Abstract
BACKGROUND
The treatment of metastatic renal cell carcinoma (RCC) with high-dose interleukin-2 (HD IL-2) has resulted in durable tumor regression in a minority of patients. The current study presents the authors’ 20-year experience administering this immunotherapeutic agent.
METHODS
Patients with metastatic RCC (n = 259) were treated with HD IL-2 alone from January 13, 1986 through December 31, 2006 at the Surgery Branch of the National Cancer Institute. Potential predictive factors for response and survival, both pretreatment and treatment-related, were first subjected to univariate analysis and then to multivariate logistic regression or a Cox proportional hazards model. Finally, the authors investigated Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic factors for survival to assess their predictive value in the patient population in the current study.
RESULTS
A total of 23 patients experienced a complete response and 30 patients achieved a partial response, for an overall objective response rate of 20%. All partial responders had developed disease recurrence at the time of last follow-up, but only 4 complete responders had experienced disease recurrence by that time. Despite toxicities, only 2 patients developed treatment-related mortalities over this same time period. A higher baseline weight (P =.05) and MSKCC prognostic factors (P = .02) were found to be the variables most associated with response. For survival >4 years and overall survival, several pretreatment and treatment-related factors maintained significance, but none more so than response (P <.0001).
CONCLUSIONS
HD IL-2 can induce complete tumor regression in a small number of patients, and many patients have experienced extended disease-free intervals. Given its relative safety, HD IL-2 should still be considered a first-line therapy in patients with metastatic RCC who have an overall good performance status.
Keywords: renal cell carcinoma, interleukin-2, immunotherapy, metastatic
By current estimates, approximately 51,000 individuals in the U.S. will have been diagnosed with renal cell carcinoma (RCC) in 2007 and 1 in 74 men and women will receive this diagnosis during their lifetime. Over the last 20 years, there has been a significant increase in the incidence of RCC. Although 5-year survival for disease diagnosed at stage I (T1a-T1bN0M0: tumor up to 7 cm in greatest dimension [T1a-T1b], no regional lymph node metastasis [N0], and no distant metastasis [M0]) is near 90%, the expected 5-year survival for patients with metastatic disease at the time of diagnosis is approximately 10%.1,2 Despite its relatively dismal prognosis, metastatic RCC has paradoxically been one of the few solid human tumors that can still be cured after metastasizing widely. Since the inception of its use in the mid-1980s, treatment with high-dose interleukin-2 (HD IL-2) has resulted in complete and durable tumor regressions and long-term survival in a small percentage of patients with metastatic RCC.
Based on our single-institution experience and the results from 21 other sites, in 1992 the U.S. Food and Drug Administration (FDA) approved the use of HD IL-2 for patients with metastatic RCC. Over the last 20 years, our group and others have attempted to enhance the effectiveness of HD IL-2 by modifying it using polyethylene glycol to extend its half-life or combining it with interferon-α (IFN-α), lymphokine-activated killer (LAK) cells, or several chemotherapy agents.3–7 In addition, attempts have been made to mitigate the toxicities of HD IL-2 by using lower intravenous doses or administering it as a subcutaneous injection.8–10 Despite these efforts, randomized studies have failed to demonstrate that any of theses various combinations were superior to HD IL-2 administered intravenously alone.11 Thus, HD IL-2 remains the standard IL-2–based treatment for eligible patients with metastatic RCC.
Multiple studies have demonstrated that HD IL-2 can produce an objective response rate of approximately 20% in patients with RCC. Complete tumor regressions can occur in 7% to 10% of patients and are generally durable, with nearly all patients remaining free of disease up to 20 years after therapy. Although more common at approximately 10% to 15%, partial responses have not been reported to be as durable and the majority of patients develop disease recurrence within 2 years.3,12,13 Given that a minority of patients achieve a major benefit, many groups have attempted to identify prognostic factors that might help predict response or clarify the biologic mechanisms involved.14,15 In the current study, we updated and reviewed our single-institution experience with HD IL-2 in the treatment of 259 patients with metastatic RCC. We reanalyzed variables that might be associated with response in this cohort receiving only HD IL-2 and added an analysis of factors associated with prolonged survival.
MATERIALS AND METHODS
Patients
Patients included in the current study all had measurable metastatic RCC and were treated at the Surgery Branch of the National Cancer Institute (NCI) between January 13, 1986 and December 31, 2006. Although only patients with RCC of clear cell histology were considered for treatment, this included tumors with sarcomatoid, granular, and mixed papillary elements. Specifically, pure papillary, collecting duct, medullary, and chromophobe tumors were excluded. All patients had received no other therapy for at least 4 weeks before beginning treatment with HD IL-2. Before enrolling, all patients were evaluated with routine physical examination, history, and screening blood studies. In the early years, radiologic workup included computed tomography (CT) scan of the chest as well as bone and liver scans, whereas in later years patients were routinely evaluated with CT studies of the chest, abdomen, and pelvis and magnetic resonance imaging (MRI) scans of the brain. In addition, patients age >50 years were required to undergo a pretreatment stress electrocardiogram or a stress radionuclide study. Those patients found to have evidence of ischemic heart disease, significant arrhythmias, or other comorbidities were excluded. All patients provided informed consent before protocol enrollment. The Institutional Review Board of the NCI approved all protocols.
Patients were not included in this review if they had received any prior treatment with IL-2, IL-2 conjugated to polyethylene glycol, or IL-2 in combination with any other agent.
Interleukin-2
Recombinant IL-2 (Cetus Oncology Division, Chiron Company, Emeryville, Calif) was reconstituted from a lyophilized powder with 5% human albumin. The drug was then administered intravenously at a dose of 720,000 IU/kg over 15 minutes every 8 hours as tolerated up to a maximum of 15 doses. In recent years, the maximum limit was reduced to 12 doses because very few patients were able to tolerate 15 consecutive doses. Patients withdrew from treatment before reaching the maximum number of doses because of their refusal to continue treatment, early signs of mental confusion, or because of grade 3 and 4 toxicities that were not easily reversed by supportive therapy (determined according to the National Cancer Institute Common Toxicity Criteria grading system).
After the initial treatment cycle, patients returned 10 to 15 days later for a second cycle administered in the same fashion if possible. Two cycles constituted 1 course of treatment. Patients with evidence of tumor regression or those with stable disease after a course of therapy would then proceed to additional courses of treatment beginning every 2 months. The majority of patients received their treatment on a general surgical ward, although some patients were transferred to the intensive care unit for monitoring or if vasopressor therapy was warranted. Common side effects from HD IL-2 were prevented or minimized by the routine administration of antipyretics, anti-inflammatories, antiemetics, anti-diarrheals, and H2 antagonists.
Evaluation of Response
All metastatic lesions were measured by either radiologic or physical examination. Initially, responses were calculated using the World Health Organization criteria. These guidelines consider a partial response to be a ≥50% reduction in the sum of the products of the maximum perpendicular dimensions of all lesions lasting at least 4 weeks with no new or growing lesions. A complete response was defined as the complete disappearance of all known lesions confirmed to last at least 4 weeks. Progressive disease was defined by WHO criteria as a 25% increase in the product of the perpendicular dimensions of any pre-existing lesions or the development of any new lesions.16 In 1998, the Response Evaluation Criteria in Solid Tumors was proposed by the RECIST group. RECIST classifies a partial response as a ≥30% decrease in the sum of the longest dimensions of the target lesions. A 20% increase in the sum of the longest dimensions of the target lesions or the development of any new lesions are classified as progressive disease.17 The current study includes patients measured using the criterion appropriate for the time during which their treatment was administered.
Statistical Analysis
Data were initially evaluated in a univariate fashion. For continuous variables, comparisons between responders and nonresponders and between those patients surviving > 4 years or <4 years were performed using the Wilcoxon rank sum test. Four years was chosen based on the stabilization of the overall survival curves at approximately this time point after the initiation of treatment. The same comparisons between dichotomous variables were performed using either the Fisher exact test or the Mehta and Patel modification to the Fisher exact test.18 Those factors, which were potentially identified to be associated with either response or survival >4 years on univariate analyses, were then included in a multivariate logistic regression model to determine whether any of these factors may have a simultaneous association with either of the 2 outcomes. All P values were 2-sided with statistical significance being considered when P was <.05. The results are reported without any formal correction for multiple comparisons because the analysis was intended to be descriptive.
While analyzing variables that might predict survival, we also incorporated the Memorial Sloan-Kettering Cancer Center (MSKCC) prognostic factors as reported by Motzer et al.19 These negative prognostic factors included poor Karnofsky performance status, high lactate dehydrogenase (LDH) level, low hemoglobin, high corrected calcium, and lack of prior nephrectomy. Normal limits for laboratory values were defined by the central laboratory of the NCI and some varied during the length of the current study as methodologies changed. Because Motzer et al.19 determined that survival with metastatic RCC differed according to the aggregate number of factors each patient possessed, the Cochran-Armitage test for trend was used to analyze the association between the actual number of factors (ie, 0, 1–2, or >3) and survival of >4 years versus <4 years.20 Finally, to determine whether these factors and others were associated with overall survival, a Cox regression analysis was performed.
RESULTS
Demographics
Between January 13, 1986 and December 31, 2006, 259 patients with metastatic RCC were treated with intravenous HD IL-2 alone (Table 1). The majority of our patients were men between the ages of 40 and 60 years. Greater than 75% of the patient population in the current study were of good performance status, with an ECOG classification of 0. Only 6 of the 259 patients had not received any previous therapy. The overwhelming majority had undergone surgery and approximately 15% had received previous immunotherapy, with the majority of these patients having received IFN-α.
TABLE 1.
Characteristics of Patients Treated With High-dose Interleukin-2
| Characteristic | No. of patients | |
|---|---|---|
| Total | 259 | 100% |
| Sex | ||
| Male | 180 | 69% |
| Female | 79 | 31% |
| Age, y | ||
| 11–20 | 2 | 1% |
| 21–30 | 11 | 4% |
| 31–40 | 32 | 12% |
| 41–50 | 92 | 36% |
| 51–60 | 92 | 36% |
| 61–70 | 30 | 12% |
| ECOG performance status | ||
| 0 | 198 | 76% |
| 1 | 50 | 19% |
| 2 | 10 | 4% |
| 3 | 1 | |
| Prior treatment | ||
| None | 6 | 2% |
| Surgery | 250 | 97% |
| Chemotherapy | 16 | 6% |
| Radiotherapy | 23 | 9% |
| Hormonal | 7 | 3% |
| Immunotherapy | 38 | 15% |
| Any 2 or more | 66 | 25% |
| Any 3 or more | 15 | 6% |
ECOG indicates Eastern Cooperative Oncology Group.
Response to Therapy and Overall Survival
Of the 259 patients in this study, 23 (9%) experienced a complete response and 30 patients (12%) achieved a partial response, for an overall objective response rate of 20%. The response durations are shown in Figure 1 and Table 2. All patients who experienced a partial response ultimately developed disease recurrence, with a median duration of response of 15.5 months. In contrast, of the 23 complete responders, only 4 had developed disease recurrence at the time of last follow-up, all within the first 4 years after the initiation of treatment (Table 2). Three other ongoing complete responders were censored at 141 months, 88 months, and 46 months because of death from other causes. Of the remaining 16 ongoing complete responders, the follow-up at the time of this report was between 24 and 221 months.
FIGURE 1.
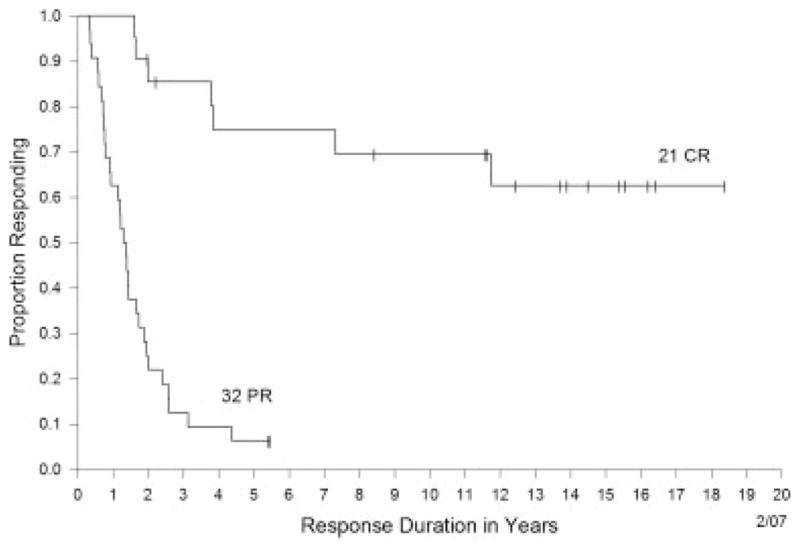
Response duration for patients with a complete response (CR) versus those with a partial response (PR).
TABLE 2.
Duration of Response in Patients Treated With High-dose Interleukin-2
| Complete response, months (n = 23)* | Partial response, months (n = 30) |
|---|---|
| 221+, 197+, 194+, 187+, 185+, 174+, 167+, 164+, 149+, 141, 139+, 139+, 101+, 88, 65+, 65+, 46, 46, 26+, 24, 24+, 20, 19 | 53, 38, 31, 31, 29, 24, 24, 23, 21, 20, 17, 17, 17, 17, 16, 15, 14, 14, 11, 11, 9, 9, 9, 9, 8, 7, 7, 5, 4, 4 |
“+”indicates ongoing response as of last follow-up (March 15, 2007).
For all 259 patients, the median overall survival was 19 months. Figure 2 demonstrates the differences in survival between the complete and partial responders and nonresponders. Based on groups that were determined retrospectively, the median survival of the partial responders and nonresponders was 39.1 months and 15.1 months, respectively, from the on-study date. The median survival of the complete responders had not yet been reached at the time of last follow-up. Response was observed at a variety of sites, including the lung, liver, bone, and lymph nodes.
FIGURE 2.
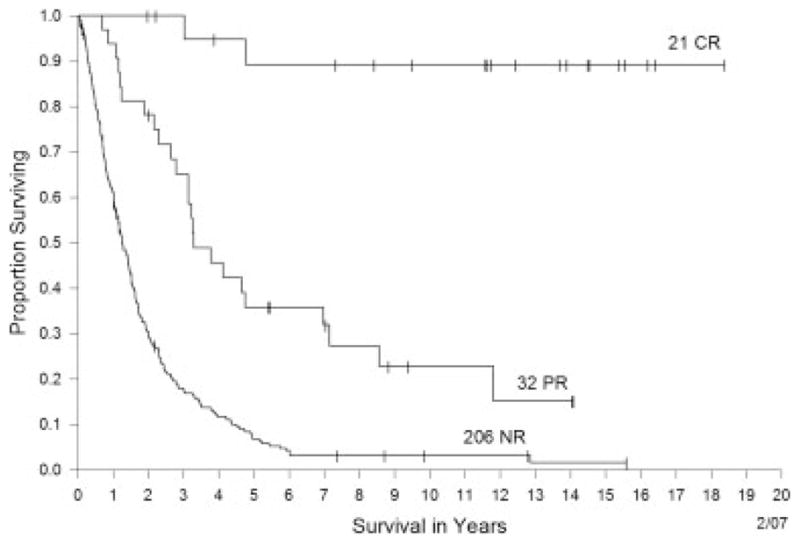
Overall survival for patients with a complete response (CR) versus those with a partial response (PR) versus nonresponders (NR).
Toxicities
There were 2 treatment-related deaths during the time period of the current study. These occurred early in the course of our experience and there has not been a death related to HD IL-2 toxicity in a patient with RCC reported in 20 years. Hematologic toxicities were generally limited to grade 1 and grade 2 and included decreases in serum hemoglobin, leukopenia, and thrombocytopenia (Table 3). Hepatic toxicities included grade 1 and grade 2 hyperbilirubinemia and elevations in aspartate aminotransferase. Other grade 1 and grade 2 toxicities included edema, chills, fatigue, dyspnea, rash, diarrhea, and nausea. Because of the capillary leak syndrome, vasodilation, and diarrhea that HD IL-2 therapy can cause, patients often experienced hypotension and oliguria. Confusion and a depressed level of consciousness were among the other major grade 3 and grade 4 toxicities. Grade 3 and grade 4 infections, both catheter-related and in general, are believed to be because of neutrophil dysfunction from IL-2 but have become relatively uncommon with the given routine use of prophylactic antibiotics for indwelling devices.21 Overall, grade 3 and grade 4 toxicities have diminished significantly since we began treating patients with HD IL-2. When comparing the first 65 and the last 64 patients who were treated, grade 3 and grade 4 toxicities were found to have diminished from 209 and 48, respectively, to 56 and 6, respectively. Finally, apart from the 2 treatment-related deaths, all toxicities were readily reversible after the discontinuation of therapy.
TABLE 3.
Toxicities*
| Adverse events | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|---|---|---|---|
| Allergic rhinitis | 8 | 9 | — | — |
| Hemoglobin decrease | 43 | 28 | 5 | — |
| Leukopenia | 8 | 21 | — | 1 |
| Thrombocytopenia | 33 | 39 | 20 | 3 |
| Edema | 41 | 47 | 2 | — |
| Hypotension | 2 | 34 | 99 | 5 |
| Myocardial ischemia | — | 2 | 3 | 1 |
| Myocarditis | — | — | 1 | — |
| Arrhythmia (ie, SVT, etc) | 56 | 10 | 11 | 3 |
| Chills | 1 | 53 | 34 | — |
| Fatigue | 20 | 63 | 34 | — |
| Rash | 26 | 32 | 16 | 2 |
| Ascites | 3 | 7 | — | — |
| Diarrhea | 38 | 26 | 58 | — |
| Mucositis | 21 | 13 | 3 | — |
| Nausea | 21 | 59 | 56 | 3 |
| Vomiting | — | 1 | — | — |
| Melena/GI bleed | — | 1 | — | — |
| Hematuria | 3 | — | — | — |
| Hemorrhage | 5 | 1 | 1 | 2 |
| Infection | 2 | 5 | 9 | 3 |
| Catheter infection | — | — | 4 | 4 |
| Increased AST | 50 | 19 | 5 | — |
| Hyperbilirubinemia | 62 | 78 | 15 | — |
| Hypocalcemia | — | — | 1 | — |
| Increased creatinine | 62 | 47 | 5 | — |
| Confusions | 11 | 15 | 21 | 14 |
| Hallucinations | — | — | 1 | — |
| Depressed level of consciousness | 8 | 8 | 13 | 8 |
| Respiratory disorder | 14 | 48 | 17 | 8 |
| Pleural effusion | 4 | 13 | 3 | — |
| Low urine output due to CLS | — | — | 38 | 11 |
| Renal failure | — | — | 1 | — |
SVT indicates supraventricular tachycardia; GI, gastrointestinal; AST, aspartate aminotransferase; CLS, capillary leak syndrome.
Determined according to the National Cancer Institute Common Toxicity Criteria grading system.
Prognostic Factors Associated with Response to IL-2
First, it should be noted that a large number of pre-treatment factors were not found to be associated with response. As can be seen in Table 4, only fewer MSKCC risk factors (P = .02), a higher baseline weight (P = .05), and a lack of prior immunotherapy (P =.05) were found to be significantly associated with response to HD IL-2. Sites of disease were studied to determine their possible association with response. Again, no individual site of disease demonstrated statistical significance. However, there was a weak trend toward a higher response rate in those patients who had undergone a prior nephrectomy (P =.12).
TABLE 4.
Interleukin-2-based Studies Analyzing Predictors of Response and Survival
| Study | No. of patients | Treatment | Predictors of response | Predictors of survival |
|---|---|---|---|---|
| Palmer 199214 | 327 | HD IL-2, HD IL-2 plus LAK cells | NA | ECOG PS (0 vs 1), DTI (>24 mo or <24 mo), no. of disease sites (1 vs >2) |
| Royal 199615 | 239 | HD IL-2, PEG IL-2, HD IL-2 plus LAK cells | Prior nephrectomy, no prior immunotherapy, lower platelet nadir, higher rebound lymphocytosis | NA |
| Fyfe 199512 | 255 | HD IL-2 alone | ECOG PS 0 | ECOG PS 0, prior nephrectomy, DTI |
| Rosenberg 199813 | 227 | HD IL-2 alone | No prior immunotherapy, total amount of IL-2 (1st course), higher rebound lymphocytosis | NA |
| Yang 200311 | 156 | HD IL-2 alone, LD IL-2, subcutaneous IL-2 | Absence of local recurrence, higher baseline weight, corrected calcium, greater no. of doses | Lower baseline platelet count, disease confined to lungs, response to IL-2 |
| Upton 200522 | 231 | HD IL-2 alone, HD IL-2 plus IFN, LD IL-2 plus IFN or 5-flourouracil | Clear cell tumors, no papillary features, alveolar features >50%, no granular features | NA |
| Atkins 200523 | 66 | HD IL-2 alone, LD IL-2 | High CAIX | High CAIX |
| Current study | 259 | HD IL-2 alone | Better MSKCC score, higher baseline weight, no prior immunotherapy | Higher baseline albumin, fewer disease sites, response, lower peak TSH, lower ECOG PS, higher rebound lymphocytosis, lower platelet nadir |
HD indicates high-dose; IL-2, interleukin-2; LAK, lymphokine-activated killer; NA, not available; ECOG PS, Eastern Cooperative Oncology Group performance status; DTI, diagnosis to treatment interval; PEG, polyethylene glycol; LD, low-dose; IFN, interferon-α; CAIX, carbonic anhydrase inhibitor; MSKCC, Memorial Sloan-Kettering Cancer Center; TSH, thyroid-stimulating hormone.
Treatment-related variables, including laboratory parameters assessed during treatment, also were evaluated for response. The analysis of the correlation between IL-2 doses and response required several refinements to avoid unintentional biases. Because the number of total doses a patient received was biased by the finding that only patients responding to treatment receive additional courses, we examined only the number of IL-2 doses in the first and second cycles of the first course, the total number of IL-2 doses in the first course, and the total IU/kg of the drug received in that first course. Of these factors, only the total IU/kg of IL-2 received during that first course was found to be associated with response (P =.04 on the univariate analysis). This variable was then included along with the previously mentioned significant pretreatment factors in a multivariate analysis, but it did not maintain its significance.
Prognostic Factors Associated with Survival >4 Years
Analyses for this outcome were restricted to the 249 patients who were not enrolled on study <4 years before December 2006 and who remained alive at the time of last follow-up because the outcomes of the other 10 patient with respect to this endpoint were not determined. Again, a multitude of pretreatment variables were assessed and found not to be associated with survival of >4 years. Of those variables that were, only fewer sites of metastatic disease (P = .0014) and a higher baseline albumin (P =.004) were found to maintain statistical significance (Table 4).
The analysis was then performed examining treatment factors. Univariate treatment factors found to be predictive of survival of ≥4 years were included along with sites of disease, baseline albumin, and response to HD IL-2 in a logistic regression model. Again, fewer sites of disease (P = .004) and a higher baseline albumin (P = .0007) remained statistically significant. In addition, a higher rebound lymphocytosis (P = .002), lower peak thyroid-stimulating hormone (TSH) (P = .012), and response (P <.0001) were found to be strongly associated with survival of >4 years. Of these 5 factors, however, a partial or complete response to HD IL-2 emerged by far as the strongest predictor of survival of >4 years with 31 of 50 responders (62%) surviving >4 years compared with just 22 of 199 nonresponders (11%) achieving that milestone. Incidentally, as with response, there was no apparent association between survival of >4 years and the total doses or IU/kg of HD IL-2 received.
Similar to the previous logistic regression model, a higher baseline albumin (P =.0063), lower peak TSH (P =.0005), response (P <.0001), lower platelet nadir (P =.0003), and better ECOG performance status (P = .0035) were factors that were considered to be predictive of longer overall survival in the Cox model (Table 5). Of most significance, there was strong association between response to HD IL-2 and long-term survival (P ≤.0001). Although the addition of the MSKCC score did not obviate the significance of any of the other 5 factors in the Cox model, it was itself not found to be sufficiently associated with survival (P = .13) to warrant inclusion in the final Cox model.
TABLE 5.
Factors Associated With Overall Survival
| Parameters | P | Hazards ratio | 95% CI |
|---|---|---|---|
| Response | <.0001 | 0.21 | 0.14–0.31 |
| Baseline albumin | .0063 | 0.64 | 0.47–0.88 |
| Platelet nadir | .0003 | 1.005 | 1.002–1.007 |
| ECOG PS (0 vs. 1) | .0035 | 1.65 | 1.18–2.31 |
| Peak TSH | .0005 | 1.02 | 1.007–1.025 |
95% CI indicates 95% confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; TSH, thyroid-stimulating hormone.
DISCUSSION
The goals of the current study were to update our 20-year experience administering HD IL-2 for the treatment of patients with metastatic RCC. As of December of 2006, we had treated 259 patients with this immunotherapy agent. Approximately 9% of our patients (n = 23) experienced a complete response and 11% (n = 30) achieved a partial response, for an overall objective response rate of 20%. With what to our knowledge is the longest follow-up currently available, this study demonstrates that the majority of patients achieving a complete response are likely cured, with 19 of 23 complete responders remaining free of disease at 24 to >221 months from the onset of treatment (Fig. 1) (Table 2). Although all partial responders do develop disease recurrence with time, these individuals also appear to derive some benefit from their treatment.
Analyzing associations between multiple parameters and clinical outcome after IL-2 therapy is hampered by relatively small sample sizes and a low frequency of response or long-term survival. Analyses of numerous parameters without corrected P values are likely to misidentify chance associations as being significant whereas strict corrections applied to P values across multiple analyses and for parameters with varying degrees of association with one another may mask genuine associations as being nonsignificant when examining the results from trials with small numbers of subjects. Therefore, it is of value to examine what parameters were found to be significantly associated with response and survival in independent studies by other investigators (Table 4).11–15,22,23 Because to our knowledge the current study encompasses the most recent and largest retrospective analysis of patients with metastatic RCC published to date, we added an analysis of the MSKCC risk factors to our study of variables associated with improved response and survival. Motzer et al.19 originally identified these risk factors in a retrospective analysis of 670 patients with metastatic RCC who were treated at a single institution over a 21-year period. Of these 670 patients, 102 had been treated with IL-2 alone or in combination with IFN-α. The primary purpose of the study was to define pretreatment factors predictive of survival. A high LDH level (>1.5 times normal), low Karnofsky performance status (<80%), low serum hemoglobin (< the lower limit of normal), high corrected calcium, and an absence of nephrectomy were considered to be risk factors for shorter survival.19 Applying this list to their patient population, the authors found that patients having none of the identified risk factors had a median survival of 20 months, whereas those with 1 or 2 risk factors had a median survival of 10.3 months; subjects with 3, 4, or 5 risk factors experienced a median survival of only 3.9 months.19
In a regression model limited to pretreatment variables, we found that fewer MSKCC risk factors were correlated more significantly with response to HD IL-2 (P =.02) rather than survival. Admittedly, MSKCC risk factors include parameters that individually were either not found to be statistically significant on the univariate analysis (ie, prior nephrectomy, LDH level, performance status, and corrected calcium) or lost significance on the multivariate analysis (ie, baseline hemoglobin). However, when applied to the current study patients as a group, these variables appear to be strong pretreatment predictors of response. With regard to the association between a higher baseline weight and response, the related factors of the total amount of IL-2 and the body mass index were found to be only marginally associated with response, suggesting that neither was the sole factor contributing to the association observed.
None of the parameters elicited during treatment proved to be significantly associated with response. Several sequential analyses by the NCI Surgery Branch have identified no prior immunotherapy and a greater rebound lymphocytosis (here, peak minus baseline lymphocyte count) as being associated with a higher frequency of response to IL-2. Furthermore, because 23 of 46 patients with prior immunotherapy had received IFN-α, we also examined this subset of patients individually and found, on the univariate analysis, the suggestion of an association between response and an absence of previous IFN-α therapy (P =.05). Thus, although there may be some suggestion that prior immunotherapy could be detrimental to achieving a response with HD IL-2, it remains unclear whether treatment with other immunotherapeutic agents before HD IL-2 should be discouraged.
Although Upton et al., Atkins et al., and others have found molecular and histologic features of clear cell renal cancer to be predictive of a higher chance of response, our treatment population was strictly limited to patients with clear cell tumors, potentially negating this consideration in the current study.22–24
The analysis of factors associated with prolonged survival after HD IL-2 therapy had not previously been performed on our entire cohort of patients receiving HD IL-2 monotherapy. Because the majority of patients do not appear to benefit in any discernable way from HD IL-2 therapy (although a minority appear to demonstrate durable survival as evidenced by stabilization of survival curves), we chose to study survival of >4 years rather than median survival as a way to determine factors that might identify those patients who receive the greatest benefit from HD IL-2 treatment. The number of MSKCC risk factors demonstrated only a trend but no significant association with prolonged survival in the current study population, but our patients were fewer and less diverse than those studied by Motzer et al,19 which hampers our ability to detect significant associations across these categories. Other significant survival-associated factors in the current study were fewer sites of disease, a higher baseline albumin, higher rebound lymphocytosis, a lower peak TSH, lower ECOG PS, and a lower platelet nadir. It was reassuring to note that a response to HD IL-2 was by far the dominant factor associated with prolonged survival, dwarfing all other factors with regard to significance and relative risk. Conversely, it was important to note that the factors associated with a higher probability of response were not sufficient to also be associated with prolonged survival. The treatment-related factors of rebound lymphocytosis and platelet nadir have previously been described, and most often associated with a superior response rate, but as known hematologic consequences of HD IL-2 administration they could simply be surrogates for a brisk response to IL-2. Similar to the current study, Palmer et al. also found fewer sites of disease to be a predictor of survival.14 To our knowledge, the significance and interpretation of the association between survival and baseline albumin and the inverse association between survival and peak TSH remain unexplained and not previously described.
Within the last few years, new agents have been approved for the treatment of metastatic RCC. These include tyrosine kinase inhibitors such as sunitinib and sorafenib, as well as the rapamycin kinase inhibitor temsirolimus. In phase 3 trials, sunitinib and sorafenib produced response rates of 31% and 10%, respectively. In addition, both demonstrated significant improvements in progression-free survival and trends toward improved survival.25,26 Temsirolimus demonstrated an improvement in overall survival in comparison with IFN-α.27 Beyond their demonstrated efficacy, the appeal of these agents is their ease of administration and lack of acute or severe toxicities. However, extensive experience has shown that they rarely, if ever, induce complete regressions of metastatic RCC and require chronic, ongoing therapy if tumor stability or regression is to be maintained. In contrast, a relatively brief course of HD IL-2 therapy can result in durable complete regressions in a small population of treated patients and no maintenance therapy is required to preserve these responses. In experienced centers, the risk of a HD IL-2 treatment-related death has been reduced to <1% and all other toxicities are subject to rapid reversal after the completion of therapy.
The results of this and other studies have shown that HD IL-2 is the only systemic treatment that can consistently cure a small percentage of patients with metastatic RCC. Because such successes are not common, efforts to identify those patients with the best chance of benefiting are important to avoid unnecessary treatment and to better triage patients between the therapeutic choices now available. Choosing a tyrosine kinase inhibitor confers a higher likelihood of achieving disease regression or stability, but current data do not support the potential of a curative response with these agents. One could argue that for patients with the highest potential for responding to HD IL-2, this should be their first-line option, with a tyrosine kinase inhibitor selected if HD IL-2 is not effective. The results of the current study would suggest such patients would be those with a superior MSKCC score and no prior immunotherapy.
References
- 1.SEER Database. Cancer of the Kidney and Renal Pelvis. Bethesda, MD: National Cancer Institute Surveillance, Epidemiology, and End Results Program; 2007. [Google Scholar]
- 2.Greene FL, Page DL, Fleming ID, et al., editors. AJCC Cancer Staging Manual. 6. Chapter 36. New York: Springer; 2002. pp. 323–328. [Google Scholar]
- 3.Rosenberg SA, Lotze MT, Yang JC, et al. Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg. 1989;210:474–485. doi: 10.1097/00000658-198910000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JC, Topalian SL, Schwartzentruber DJ, et al. The use of polyethylene glycol-modified interleukin-2 (PEG-IL-2) in the treatment of patients with metastatic renal cell carcinoma and melanoma: a Phase I study and a randomized prospective study comparing IL-2 alone versus IL-2 combined with PEG-IL-2. Cancer. 1995;76:687–694. doi: 10.1002/1097-0142(19950815)76:4<687::aid-cncr2820760424>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg SA, Lotze MT, Yang JC, et al. Combination therapy with interleukin-2 and alpha-interferon for the treatment of patients with advanced cancer. J Clin Oncol. 1989;7:1863–1874. doi: 10.1200/JCO.1989.7.12.1863. [DOI] [PubMed] [Google Scholar]
- 6.Atkins M, Sparano J, Fisher RI, et al. Randomized Phase II trial of high-dose interleukin-2 either alone or in combination with interferon alfa-2b in advanced renal cell carcinoma. J Clin Oncol. 1993;11:661–670. doi: 10.1200/JCO.1993.11.4.661. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Lotze MT, Yang JC, et al. Prospective randomized trial of high-dose interleukin-2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst. 1993;85:622–632. doi: 10.1093/jnci/85.8.622. [DOI] [PubMed] [Google Scholar]
- 8.Sleijfer DT, Janssen RA, Buter J, deVries EG, Willemse PH, Mulder NH. Phase II study of subcutaneous interleukin-2 in unselected patients with advanced renal cell cancer on an outpatient basis. J Clin Oncol. 1992;10:1119–1123. doi: 10.1200/JCO.1992.10.7.1119. [DOI] [PubMed] [Google Scholar]
- 9.Atzpodien J, Hanninen EL, Kirchner H, et al. Multiinstitutional hometherapy trial of recombinant human interleukin-2 and interferon alfa-2 in progressive metastatic renal cell carcinoma. J Clin Oncol. 1995;13:497–501. doi: 10.1200/JCO.1995.13.2.497. [DOI] [PubMed] [Google Scholar]
- 10.Vogelzang NJ, Lipton A, Figlin RA. Subcutaneous interleukin-2 plus interferon alfa-2a in metastatic renal cancer: an outpatient multicenter trial. J Clin Oncol. 1993;11:1809–1816. doi: 10.1200/JCO.1993.11.9.1809. [DOI] [PubMed] [Google Scholar]
- 11.Yang JC, Sherry RM, Steinberg SM, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with meta-static renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA, Yang JC, White DE, Steinberg SM. Durability of complete responses in patients with metastatic cancer treated with high-dose interleukin-2: identification of the antigens mediating response. Ann Surg. 1998;228:307–317. doi: 10.1097/00000658-199809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palmer PA, Vinke J, Philip T, et al. Prognostic factors for survival in patients with advanced renal cell carcinoma treated with recombinant interleukin-2. Ann Oncol. 1992;3:475–480. doi: 10.1093/oxfordjournals.annonc.a058239. [DOI] [PubMed] [Google Scholar]
- 15.Royal RE, Steinberg SM, Krouse RS, et al. Correlates of response to IL-2 therapy in patients treated for metastatic renal cancer and melanoma. Cancer J Sci Am. 1996;2:91–98. [PubMed] [Google Scholar]
- 16.Miller AB, Hoogstraten B, Staquet M. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Mehta CR, Patel NR. A network algorithm for performing Fisher’s exact test in r × c contingency tables. J Am Stat Assoc. 1983;78:427–434. [Google Scholar]
- 19.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17:2530–2540. doi: 10.1200/JCO.1999.17.8.2530. [DOI] [PubMed] [Google Scholar]
- 20.Agresti A. Categorical Data Analysis. New York: John Wiley and Sons, Inc; 1990. [Google Scholar]
- 21.Bock SN, Lee RE, Fisher B, et al. A prospective randomized trial evaluating prophylactic antibiotics to prevent triple-lumen catheter-related sepsis in patients treated with immunotherapy. J Clin Oncol. 1990;8:161–169. doi: 10.1200/JCO.1990.8.1.161. [DOI] [PubMed] [Google Scholar]
- 22.Upton MP, Parker RA, Youmans A, McDermott DF, Atkins MB. Histologic predictors of renal cell carcinoma response to interleukin-2 based therapy. J Immunother. 2005;28:488–495. doi: 10.1097/01.cji.0000170357.14962.9b. [DOI] [PubMed] [Google Scholar]
- 23.Atkins MB, Regan M, McDermott D, et al. Carbonic anhydrase IX expression predicts outcome of interleukin-2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 24.Bui MH, Seligson D, Han KR, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]
- 25.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 26.Escudier B, Eisen J, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 27.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]


