Abstract
Poly(amidoamine) (PAMAM) (G3) dendrimer was modified into quaternary ammonium salts using tertiary amines with different chain lengths: dimethyldodecyl amine, dimethylhexyl amine, and dimethylbutyl amine using an efficient synthetic route. The antimicrobial activity of these dendrimer ammonium salts against Staphylococcus and E-coli bacteria was examined using the disc diffusion method. It was found that quaternary ammonium salt prepared with the dimethyldodecyl amine exhibits antimicrobial efficacy against Staphalococus and E.coli bacteria.
Keywords: PAMAM Dendrimer, Surface Modification, Staphalococus and E.coli bacteria
Introduction
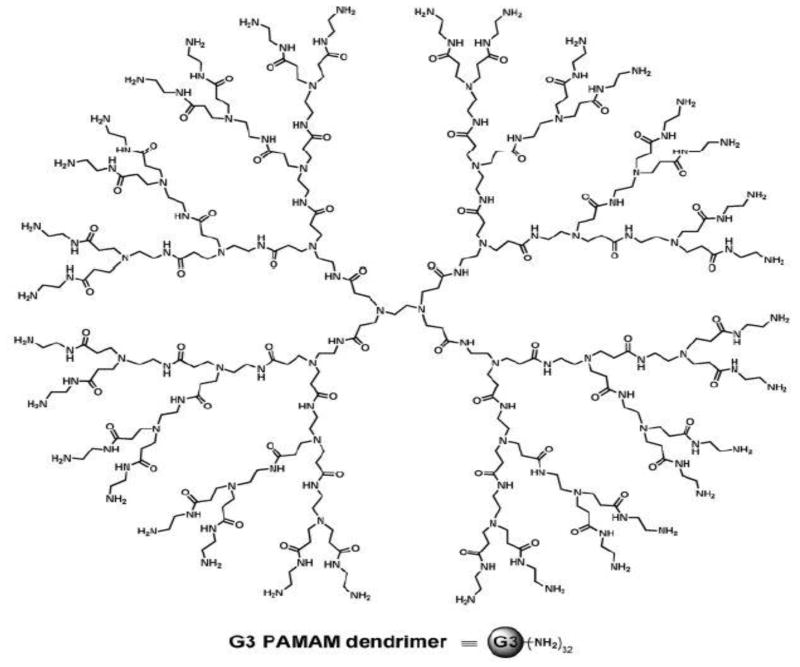
Dendrimers are monodispersed well-defined highly branched macromolecules. The dendrimers exhibit an exponential increase in functional groups with generations and they are usually monodispersed macromolecules with a regular and highly branched three-dimensional architecture.1–10 Each layer of dendrimer makes up a single generation and the structure of one such dendrimer, poly(amidoamine) (PAMAM), is displayed in Figure 1. Attention has been shifted recently to the modification and functionalization of dendrimers due to interest in various applications such as gene therapy, catalysts, and drug-delivery systems.11–14 The nature of outer functional groups determines the solubility and reactivity of dendrimers. Recently, the acetylation and quaternization of dendrimers have been reported.15–16 Monovalent and bivalent biocides are used extensively for biomedical applications; however, polyvalent biocides are lacking. It is expected that polyvalent biocides will have high activities compared to mono or bivalent biocides.17–20 Polymeric biocides were prepared by other researchers and tested for antimicrobial properties.21–27 It has been shown that dendrimers with amine functional groups could be converted as effective antimicrobial agents due to their dense primary amine functional groups.16 It is well known that quaternary ammonium salts are effective antimicrobial agents.28–29 Dendrimers can also be tailored to generate tunable cavities with ammonium or phosphonium functionalities.16,21 Because of their globular shape and tunable cavities, dendrimers have some unique properties.1–8 It has also been shown that dendrimers are able to form complexes with a variety of ions and compounds and act as a template to fabricate metal nanoparticles. The synthesis of silver/dendrimer nanocomposite and silver complexes has been reported.30–32
Figure 1.
Structure of G3 PAMAM dendrimer
Antimicrobial agents are mainly made up of small molecules. The use of antimicrobial polymers was explored and it has been shown that antimicrobial polymers have high efficiency compared to existing antimicrobial agents.21–26 The use of dendrimers as antimicrobial agents could be beneficial because of their unique structures.16,31 Thousands of compounds containing ammonium and phosphonium functionalities were synthesized and tested for antimicrobial properties. However, very few with adequate biological activities have been discovered. We report herein the synthesis of PAMAM dendrimer ammonium salts by two different routes for potential use as an effective antimicrobial agent and tested against Gram-positive and Gram-negative bacteria.
Results and Discussion
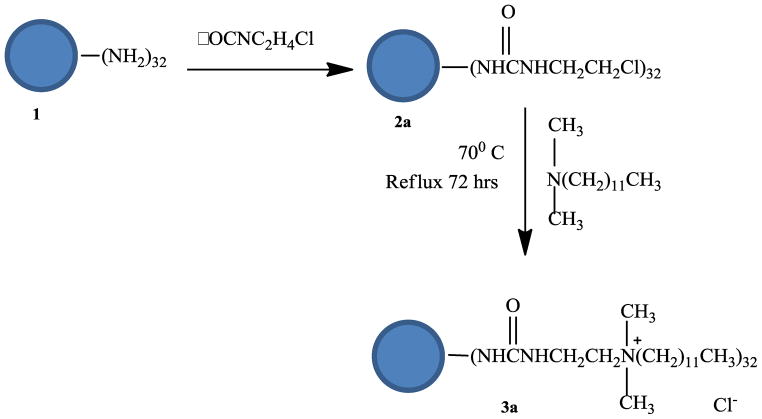
In the first method, Generation 3 (G3) PAMAM dendrimer 1 was converted into a quaternary ammonium salt by adding 2-chloroethyl isocyanate to the G3 PAMAM dendrimer to the G3 PAMAM chlorinated dendrimer 2a.16 The chlorinated dendrimer was then reacted with N,N-dimethyldodecylamine to prepare G3 quaternary ammonium dendrimer salt 3a. Scheme 1 shows the reaction scheme for the modification of G3 PAMAM dendrimer into quaternary ammonium dendrimer salt.33 The product was isolated using a 10 K cut-off ultrafiltration membrane. The final product 3a was isolated as a yellow solid in only a 25% yield.
Scheme 1.
Synthesis scheme for the synthesis of G3 quaternary dendrimer ammonium salt by method 1
The quaternary ammonium dendrimer salt 3a was characterized by NMR and FTIR spectroscopies. The 13C-NMR spectrum of PAMAM dendrimer displays a carbonyl resonance at 173 ppm and -CH2 resonances from 30 to 60 ppm whereas the 13C-NMR spectrum of G3 quaternary ammonium salt of PAMAM dendrimer shows an additional carbonyl resonance at 160 ppm, which was attributed to the urea carbonyl of the G3 quaternary ammonium salt of the PAMAM dendrimer. In addition, several new resonances are seen from 15 to 30 ppm in the 13C-NMR spectrum of the modified dendrimer, which suggested that long alkyl chains and a CH3 have been added to the dendrimer.
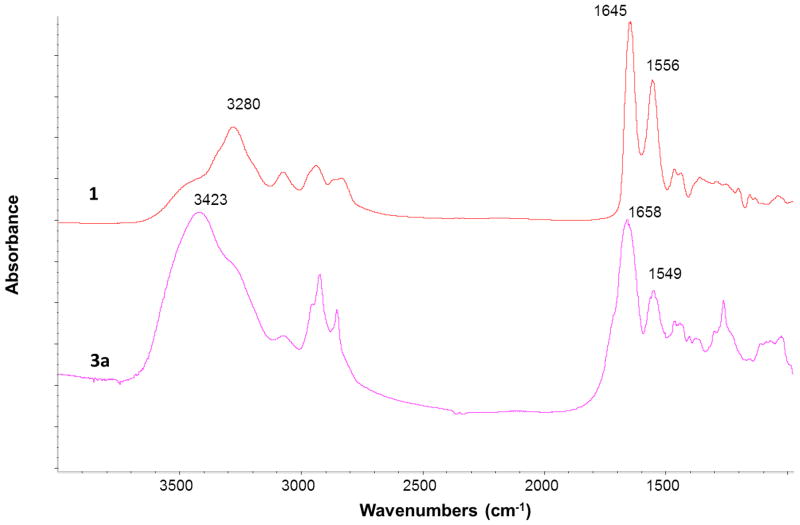
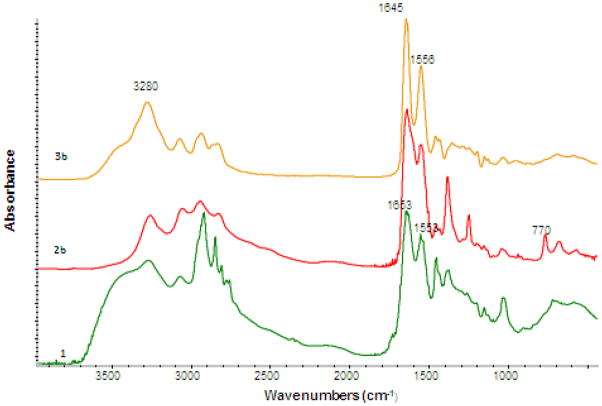
FTIR spectra of the G3 PAMAM dendrimer 1 and quaternary ammonium dendrimer salt 3a in the region from 1000 to 4000 cm−1 were compared in Figure 2. The PAMAM dendrimer 1 showed a N-H band at 3280 cm−1, C=O band at 1645 cm−1 and N-H bending at 1556 cm−1. These bands were shifted to 3423, 1658 and 1549 cm−1, respectively in the FTIR spectrum of quaternary ammonium dendrimer salt 3a. The PAMAM dendrimer showed weak bands around 2800–3000 cm−1 whereas the quaternary ammonium dendrimer salt 3a showed strong bands around 2800–3000 cm−1, confirmed that long alkyl groups have been added to the PAMAM dendrimer (see supporting information for details).
Figure 2.
FTIR Spectra of G3 PAMAM dendrimer (1) and quaternary ammonium dendrimer salt (3a) in the region 700–1800 cm−1
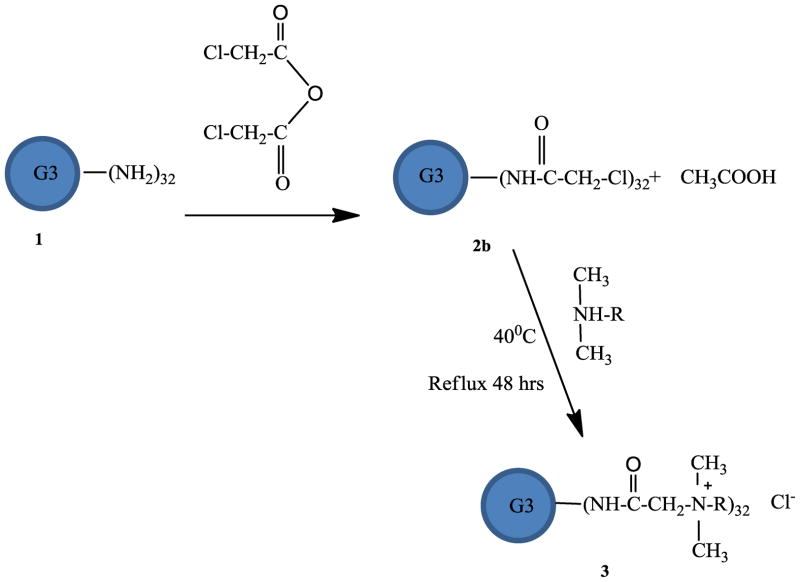
In order to improve the yield, G3-PAMAM dendrimer 1 was modified using an alternative method to modify the surfaces with an ammonium group. The synthetic scheme is illustrated in Scheme 2. In this method, G3 PAMAM dendrimer 1 was first chloroacetylated with chloroacetic anhydride in methanol to form chloroacetylated G3 PAMAM dendrimer 2b and then reacted with N,N- dimethyldodecylamine, N,N- dimethylhexyl amine and N,N- dimethylbutylamine to prepare G3 quaternary ammonium dendrimer salts 3b, 3c, 3d. This reaction provided dendrimer salt with a 70% to 80% yield after purification. 34, 35
Scheme 2.
Synthesis scheme for the synthesis of G3 quaternary dendrimer ammonium salt by method 2
To ensure successful conversion of the PAMAM dendrimer to chloroacetylated dendrimer, FTIR and NMR spectroscopies were utilized. FTIR spectra of compound 1 and 2b are shown in Figure 3. FTIR spectra of compounds 3b, 3c, and 3d are identical and therefore the FTIR spectrum of one of the quaternary ammonium salts 3b is included in Figure 3. FTIR spectrum of compound 1 shows a broad band at 3280 cm−1, which is attributed to the N-H stretching vibration from amide group. IR bands at 3480 and 3220 cm−1 are assigned to the N-H stretching vibration of primary amines. As the PAMAM dendrimer is chloroacetylated, the IR bands at 3480 and 3220 cm−1 almost disappeared, suggesting that most of the primary amines have been converted to secondary amines. Compound 1 shows a strong band at 1556 cm−1, attributed to N-H bending vibration of the primary amine (amide II). Compound 2b and 3b shows weak amide II vibrations at 1570 cm−1 and 1560 cm−1, respectively. This band is attributed to the N-H bending vibration of the secondary amide. FTIR spectra of compound 1 and 2b shows small IR bands associated with C-H stretching vibrations. around 2800–3000 cm−1 region. However, IR spectrum of compound 3b shows very strong bands in the region from 2800 to 3000 cm−1, suggesting that long alkyl chains have been added to the dendrimer.
Figure 3.
FTIR Spectra of G3 PAMAM dendrimer (1), chloroacetylated dendrimer (2b) and quaternary ammonium dendrimer salt (3b) in the region 700–1800 cm−1
Compound 1 shows a single band at 1645 cm−1, which it is assigned to C = O stretching of the amide component (R-CONHR1). FTIR spectra of compounds 2b shows the same characteristic peak at 1645 cm−1 along with an additional carbonyl band in the same region, due to an additional carbonyl group that was introduced during chloroaceylation (R-NHCOCH2Cl). The C = O stretching shifts to a higher wavenumber of 1652 cm−1 for the amide component (R-NHCOCH2NR3+) in the IR spectrum of compound 3b. FTIR spectrum of compound 2b shows a band at 770 cm−1, which is attributed to C-Cl stretching vibration, suggesting that chlorine is incorporated into the compound 2b. It is apparent in the FTIR spectrum of compound 3b that the absorbance of C-Cl band has become weaker suggesting chloroacetylated dendrimer had been converted to a salt.
13C-NMR spectroscopy was used to confirm the surface modification of the PAMAM dendrimer to chloroacetylated dendrimer and quaternary ammonium salts (see supporing information). It appears that compound 1 shows resonance at 174.4 ppm, which is attributed to carbonyl group of the dendrimer. The 13C-NMR spectrum of compound 1 has resonances in the region from 30–54 ppm representing the CH2 groups of the dendrimer. There is a resonance at 53 ppm, which represents the α carbons and the resonance at 51 ppm represents the β carbons, and so forth. Since carbonyls are electron withdrawing, the -CH2 group directly attached to the carbonyl will have a resonance more downfield than the ones further from the carbonyl group. The CH2 groups between the two nitrogen atoms have a chemical shift of 34.7 ppm and 38.6 ppm respectively.
The 13C-NMR spectrum of compound 2b shows a single resonance at 174.4 ppm, which is attributed to the carbonyls in the dendrimer. However, the carbonyls that were added to the dendrimer show a resonance further downfield at 175.4 ppm. Chlorine is an electron-withdrawing group and therefore a shift further downfield would confirm the addition of chlorine to the dendrimer. The 13C-NMR spectrum of compound 2b shows an additional resonance at 45.3 ppm compared to the 13C-NMR spectrum of compound 1. It was attributed to the -CH2 group from the newly introduced -COCH2Cl component.
The 13C-NMR spectrum of compound 3b has several additional resonances, ranging from 14 to 67 ppm, which confirms that long alkyl chains have been added to the compound. A resonance at 14.6 ppm represents the CH3 of the alkyl chains. The resonances from 23–30 ppm are attributed to CH2s closer to the methyl groups whereas the resonances from 59–65 ppm are attributed to the CH2s that are closer to the ammonium ion. The resonance at 67.0 ppm is the CH2s between the carbonyl and the ammonium ion. The quaternary ammonium salt 3b displays a resonance at 174 ppm, which is associated with carbonyls in the inner region of the dendrimer and a resonance at 166.7 ppm is from carbonyls in the outer region of the modified dendrimer. Compounds 3c and 3d showed similar 13C NMR spectra, suggesting structure of these salts are similar to compound 3b. MALDI-TOF of 3b presents its molecular peak at ~13,300, which is reasonably close to calculated molecular weight of ~14,300. This observation confirmed most of the amine groups in the dendrimer are quartenized.
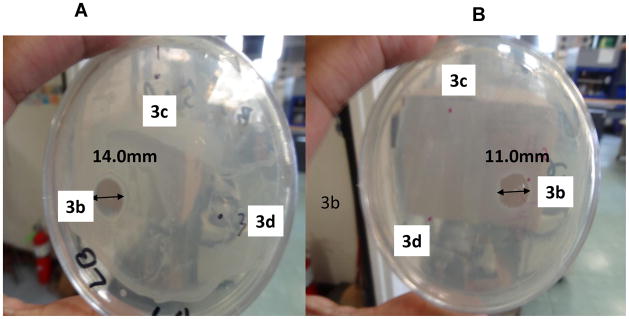
The disc diffusion method was used for antimicrobial testing of all ammonium salts against Gram-positive (Staphalococus) and Gram-negative (E. coli) bacteria. Accordingly, 100 μl of Staphalococus bacteria was spread into the agar plate. A 5.0 μL solution of compounds 3b, 3c, and 3d (2 mg/mL) was injected into the agar plate in three different spots and the seed culture was incubated for 18 hours at 45 °C. This procedure was repeated for E. coli bacteria. The compound 3b showed a zone of inhibition of 14.0 mm for E. coli and a zone of inhibition of 11.0 mm for Staphalococus, shown in Figure 4. From the disc diffusion method, we can see that E. coli showed a larger zone of inhibition than Staphalococus, which implies molecule 3b was more attracted to the Gram-negative bacteria than the Gram-positive bacteria. In order to see the effect of concentration of salt on antimicrobial activity, 10.0 μL solutions of compounds 3b, 3c, and 3d were injected. The zone of inhibition increased from 14.0 mm to 18.0 mm for E. coli and a zone of inhibition increased from 11.0 mm to 16.0 mm for Staphalococus. It is generally accepted that the mode of antimicrobial action of the ammonium groups follows as a sequence of activity: adsorption of ammonium groups onto the cell surface, followed by diffusion through the cell wall, and then binding to the cytoplasmic membranes, causing disruption of membranes, concluding with release of cytoplasmic constituents such as DNA, RNA, and K+ and followed by death of the cell.16,21 It appears that the adsorption of ammonium groups on the surface of Gram-negative bacteria is greater than on Gram-positive bacteria and this may be attributed to a high charge density on the surface of Gram-negative bacteria. It should be noted that silver nitrate dendrimer complexes and MesoSilver dendrimer complexes were prepared and tested against Staphalococus. The silver nitrate complex and Meso silver complex showed zones of inhibition of 2.6 and 2.65 mm, respectively, and they were not as effective as the modified dendrimer salt.27
Figure 4.
Images of agar plates after studied using disc diffusion method after 24 hours of incubation of Gram-negative (E. coli) bacteria (A) and Gram-positive (Staphalococus) (B)
In summary, we have synthesized poly(amidoamine) (PAMAM) (G3) dendrimer quaternary ammonium salts using tertiary amines with different chain lengths: dimethyldodecyl amine, dimethylhexyl amine, and dimethylbutyl amine using an efficient synthetic route. The antimicrobial activity of these dendrimer ammonium salts against Staphylococcus and E-coli bacteria was examined using the disc diffusion method. It was found that quaternary ammonium salt prepared with dimethyldodecyl amine exhibits significant antimicrobial efficacy against Staphalococus and E.coli bacteria and salts prepared using dimethylhexyl amine and dimethylbutyl amine exhibit no antimicrobial efficacy against Staphalococus and E.coli bacteria.
Supplementary Material
Acknowledgments
We thank Long Island University and Army Research Laboratory for financial support for this research. We also thank Ms Anusha Reddy for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Tomalia DA, Baker H, Dewald JR, Hall M, Kallos G, Martin S, Roek J, Ryder J, Smith P. Polym Journal. 1985;17:117. [Google Scholar]
- 2.Hodge P. Nature. 1993;362:18. [Google Scholar]
- 3.Newkome GR, Yao ZQ, Baker GR, Gupta VK. J Org Chem. 1985;50:2003. [Google Scholar]
- 4.Murat M, Crest G. Macromolecules. 1996;29:1278. [Google Scholar]
- 5.Bossman AW, Janssen HM, Meijer EW. Chem Rev. 1999;9:1665. doi: 10.1021/cr970069y. [DOI] [PubMed] [Google Scholar]
- 6.Hawker CJ, Frechet JMJ. J Am Chem Soc. 1990;112:7638. [Google Scholar]
- 7.Zhao M, Crooks RM. Chem Mater. 1999;11:3379. [Google Scholar]
- 8.Balogh L, Swanson DR, Spindler R, Tomalia DA. Proc ACS PMSE. 1997;77:118–119. [Google Scholar]
- 9.Balogh L, Tomalia DA. J Am Chem Soc. 1998;120:7355. [Google Scholar]
- 10.Zhao M, Sun L, Crooks RM. J Am Chem Soc. 1998;120:4877. [Google Scholar]
- 11.Singh P. Bioconjugate Chem. 1998;9:54. doi: 10.1021/bc970048a. [DOI] [PubMed] [Google Scholar]
- 12.Santo M, Fox MA. J Phy Org Chem. 1999;12:293. [Google Scholar]
- 13.Frechet JMJ. Science. 1994;263:1710. doi: 10.1126/science.8134834. [DOI] [PubMed] [Google Scholar]
- 14.Reddy JA, Dean D, Kennedy MD, Low PS. J Pharm Sci. 1999;12:293. [Google Scholar]
- 15.Majoros IJ, Keszler B, Woehler S, Bull T, Baker JR. Macromolecules. 2003;36:5526. [Google Scholar]
- 16.Chen CZ, Beck-Tan NC, Dhurjati P, van Dyk TK, Larossa RA, Cooper SL. Biomacromolecules. 2000;1:473. doi: 10.1021/bm0055495. [DOI] [PubMed] [Google Scholar]
- 17.Donaruma LG, Vogl O, Ottenbrite RM. Anionic Polymer Drugs. John Wiley & Son; New York: 1980. [Google Scholar]
- 18.Yuan HL, Tazuke S. Polym J. 1983;15:125. [Google Scholar]
- 19.Kaazawa K, Ikeda T, Endo T. J Polym Sci, Polym Chem. 1993;31:1467. [Google Scholar]
- 20.Mammen HC, Choi SK, Whitesides GM. Angew Chem, Int Ed. 1998;37:2754. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Kenaway ER, Worley SD, Broughton R. Biomacromolecules. 2007;8:1359. doi: 10.1021/bm061150q. [DOI] [PubMed] [Google Scholar]
- 22.Kanazawa A, Ikeda T, Endo T. J Polym Sci, Part A: Polym Chem. 1993;31:1441. [Google Scholar]
- 23.Kanazawa A, Ikeda T, Endo T. J Polym Sci, Part A: Polym Chem. 1993;31:335–343. [Google Scholar]
- 24.Dizman B, Elasri MO, Mathias LJ. J Appl Polym Sci. 2004;94:635. [Google Scholar]
- 25.Kanazawa A, Ikeda T, Endo T. J Polym Sci, Part A: Polym Chem. 1993;31:3031. [Google Scholar]
- 26.Tokura S, Ueno K, Miyazaki S, Nishi N. Makromol Symp. 1997:120. [Google Scholar]
- 27.Ghosh S, Yadev S, Vasanthan N, Sekosan G. J Appl Polym Sci. 2010;215:716. [Google Scholar]
- 28.Tan S, Li G, Shen J, Liu Y, Zong M. J Appl Polym Sci. 2000;77:1869. [Google Scholar]
- 29.Wang C, Nakamura J Polym Sci, Polym Chem. 1996;34:2517. [Google Scholar]
- 30.Ottaviani MF, Valluzzi R, Balogh L. Macromolecules. 2002;35:5105. [Google Scholar]
- 31.Saito R, Okamura SI, Ishizu K. Polymer. 1996;37:5255. [Google Scholar]
- 32.Balogh L, Valluzi Hagnauer GL, Lavendure KS, Gido SP, Tomalia DA. J Nanoparticle Res. 1991;1:353. [Google Scholar]
- 33.General procedure for preparation of dendrimer 3a: 0.500 grams of PAMAM (G3) dendrimer (1) was dissolved in anhydrous 2-methyl-2-pyrrolidinone in a dried round bottom flask. 1.1 equivalent of 2-chloroethyl isocyanate was added drop wise at ambient temperature and stirred at room temperature for 12 hours to complete the reaction. 1 g (5 equiv.) of dimethyl dodecylamine was added to the reaction mixture, slowly heated to 80°C and refluxed for 72 hours. The reaction mixture was concentrated and poured into acetone for precipitation. The product obtained was a yellow solid 3a. 13C NMR (Methanol-D4, 100 MHz, ppm vs. TMS): 175.4, 160.0, 171.5, 53.7, 51.2, 45.3, 40.8, 38.4, 37.9 and 33.5.
- 34.General procedure for preparation of dendrimer 2b; 3.0 mL (15.85% w/w) of PAMAM dendrimer (1) was dried and dissolved in 10.0 mL of methanol. 0.2934 g of chloroacetic anhydride was dissolved in 5.0 mL of methanol and added to the dendrimer solution in a drop wise manner. The mixture was then stirred for 2 hours to obtain chloacetylated dendrimer product (2b). 1H NMR (δ ppm, 400 MHz, Methanol-D4) : δ 2.32 (t, J= 6.4 Hz), (-NCH2CH2O-), 2.54 (d, J=6 Hz, CONHCH2CH2N-), 2.67 (t, J= 6 Hz, CONHCH2CH2NH2-), 2.74 (t, J= 6.4 Hz, -NCH2CH2CO-), 3.21 (d, J= 5.2 Hz) (-CONHCH2CH2NH2-) and 3,70 (s) (–CO-CH2-Cl). 13C NMR (Methanol-D4, 100 MHz, ppm vs. TMS): 175.4, 174.4, 171.5, 53.7, 51.2, 45.3, 40.8, 38.4, 37.9 and 33.5.
- 35.General procedure for preparation of dendrimer 3b, 3c, 3d. 0.5 mL of N,N-dimethyldodecylamine was then added to the 3 neck-flask containing chloacetylated dendrimer (2) equipped with thermometer and a condenser. The mixture was refluxed for 2 days at constant temperature of 40° C. After reaction was complete, reaction mixture was cooled to room temperature. The product was isolated using a 10 K cut-off ultrafiltration membrane. The final product 3b was isolated. Two similar experiments were carried out using N,N-dimethylhexylamine, and N,N-dimetylbutylamine. Spectral data for 3b: 1H NMR (δ ppm, 400 MHz, Methanol-D4) : 13C NMR (Methanol-D4, 100 MHz, ppm vs. TMS): 174.2, 169.2., 166.7, 67.0, 65.7, 64.9, 62.1, 59.2, 53.7, 52.3, 51.1, 44.8, 43.5, 41.9, 33.2, 30.5, 27.4, 25.8, 23.7, and 14.6.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.