and stimulates the conversion of guanosine triphosphate (GTP) into cyclic guanosine monophosphate (cGMP). Signaling through this pathway regulates muscle contractility mediated by protein kinase G (PKG).
Figure 3.
The role of different isoforms of nNOS, namely nNOSµ and nNOSβ in skeletal muscle, is illustrated. Two binding sites of nNOSµ can be seen: at the dystrophin rod domain and at α-syntrophin (a member of dystrophin-glycoprotein complex). NO derived from nNOSµ enhances blood flow to muscle during activity by preventing vasoconstriction of nearby blood vessels during muscle contraction; the increased blood flow is a source of oxygen supply to the muscle during exercise. In contrast, nNOSβ signaling at the Golgi complex regulates force generation during and after exercise generating cGMP dependent protein kinase G (PKG). (Adapted with permission of the American Society for Clinical Investigation from Percival et al. 2010 [117]). Abbreviations: nNOS- nitric oxide synthase; BV-blood vessel; aDG-alpha-dystroglycan; b-DG- beta-dystroglycan; NO- nitric oxide; Syn –syntrophin; GTP- guanosine triphosphate; sGC- soluble guanylyl cyclase; cGMP- cyclic guanosine monophosphate
In DMD the absence of dystrophin leads to mislocalization of nNOSµ because of the loss α-syntrophin [118], its ligand to the dystrophin-glycoprotein complex. nNOSµ expression is substantially reduced (>80%) in dystrophin-deficient muscles [118]. Validation of the DMD-associated aberrant response in this pathway has been illustrated by the decrease in muscle oxygenation observed in exercising skeletal muscle caused by unopposed sympathetic vasoconstriction [34]. This contrasts with responses in healthy children with normal nNOS where blood flow is maintained or increased due to vasodilatation.
At present, it is not possible to selectively increase nNOSµ expression using a pharmacological approach. It is, however, possible to enhance GMP signaling by inhibiting the activity of GMP-hydrolyzing phosphodiesterases (PDEs). PDE5A inhibitors that are currently available for clinical use include sildenafil (Viagra®, Revatio®) or tadalafil (Cialis®). Both block cGMP breakdown, thereby raising cellular cGMP concentrations in many tissues, including smooth and skeletal muscle, and probably cardiac muscle as well. This class of drugs has been approved for treatment of pulmonary hypertension in children paving the way for clinical trials in DMD patients.
Pre-clinical studies in the mdx mice further support bringing PDE5A inhibitors forward for clinical trials. Both tadalafil and sildenafil restore blood supply to skeletal muscles after exercise [119, 120] and protect the dystrophin-deficient skeletal muscle from contraction-induced injury [119]. In addition, a protective effect is further illustrated by the reduction in serum CK and improved histopathological appearance of muscle. These findings demonstrate that PDE5A inhibitors can compensate for the loss of vasomodulatory function related to nNOSµ depletion. Cardiac muscle of the mdx mouse likewise shows benefit from PDE5A inhibition. Sildenafil reduces Evan’s Blue dye uptake in mdx cardiomyocytes, indicative of improved membrane stability, less susceptible to contraction-induced damage [121].
Currently PDE5A inhibitors are in clinical trial and centers are openly recruiting DMD and BMD subjects for short and long-term studies. Clinical trial details and opportunities are published at http://www.Clinicaltrials.Gov. A potential caveat for clinical improvement is the severe loss of nNOS in DMD muscle because of degradation of the dystrophin-glycoprotein complex and loss of the α-syntrophin ligand [122, 123]. It is assumed that the Golgi-associated nNOSβ is also a source of NO for the vasculature helping to rescue the muscle through multiple mechanisms.
5.7 CINODS (COX inhibiting nitric oxide-donators)
Inhibition of cyclooxygenase (COX) enzymes is the basic mode of action of non-steroidal anti-inflammatory drugs (NSAIDs). CINODS represent chemical entities that are cleaved in the body yielding a nitric oxide-donating moiety and a balanced COX-1/COX-2 inhibiting non-steroidal anti-inflammatory agent. Naproxcinod (HCT 3012) is a derivative of naproxen with a nitroxybutyl ester to allow it to also act as a nitric oxide (NO) donor. The therapeutic potential derived from the NO regulation of blood flow to muscle has been fully discussed using phosphodiesterase inhibitors (PGE5A inhibition). Added efficacy results from the enhanced capacity of NO to improve muscle function through activation of satellite precursor cells facilitating muscle repair and regeneration [124–126]. Combining NO with an anti-inflammatory agent enhances the potential for the treatment of DMD. Multiple lines of evidence demonstrate the efficacy of reducing inflammation in DMD. The benefit of prednisone and deflazacort has been reviewed (see Current Treatment above). Other approaches reinforce the protective effects of anti-inflammatory products. For example, antibody to TNF-α in mdx mice prevents the early acute phase of myofiber necrosis [108, 127]. Skeletal muscle benefits are further illustrated by preventing mast cell degranulation and inhibiting release of inflammatory mediators resulting from NF-κB signaling [94, 106].
The safety of Naproxcinod has been demonstrated in phase II and phase III clinical trials that include more than 4000 subjects with osteoarthritis treated for up to 65 weeks [128, 129]. In these studies gastrointestinal side effects and hypertension, commonly seen with NSAIDs, were kept to a minimum through the release of nitric oxide [130, 131].
Proof of principle studies in the mdx and alpha-sarcoglycan knockout mice using HCT 1026, an alternative CINOD product, combining the NSAID flurbiprofen with a NO-donor moiety, have demonstrated favorable results [132]. In both models, HCT 1026 significantly ameliorated the morphological, biochemical, and functional phenotype in the absence of significant adverse effects. Evidence that there was slowing of disease progression was demonstrated by reduced muscle damage, accompanied by decreased cellular inflammation, a drop in serum CK and preservation of the number of satellite cells. HCT 1026 was significantly more effective than a comparative cohort taking prednisolone. An additional beneficial effect of HCT 1026 was the enhanced therapeutic efficacy of arterially delivered donor stem cells capable of a 4-fold increase in their ability to migrate and reconstitute muscle fibers.
These studies lay the groundwork for moving CINODS to clinical trial in DMD. Naproxcinod is the preferred drug because of its excellent safety record in the treatment of osteoarthritis. Perhaps wanting for this particular CINOD are more pre-clinical studies in dystrophin-deficient mice to reinforce naproxcinod efficacy in DMD-related muscular dystrophy. As noted above most of the pre-clinical work using CINODs has been done with HCT 1026. Presently NicOx S.A., a French pharmaceutical company, is planning a stepped up program to bring HCT 3012 into the clinical arena (TREAT-NMD Website). There has also been a 12-month clinical trial in in DMD, BMD and LGMD patients in Italy using isosorbide dinitrate as the NO donor combined with ibuprofen. The Italian study demonstrated a favorable safety and tolerability threshold and preliminary evidence of efficacy using a combined motor function scale [133].
6. Potential Developmental Issues
Several challenges for pharmacologic treatment of DMD must be considered. Given that that the use of glucocorticoids (prednisone or deflazacort) has become the standard of care in most regions of the USA and in many parts of Europe, the design and interpretation of any prospective study is more difficult. In most instances, contemporary clinical protocols are directly comparing one cohort taking glucocorticoid alone versus a second cohort taking glucocorticoid plus test product. A further challenge then becomes the risk that the glucocorticoid could obscure the efficacy of the test product.
Inherently there exists a potential bias toward the use of molecular-based therapies, however, to date the benefits have been modest. In the case of exon skipping, the translational product will depend on the specific exon(s) skipped. This is not unexpected based on observations in BMD where different deletion mutations (i.e., diverse truncated dystrophin proteins) result in variable severity of disease related to the ability of the truncated dystrophin peptide to correct the underlying defect [5]. With regard to stop codon readthrough (mutation suppression) the FDA has continued to challenge the results of the first clinical trial and asked for additional information before approving Ataluren for clinical use. The other major category of molecular therapy, utrophin upregulation, appears to hold promise but agents capable of achieving this goal are in the early stages of planned clinical trials.
Muscle growth factors based on myostatin inhibition have not shown efficacy (MYO-29) [63] or have been encumbered by side effects that stopped the clinical trial (ACE-031; http://www/Clinicaltrials.gov). The potential pharmacologic approaches to combat fibrosis are hampered by several factors. The failure to reproduce the findings of losartan in the mdx mouse [78, 79] and modest benefits of other agents like pirfenidone dampens enthusiasm for bringing these agents forward for clinical trials. Other products found to be effective in mdx mice such as anti-neoplastic drugs (suramin or Imatinib mesylate ) are not well positioned for clinical trials considering the risk benefit ratio.
Pre-clinical data emphasize the potential for efficacy with PDE5A inhibitors. Clinical trials are currently underway but focus on the cardiac outcome measures in dystrophinopathy patients 15 years and older (http:\\www.Clinicaltrials.gov). There is room for PDE5A clinical trials in younger boys targeting primary outcomes in skeletal muscle. The introduction of novel pharmacologic products like CINODS that combine inhibition of COX enzymes with a NO donor (Naproxcinod) are putatively attractive but will likely require further pre-clinical studies in dystrophin-deficient mice or dogs prior to clinical testing.
7. Conclusion
The targets for pharmacologic therapies are well defined and have been presented in this review. It is important to convey that the barriers to successful treatment of DMD are significant and have been emphasized in the discussion of each proposed agent. It may be that any single approach will not prove to be satisfactory and that multiple drugs, targeting more than one pathway, may be required. An unequivocal goal for any future treatment for DMD is a demonstrated efficacy equal or better than glucocorticoids. If any drug undergoing clinical testing demonstrates efficacy equal to glucocorticoids with a lower side effect profile, it will be poised for immediate clinical use. It would refreshing to relieve DMD boys of the obesity that accompanies steroid therapy, along with other side effects that include an increase in long bone and vertebral body fractures, cataracts, hypertension, increased insulin resistance, growth retardation, and delayed puberty.
8. Expert Opinion
The goals for future drug development have been established: at minimum there must be equal or better efficacy than glucocorticoids with a lower side effect profile. A drug can also qualify for marketing if a significant steroid-sparing component is demonstrated. At the time of writing this review, the neuromuscular community is excited by the potential for exon skipping. This is clearly appropriate but the challenges have been outlined in this review. Alternative approaches to pharmacologic treatment of DMD that may have an important future role have not been discussed here. These include gene replacement or gene repair and stem cell therapy, all of which have challenges. DMD gene replacement is limited because of the size of the full length transcript (14 kb) that cannot fit the <5 kb packaging limitations of AAV. This mandates the use of mini-dystrophin genes and the clinical efficacy of these attenuated gene products remains unknown. In addition, in the only human gene therapy study done to date in DMD, an immune response was encountered that has to be considered in future studies [36]. It was mentioned above that the repertoire of drugs available to reduce fibrosis in muscle is relatively restricted raising the possibility of a gene repair strategy employing mRNAs (miRNAs). Evidence from DMD patient muscle biopsies supports this concept based on the finding of down regulation of miRNAs contributing to the fibrotic process in DMD muscle biopsies [89]. In addtion, pre-clinical studies in mdx mice show that electroporation of miR-29 reduces collagen deposition in muscle [90]. More sustained delivery of miR-29 could be achieved using rAAV. Finally, it can be said that some investigators are enthusiastic about using stem cells to correct the underlying defect in DMD. Obstacles to this approach center around challenges in targeted skeletal muscle delivery, and which lineage of stem cells would be most appropriate for clinical application [37]. The most promising results have been achieved in the dystrophic dog model using a type of stem cell variant derived from mesoangioblasts (pericytes) [134]. A phase I trial using mesangioblasts has been proposed and is in the planning stages.
In the final analysis, all approaches to DMD treatment are still relatively early in development. In this review we have attempted to provide the most up to date information regarding the potential of pharmacologic agents to show efficacy and some index of their potential to ameliorate the disease.
Acknowledgments
JM is supported by grants from the NIH/NINDS (grant no. U54NS055958), NIH/NIDDK (grant no. 1U01NS058451-01), NIH/NINDS (1U01NS066914-01A1), NIH/NIAMS (grant no. 1R01AR060911-01), the Myositis Association, Parent Project Muscular Dystrophy, the Muscular Dystrophy Association (grant no. 158784 and the MDA DMD Clinical Research Network Project Grant), and Ben’s Dream-Sanfilippo Research Foundation.
Footnotes
All other authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- 1.Prevalence of Duchenne/Becker muscular dystrophy among males aged 5–24 years - four states, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:1119–1122. [PubMed] [Google Scholar]
- 2. Mendell JR, Shilling C, Leslie ND, et al. Evidence-based path to newborn screening for duchenne muscular dystrophy. Ann Neurol. 2012;71:304–313. doi: 10.1002/ana.23528. First report of two-tier system using dried blood spots taken at birth to identify DMD cases. CK is first determined and if it meets a threshold preditive of DMD gene mutations, DNA testing will follow on the same dried blood spot sample.
- 3.Brooke MH, Fenichel GM, Griggs RC, et al. Clinical investigation in Duchenne dystrophy: 2. Determination of the "power" of therapeutic trials based on the natural history. Muscle Nerve. 1983;6:91–103. doi: 10.1002/mus.880060204. [DOI] [PubMed] [Google Scholar]
- 4.Eagle M, Baudouin SV, Chandler C, et al. Survival in Duchenne muscular dystrophy: improvements in life expectancy since 1967 and the impact of home nocturnal ventilation. Neuromuscul Disord. 2002;12:926–929. doi: 10.1016/s0960-8966(02)00140-2. [DOI] [PubMed] [Google Scholar]
- 5. England SB, Nicholson LV, Johnson MA, et al. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. Case report of interest demonstrating a mild case of Becker muscular dystrophy with almost one-half of the DMD gene deleted.
- 6.Muntoni F, Torelli S, Ferlini A. Dystrophin and mutations: one gene, several proteins, multiple phenotypes. Lancet Neurol. 2003;2:731–740. doi: 10.1016/s1474-4422(03)00585-4. [DOI] [PubMed] [Google Scholar]
- 7. Koenig M, Hoffman EP, Bertelson CJ, et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. Describes the first cloning of the DMD gene. Historically important article.
- 8.Bonilla E, Samitt CE, Miranda AF, et al. Duchenne muscular dystrophy: deficiency of dystrophin at the muscle cell surface. Cell. 1988;54:447–452. doi: 10.1016/0092-8674(88)90065-7. [DOI] [PubMed] [Google Scholar]
- 9.Oudet C, Hanauer A, Clemens P, et al. Two hot spots of recombination in the DMD gene correlate with the deletion prone regions. Hum Mol Genet. 1992;1:599–603. doi: 10.1093/hmg/1.8.599. [DOI] [PubMed] [Google Scholar]
- 10. Chamberlain JS, Gibbs RA, Ranier JE, et al. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988;16:11141–11156. doi: 10.1093/nar/16.23.11141. First routine methodology clinically implemented for screening of the DMD gene for mutations.
- 11.Beggs AH, Koenig M, Boyce FM, et al. Detection of 98% of DMD/BMD gene deletions by polymerase chain reaction. Hum Genet. 1990;86:45–48. doi: 10.1007/BF00205170. [DOI] [PubMed] [Google Scholar]
- 12. Lalic T, Vossen RH, Coffa J, et al. Deletion and duplication screening in the DMD gene using MLPA. Eur J Hum Genet. 2005;13:1231–1234. doi: 10.1038/sj.ejhg.5201465. Highly accurate method for DMD gene mutation screening.
- 13.Dent KM, Dunn DM, von Niederhausern AC, et al. Improved molecular diagnosis of dystrophinopathies in an unselected clinical cohort. Am J Med Genet A. 2005;134:295–298. doi: 10.1002/ajmg.a.30617. [DOI] [PubMed] [Google Scholar]
- 14. Flanigan KM, von Niederhausern A, Dunn DM, et al. Rapid direct sequence analysis of the dystrophin gene. Am J Hum Genet. 2003;72:931–939. doi: 10.1086/374176. Important method of DMD gene screening to identify point mutations.
- 15.Zebracki K, Drotar D. Pain and activity limitations in children with Duchenne or Becker muscular dystrophy. Dev Med Child Neurol. 2008;50:546–552. doi: 10.1111/j.1469-8749.2008.03005.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen JY, Clark MJ. Family function in families of children with Duchenne muscular dystrophy. Fam Community Health. 2007;30:296–304. doi: 10.1097/01.FCH.0000290542.10458.f8. [DOI] [PubMed] [Google Scholar]
- 17.Mendell JR, Vaughn AJ. Duchenne muscular dystrophy: ethical and emotional considerations in long-term management. Semin Neurol. 1984;4:98–103. doi: 10.1055/s-2008-1041537. [DOI] [PubMed] [Google Scholar]
- 18. Mendell JR, Moxley RT, Griggs RC, et al. Randomized, double-blind six-month trial of prednisone in Duchenne's muscular dystrophy. N Engl J Med. 1989;320:1592–1597. doi: 10.1056/NEJM198906153202405. First description of glucocorticoid efficacy in DMD. Remains the only established method of treatment for this disease.
- 19. Biggar WD, Harris VA, Eliasoph L, et al. Long-term benefits of deflazacort treatment for boys with Duchenne muscular dystrophy in their second decade. Neuromuscul Disord. 2006;16:249–255. doi: 10.1016/j.nmd.2006.01.010. Describes efficacy of sodium sparing glucocorticoid therapy for DMD.
- 20.Griggs RC, Moxley RT, 3rd, Mendell JR, et al. Prednisone in Duchenne dystrophy. A randomized, controlled trial defining the time course and dose response. Clinical Investigation of Duchenne Dystrophy Group. Arch Neurol. 1991;48:383–388. doi: 10.1001/archneur.1991.00530160047012. [DOI] [PubMed] [Google Scholar]
- 21.Griggs RC, Moxley RT, 3rd, Mendell JR, et al. Duchenne dystrophy: randomized, controlled trial of prednisone (18 months) and azathioprine (12 months) Neurology. 1993;43:520–527. doi: 10.1212/wnl.43.3_part_1.520. [DOI] [PubMed] [Google Scholar]
- 22. Escolar DM, Hache LP, Clemens PR, et al. Randomized, blinded trial of weekend vs daily prednisone in Duchenne muscular dystrophy. Neurology. 2011;77:444–452. doi: 10.1212/WNL.0b013e318227b164. Describes efficacy of weekend treatment of DMD and compares directly with daily dosing.
- 23.Balaban B, Matthews DJ, Clayton GH, et al. Corticosteroid treatment and functional improvement in Duchenne muscular dystrophy: long-term effect. Am J Phys Med Rehabil. 2005;84:843–850. doi: 10.1097/01.phm.0000184156.98671.d0. [DOI] [PubMed] [Google Scholar]
- 24. King WM, Ruttencutter R, Nagaraja HN, et al. Orthopedic outcomes of long-term daily corticosteroid treatment in Duchenne muscular dystrophy. Neurology. 2007;68:1607–1613. doi: 10.1212/01.wnl.0000260974.41514.83. Reports the reduction in scoliosis coincident with glucocorticoid treatment of DMD.
- 25. van Deutekom JC, Janson AA, Ginjaar IB, et al. Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med. 2007;357:2677–2686. doi: 10.1056/NEJMoa073108. First report of efficacy of exon skipping in DMD using 2’O-methyl-ribo-oligonucleoside-phoshophorothioate (2OMe) oligomer.
- 26. Kinali M, Arechavala-Gomeza V, Feng L, et al. Local restoration of dystrophin expression with the morpholino oligomer AVI-4658 in Duchenne muscular dystrophy: a single-blind, placebo-controlled, dose-escalation, proof-of-concept study. Lancet Neurol. 2009;8:918–928. doi: 10.1016/S1474-4422(09)70211-X. Reports efficacy of exon skipping in DMD using a different antisense oligomer [phosphorodiamidate morpholino oligomers (PMOs)]
- 27.Davies J, Gilbert W, Gorini L. Streptomycin, Suppression, and the Code. Proc Natl Acad Sci U S A. 1964;51:883–890. doi: 10.1073/pnas.51.5.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson WF, Gorini L, Breckenridge L. Role of ribosomes in streptomycin-activated suppression. Proc Natl Acad Sci U S A. 1965;54:1076–1083. doi: 10.1073/pnas.54.4.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Squire S, Raymackers JM, Vandebrouck C, et al. Prevention of pathology in mdx mice by expression of utrophin: analysis using an inducible transgenic expression system. Hum Mol Genet. 2002;11:3333–3344. doi: 10.1093/hmg/11.26.3333. Report of the potential efficacy of utrophin upregulation as a treatment for DMD.
- 30.Nethery D, Callahan LA, Stofan D, et al. PLA(2) dependence of diaphragm mitochondrial formation of reactive oxygen species. J Appl Physiol. 2000;89:72–80. doi: 10.1152/jappl.2000.89.1.72. [DOI] [PubMed] [Google Scholar]
- 31. Acharyya S, Villalta SA, Bakkar N, et al. Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy. J Clin Invest. 2007;117:889–901. doi: 10.1172/JCI30556. Describes the potential for therapeutic efficacy of nemo binding peptide in DMD.
- 32. Haidet AM, Rizo L, Handy C, et al. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci U S A. 2008;105:4318–4322. doi: 10.1073/pnas.0709144105. First report showing potential efficacy of follistatin delivered by rAAV.
- 33.Rodino-Klapac LR, Haidet AM, Kota J, et al. Inhibition of myostatin with emphasis on follistatin as a therapy for muscle disease. Muscle Nerve. 2009;39:283–296. doi: 10.1002/mus.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sander M, Chavoshan B, Harris SA, et al. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci U S A. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desguerre I, Mayer M, Leturcq F, et al. Endomysial fibrosis in Duchenne muscular dystrophy: a marker of poor outcome associated with macrophage alternative activation. J Neuropathol Exp Neurol. 2009;68:762–773. doi: 10.1097/NEN.0b013e3181aa31c2. [DOI] [PubMed] [Google Scholar]
- 36. Mendell JR, Campbell K, Rodino-Klapac L, et al. Dystrophin immunity in Duchenne's muscular dystrophy. N Engl J Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. Immunity to mini-dystrophin was seen following gene therapy for DMD.
- 37.Meng J, Muntoni F, Morgan JE. Stem cells to treat muscular dystrophies - where are we? Neuromuscul Disord. 2011;21:4–12. doi: 10.1016/j.nmd.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Winnard AV, Mendell JR, Prior TW, et al. Frameshift deletions of exons 3–7 and revertant fibers in Duchenne muscular dystrophy: mechanisms of dystrophin production. Am J Hum Genet. 1995;56:158–166. [PMC free article] [PubMed] [Google Scholar]
- 39.Burrow KL, Coovert DD, Klein CJ, et al. Dystrophin expression and somatic reversion in prednisone-treated and untreated Duchenne dystrophy. CIDD Study Group. Neurology. 1991;41:661–666. doi: 10.1212/wnl.41.5.661. [DOI] [PubMed] [Google Scholar]
- 40. McClorey G, Fletcher S, Wilton S. Splicing intervention for Duchenne muscular dystrophy. Curr Opin Pharmacol. 2005;5:529–534. doi: 10.1016/j.coph.2005.06.001. Provides good overview of exon skipping as a preview to clinical trials.
- 41. Malik V, Rodino-Klapac LR, Viollet L, et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann Neurol. 2010;67:771–780. doi: 10.1002/ana.22024. Clinical report of an aminoglycoside -induced stop codon readthrough in DMD.
- 42.Hirawat S, Welch EM, Elfring GL, et al. Safety, tolerability, and pharmacokinetics of PTC124, a nonaminoglycoside nonsense mutation suppressor, following single- and multiple-dose administration to healthy male and female adult volunteers. J Clin Pharmacol. 2007;47:430–444. doi: 10.1177/0091270006297140. [DOI] [PubMed] [Google Scholar]
- 43.Mann CJ, Honeyman K, Cheng AJ, et al. Antisense-induced exon skipping and synthesis of dystrophin in the mdx mouse. Proc Natl Acad Sci U S A. 2001;98:42–47. doi: 10.1073/pnas.011408598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goyenvalle A, Babbs A, Powell D, et al. Prevention of dystrophic pathology in severely affected dystrophin/utrophin-deficient mice by morpholino-oligomer-mediated exon-skipping. Mol Ther. 2010;18:198–205. doi: 10.1038/mt.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yokota T, Lu QL, Partridge T, et al. Efficacy of systemic morpholino exon-skipping in Duchenne dystrophy dogs. Ann Neurol. 2009;65:667–676. doi: 10.1002/ana.21627. Describes the efficacy of morpholino-based exon skipping in the dystrophic dog with potential insight into benefit for patients.
- 46. Goemans NM, Tulinius M, van den Akker JT, et al. Systemic administration of PRO051 in Duchenne's muscular dystrophy. N Engl J Med. 2011;364:1513–1522. doi: 10.1056/NEJMoa1011367. Systemically administered exon skipping antisense oligomer demonstrates clinical efficacy.
- 47. Cirak S, Arechavala-Gomeza V, Guglieri M, et al. Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet. 2011;378:595–605. doi: 10.1016/S0140-6736(11)60756-3. Systemically administered morpholino in DMD demonstrated dystrophin expression.
- 48.Barton-Davis ER, Cordier L, Shoturma DI, et al. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Invest. 1999;104:375–381. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner KR, Hamed S, Hadley DW, et al. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann Neurol. 2001;49:706–711. [PubMed] [Google Scholar]
- 50.Politano L, Nigro G, Nigro V, et al. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 2003;22:15–21. [PubMed] [Google Scholar]
- 51.Welch EM, Barton ER, Zhuo J, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 52.Clinical Trials. < http://www.clinicaltrials.gov/ct2/results?term=ptc124>.
- 53.Rowe SM, Clancy JP. Pharmaceuticals targeting nonsense mutations in genetic diseases: progress in development. BioDrugs. 2009;23:165–174. doi: 10.2165/00063030-200923030-00003. [DOI] [PubMed] [Google Scholar]
- 54.Wilschanski M, Miller LL, Shoseyov D, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J. 2011;38:59–69. doi: 10.1183/09031936.00120910. [DOI] [PubMed] [Google Scholar]
- 55. Tinsley J, Deconinck N, Fisher R, et al. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med. 1998;4:1441–144. doi: 10.1038/4033. Demonstrates the potential for utrophin upregulation as a treatment for DMD.
- 56.Gilbert R, Nalbantoglu J, Petrof BJ, et al. Adenovirus-mediated utrophin gene transfer mitigates the dystrophic phenotype of mdx mouse muscles. Hum Gene Ther. 1999;10:1299–1310. doi: 10.1089/10430349950017987. [DOI] [PubMed] [Google Scholar]
- 57.Tinsley JM, Fairclough RJ, Storer R, et al. Daily treatment with SMTC1100, a novel small molecule utrophin upregulator, dramatically reduces the dystrophic symptoms in the mdx mouse. PLoS One. 2011;6:e19189. doi: 10.1371/journal.pone.0019189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moorwood C, Lozynska O, Suri N, et al. Drug discovery for Duchenne muscular dystrophy via utrophin promoter activation screening. PLoS One. 2011;6:e26169. doi: 10.1371/journal.pone.0026169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krag TO, Bogdanovich S, Jensen CJ, et al. Heregulin ameliorates the dystrophic phenotype in mdx mice. Proc Natl Acad Sci U S A. 2004;101:13856–13860. doi: 10.1073/pnas.0405972101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Amenta AR, Yilmaz A, Bogdanovich S, et al. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci U S A. 2011;108:762–767. doi: 10.1073/pnas.1013067108. Potential therapeutic agent upregulating utrophin through a novel approach.
- 61. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. Important paper demonstrating the potential for myostatin inhibition.
- 62. Schuelke M, Wagner KR, Stolz LE, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350:2682–2688. doi: 10.1056/NEJMoa040933. Interesting report demonstrating a mutation of the myostatin gene causing muscle hypertrophy in an infant.
- 63.Wagner KR, Fleckenstein JL, Amato AA, et al. A phase I/IItrial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- 64.Bogdanovich S, Krag TO, Barton ER, et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418–421. doi: 10.1038/nature01154. [DOI] [PubMed] [Google Scholar]
- 65.Cadena SM, Tomkinson KN, Monnell TE, et al. Administration of a soluble activin type IIB receptor promotes skeletal muscle growth independent of fiber type. J Appl Physiol. 2010;109:635–642. doi: 10.1152/japplphysiol.00866.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SJ, Lee YS, Zimmers TA, et al. Regulation of muscle mass by follistatin and activins. Mol Endocrinol. 2010;24:1998–2008. doi: 10.1210/me.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Amthor H, Macharia R, Navarrete R, et al. Lack of myostatin results in excessive muscle growth but impaired force generation. Proc Natl Acad Sci U S A. 2007;104:1835–1840. doi: 10.1073/pnas.0604893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fryburg DA. Insulin-like growth factor I exerts growth hormone- and insulin-like actions on human muscle protein metabolism. Am J Physiol. 1994;267:E331–E336. doi: 10.1152/ajpendo.1994.267.2.E331. [DOI] [PubMed] [Google Scholar]
- 69.Barton ER, Morris L, Musaro A, et al. Muscle-specific expression of insulin-like growth factor I counters muscle decline in mdx mice. J Cell Biol. 2002;157:137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shavlakadze T, White J, Hoh JF, et al. Targeted expression of insulin-like growth factor-I reduces early myofiber necrosis in dystrophic mdx mice. Mol Ther. 2004;10:829–843. doi: 10.1016/j.ymthe.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 71.Lynch GS, Cuffe SA, Plant DR, et al. IGF-I treatment improves the functional properties of fast- and slow-twitch skeletal muscles from dystrophic mice. Neuromuscul Disord. 2001;11:260–268. doi: 10.1016/s0960-8966(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 72.Gregorevic P, Plant DR, Leeding KS, et al. Improved contractile function of the mdx dystrophic mouse diaphragm muscle after insulin-like growth factor-I administration. Am J Pathol. 2002;161:2263–2272. doi: 10.1016/S0002-9440(10)64502-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bernasconi P, Torchiana E, Confalonieri P, et al. Expression of transforming growth factor-beta 1 in dystrophic patient muscles correlates with fibrosis. Pathogenetic role of a fibrogenic cytokine. J Clin Invest. 1995;96:1137–1144. doi: 10.1172/JCI118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun G, Haginoya K, Wu Y, et al. Connective tissue growth factor is overexpressed in muscles of human muscular dystrophy. J Neurol Sci. 2008;267:48–56. doi: 10.1016/j.jns.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 75.Gosselin LE, Williams JE, Deering M, et al. Localization and early time course of TGF-beta 1 mRNA expression in dystrophic muscle. Muscle Nerve. 2004;30:645–653. doi: 10.1002/mus.20150. [DOI] [PubMed] [Google Scholar]
- 76.Passerini L, Bernasconi P, Baggi F, et al. Fibrogenic cytokines and extent of fibrosis in muscle of dogs with X-linked golden retriever muscular dystrophy. Neuromuscul Disord. 2002;12:828–835. doi: 10.1016/s0960-8966(02)00071-8. [DOI] [PubMed] [Google Scholar]
- 77. Cohn RD, van Erp C, Habashi JP, et al. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. Report aroused interest in the clinical value of angiotensin receptor blockade for the treatment of DMD.
- 78. Spurney CF, Sali A, Guerron AD, et al. Losartan decreases cardiac muscle fibrosis and improves cardiac function in dystrophin-deficient mdx mice. J Cardiovasc Pharmacol Ther. 2011;16:87–95. doi: 10.1177/1074248410381757. Losartan benefits the heart in dystrophin-deficient mice by reducing fibrosis.
- 79.Bish LT, Yarchoan M, Sleeper MM, et al. Chronic losartan administration reduces mortality and preserves cardiac but not skeletal muscle function in dystrophic mice. PLoS One. 2011;6:e20856. doi: 10.1371/journal.pone.0020856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McGaha TL, Phelps RG, Spiera H, et al. Halofuginone, an inhibitor of type-I collagen synthesis and skin sclerosis, blocks transforming-growth-factor-beta-mediated Smad3 activation in fibroblasts. J Invest Dermatol. 2002;118:461–470. doi: 10.1046/j.0022-202x.2001.01690.x. [DOI] [PubMed] [Google Scholar]
- 81.Nagler A, Firman N, Feferman R, et al. Reduction in pulmonary fibrosis in vivo by halofuginone. Am J Respir Crit Care Med. 1996;154:1082–1086. doi: 10.1164/ajrccm.154.4.8887611. [DOI] [PubMed] [Google Scholar]
- 82.Huebner KD, Jassal DS, Halevy O, et al. Functional resolution of fibrosis in mdx mouse dystrophic heart and skeletal muscle by halofuginone. Am J Physiol Heart Circ Physiol. 2008;294:H1550–H1561. doi: 10.1152/ajpheart.01253.2007. [DOI] [PubMed] [Google Scholar]
- 83. Turgeman T, Hagai Y, Huebner K, et al. Prevention of muscle fibrosis and improvement in muscle performance in the mdx mouse by halofuginone. Neuromuscul Disord. 2008;18:857–868. doi: 10.1016/j.nmd.2008.06.386. Halofuginone has potential as a therapeutic agent for DMD.
- 84.Carter NJ. Pirfenidone: in idiopathic pulmonary fibrosis. Drugs. 2011;71:1721–1732. doi: 10.2165/11207710-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 85.Gosselin LE, Williams JE, Personius K, et al. A comparison of factors associated with collagen metabolism in different skeletal muscles from dystrophic (mdx) mice: impact of pirfenidone. Muscle Nerve. 2007;35:208–216. doi: 10.1002/mus.20681. [DOI] [PubMed] [Google Scholar]
- 86.Taniguti AP, Pertille A, Matsumura CY, et al. Prevention of muscle fibrosis and myonecrosis in mdx mice by suramin, a TGF-beta1 blocker. Muscle Nerve. 2011;43:82–87. doi: 10.1002/mus.21869. [DOI] [PubMed] [Google Scholar]
- 87.Bizario JC, Cerri DG, Rodrigues LC, et al. Imatinib mesylate ameliorates the dystrophic phenotype in exercised mdx mice. J Neuroimmunol. 2009;212:93–101. doi: 10.1016/j.jneuroim.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Huang P, Zhao XS, Fields M, et al. Imatinib attenuates skeletal muscle dystrophy in mdx mice. FASEB J. 2009;23:2539–2548. doi: 10.1096/fj.09-129833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eisenberg I, Eran A, Nishino I, et al. Distinctive patterns of microRNA expression in primary muscular disorders. Proc Natl Acad Sci U S A. 2007;104:17016–17021. doi: 10.1073/pnas.0708115104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cacchiarelli D, Martone J, Girardi E, et al. MicroRNAs involved in molecular circuitries relevant for the Duchenne muscular dystrophy pathogenesis are controlled by the dystrophin/nNOS pathway. Cell Metab. 2010;12:341–351. doi: 10.1016/j.cmet.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Hauser E, Hoger H, Bittner R, et al. Oxyradical damage and mitochondrial enzyme activities in the mdx mouse. Neuropediatrics. 1995;26:260–262. doi: 10.1055/s-2007-979768. [DOI] [PubMed] [Google Scholar]
- 92.Whitehead NP, Yeung EW, Froehner SC, et al. Skeletal muscle NADPH oxidase is increased and triggers stretch-induced damage in the mdx mouse. PLoS One. 2010;5:e15354. doi: 10.1371/journal.pone.0015354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tidball JG, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J Appl Physiol. 2007;102:1677–1686. doi: 10.1152/japplphysiol.01145.2006. Good review of free radical toxicity as a pathogenic mechanism in DMD.
- 94.Messina S, Bitto A, Aguennouz M, et al. Nuclear factor kappa-B blockade reduces skeletal muscle degeneration and enhances muscle function in Mdx mice. Exp Neurol. 2006;198:234–241. doi: 10.1016/j.expneurol.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 95.Whitehead NP, Pham C, Gervasio OL, et al. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J Physiol. 2008;586:2003–2014. doi: 10.1113/jphysiol.2007.148338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nakae Y, Hirasaka K, Goto J, et al. Subcutaneous injection, from birth, of epigallocatechin-3-gallate, a component of green tea, limits the onset of muscular dystrophy in mdx mice: a quantitative histological, immunohistochemical and electrophysiological study. Histochem Cell Biol. 2008;129:489–501. doi: 10.1007/s00418-008-0390-2. [DOI] [PubMed] [Google Scholar]
- 97.Dorchies OM, Wagner S, Vuadens O, et al. Green tea extract and its major polyphenol (-)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am J Physiol Cell Physiol. 2006;290:C616–C625. doi: 10.1152/ajpcell.00425.2005. [DOI] [PubMed] [Google Scholar]
- 98.Evans NP, Call JA, Bassaganya-Riera J, et al. Green tea extract decreases muscle pathology and NF-kappaB immunostaining in regenerating muscle fibers of mdx mice. Clin Nutr. 2010;29:391–398. doi: 10.1016/j.clnu.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hibaoui Y, Reutenauer-Patte J, Patthey-Vuadens O, et al. Melatonin improves muscle function of the dystrophic mdx5Cv mouse, a model for Duchenne muscular dystrophy. J Pineal Res. 2011;51:163–171. doi: 10.1111/j.1600-079X.2011.00871.x. [DOI] [PubMed] [Google Scholar]
- 100.Chahbouni M, Escames G, Lopez LC, et al. Melatonin treatment counteracts the hyperoxidative status in erythrocytes of patients suffering from Duchenne muscular dystrophy. Clin Biochem. 2011;44:853–858. doi: 10.1016/j.clinbiochem.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 101.Sanchez-Barcelo EJ, Mediavilla MD, Tan DX, et al. Clinical uses of melatonin: evaluation of human trials. Curr Med Chem. 2010;17:2070–2095. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- 102.Gillis JC, Benefield P, McTavish D. Idebenone. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in age-related cognitive disorders. Drugs Aging. 1994;5:133–152. doi: 10.2165/00002512-199405020-00007. [DOI] [PubMed] [Google Scholar]
- 103.Buyse GM, Van der Mieren G, Erb M, et al. Long-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: cardiac protection and improved exercise performance. Eur Heart J. 2009;30:116–124. doi: 10.1093/eurheartj/ehn406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buyse GM, Goemans N, van den Hauwe M, et al. Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: results from a 12 month, double-blind, randomized placebo-controlled trial. Neuromuscul Disord. 2011;21:396–405. doi: 10.1016/j.nmd.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 105.Lawler JM. Exacerbation of pathology by oxidative stress in respiratory and locomotor muscles with Duchenne muscular dystrophy. J Physiol. 2011;589:2161–2170. doi: 10.1113/jphysiol.2011.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Peterson JM, Guttridge DC. Skeletal muscle diseases, inflammation, and NF-kappaB signaling: insights and opportunities for therapeutic intervention. Int Rev Immunol. 2008;27:375–387. doi: 10.1080/08830180802302389. Review of inflammation and NF-kappaB signaling in relation to DMD pathogenesis and possible treatment opportunities.
- 107.Du J, Mitch WE, Wang X, et al. Glucocorticoids induce proteasome C3 subunit expression in L6 muscle cells by opposing the suppression of its transcription by NF-kappa B . J Biol Chem. 2000;275:19661–19666. doi: 10.1074/jbc.M907258199. [DOI] [PubMed] [Google Scholar]
- 108.Grounds MD, Torrisi J. Anti-TNFalpha (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 2004;18:676–682. doi: 10.1096/fj.03-1024com. [DOI] [PubMed] [Google Scholar]
- 109.Pierno S, Nico B, Burdi R, et al. Role of tumour necrosis factor alpha, but not of cyclo-oxygenase-2-derived eicosanoids, on functional and morphological indices of dystrophic progression in mdx mice: a pharmacological approach. Neuropathol Appl Neurobiol. 2007;33:344–359. doi: 10.1111/j.1365-2990.2007.00798.x. [DOI] [PubMed] [Google Scholar]
- 110.Hodgetts S, Radley H, Davies M, et al. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFalpha function with Etanercept in mdx mice. Neuromuscul Disord. 2006;16:591–602. doi: 10.1016/j.nmd.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 111.Piers AT, Lavin T, Radley-Crabb HG, et al. Blockade of TNF in vivo using cV1q antibody reduces contractile dysfunction of skeletal muscle in response to eccentric exercise in dystrophic mdx and normal mice. Neuromuscul Disord. 2011;21:132–141. doi: 10.1016/j.nmd.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 112.Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358:241–251. doi: 10.1056/NEJMoa066886. [DOI] [PubMed] [Google Scholar]
- 113.Otten MH, Prince FH, Armbrust W, et al. Factors associated with treatment response to etanercept in juvenile idiopathic arthritis. JAMA. 2011;306:2340–2347. doi: 10.1001/jama.2011.1671. [DOI] [PubMed] [Google Scholar]
- 114. Peterson JM, Kline W, Canan BD, et al. Peptide-based inhibition of NF-kappaB rescues diaphragm muscle contractile dysfunction in a murine model of Duchenne muscular dystrophy. Mol Med. 2011;17:508–515. doi: 10.2119/molmed.2010.00263. Additional evidence that nemo binding peptide to block NF-kappaB is beneficial in dystrophin deficiency.
- 115.Delfin DA, Xu Y, Peterson JM, et al. Improvement of cardiac contractile function by peptide-based inhibition of NF-kappaB in the utrophin/dystrophin-deficient murine model of muscular dystrophy. J Transl Med. 2011;9:68. doi: 10.1186/1479-5876-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mendell JR, Engel WK, Derrer EC. Duchenne muscular dystrophy: functional ischemia reproduces its characteristic lesions. Science. 1971;172:1143–1145. doi: 10.1126/science.172.3988.1143. [DOI] [PubMed] [Google Scholar]
- 117.Percival JM, Anderson KN, Huang P, et al. Golgi and sarcolemmal neuronal NOS differentially regulate contraction-induced fatigue and vasoconstriction in exercising mouse skeletal muscle. J Clin Invest. 2010;120:816–826. doi: 10.1172/JCI40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chang WJ, Iannaccone ST, Lau KS, et al. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci U S A. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. nNOS is shown to be an integral component of the dystrophin-glycoprotein complex validated by affect of nNOS mRNA on dystrophin expression.
- 119.Asai A, Sahani N, Kaneki M, et al. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One. 2007;2:e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kobayashi YM, Rader EP, Crawford RW, et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. Findings suggested that the exaggerated fatigue response to mild exercise is a lack of contraction-induced signalling from sarcolemma-localized nNOS.
- 121.Khairallah M, Khairallah RJ, Young ME, et al. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci U S A. 2008;105:7028–7033. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagayama T, Zhang M, Hsu S, et al. Sustained soluble guanylate cyclase stimulation offsets nitric-oxide synthase inhibition to restore acute cardiac modulation by sildenafil. J Pharmacol Exp Ther. 2008;326:380–387. doi: 10.1124/jpet.108.137422. [DOI] [PubMed] [Google Scholar]
- 123.Fernhoff NB, Derbyshire ER, Marletta MA. A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc Natl Acad Sci U S A. 2009;106:21602–21607. doi: 10.1073/pnas.0911083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang G, Burczynski FJ, Hasinoff BB, et al. Development of a nitric oxide-releasing analogue of the muscle relaxant guaifenesin for skeletal muscle satellite cell myogenesis. Mol Pharm. 2009;6:895–904. doi: 10.1021/mp800226z. [DOI] [PubMed] [Google Scholar]
- 125.Pisconti A, Brunelli S, Di Padova M, et al. Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol. 2006;172:233–244. doi: 10.1083/jcb.200507083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gordon JR, Galli SJ. Mast cells as a source of both preformed and immunologically inducible TNF-alpha/cachectin. Nature. 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 128.Baerwald C, Verdecchia P, Duquesroix B, et al. Efficacy, safety, and effects on blood pressure of naproxcinod 750 mg twice daily compared with placebo and naproxen 500 mg twice daily in patients with osteoarthritis of the hip: a randomized, double-blind, parallel-group, multicenter study. Arthritis Rheum. 2010;62:3635–3644. doi: 10.1002/art.27694. [DOI] [PubMed] [Google Scholar]
- 129.Schnitzer TJ, Hochberg MC, Marrero CE, et al. Efficacy and safety of naproxcinod in patients with osteoarthritis of the knee: a 53-week prospective randomized multicenter study. Semin Arthritis Rheum. 2011;40:285–297. doi: 10.1016/j.semarthrit.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 130.Townsend R, Bittar N, Rosen J, et al. Blood pressure effects of naproxcinod in hypertensive patients. J Clin Hypertens (Greenwich) 2011;13:376–384. doi: 10.1111/j.1751-7176.2010.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.White WB, Schnitzer TJ, Bakris GL, et al. Effects of naproxcinod on blood pressure in patients with osteoarthritis. Am J Cardiol. 2011;107:1338–1345. doi: 10.1016/j.amjcard.2010.12.046. [DOI] [PubMed] [Google Scholar]
- 132.Brunelli S, Sciorati C, D'Antona G, et al. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci U S A. 2007;104:264–269. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. D'Angelo MG, Gandossini S, Martinelli Boneschi F, et al. Nitric oxide donor and non steroidal anti inflammatory drugs as a therapy for muscular dystrophies: Evidence from a safety study with pilot efficacy measures in adult dystrophic patients. Pharmacol Res. 2012;65:472–479. doi: 10.1016/j.phrs.2012.01.006. Demonstrates additive benefit of a nitric oxide donor combined with an NSAIDs as a therapeutic modality for DMD.
- 134. Sampaolesi M, Blot S, D'Antona G, et al. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. Demonstration of the potential for stem cell therapy in DMD.