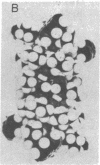
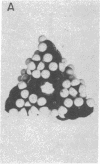
Abstract
By means of 23Na NMR, two ion binding sites were observed in phospholipid-packaged gramicidin channels and the four associated rate constants were approximated. Limits also were placed on a fifth rate constant for an intrachannel ion translocation. By using Eyring rate theory to introduce voltage dependence, these rate constants were used in steady-state-current equations for calculation of gramicidin single-channel currents for two- and three-site models. Calculated single-channel currents are compared with previously published experimental single-channel currents obtained by electrical measurements on Na+ transport across gramicidin-doped planar lipid bilayers. The calculated results for the two- and three-site models compare favorably with the experimental results. Accordingly, it is demonstrated that NMR-derived rate constants can be coupled with Eyring rate theory to calculate currents through a transmembrane channel and to do so within levels of variation that compare with the differences obtained on planar lipid bilayers formed with different lipids.
Full text
PDF
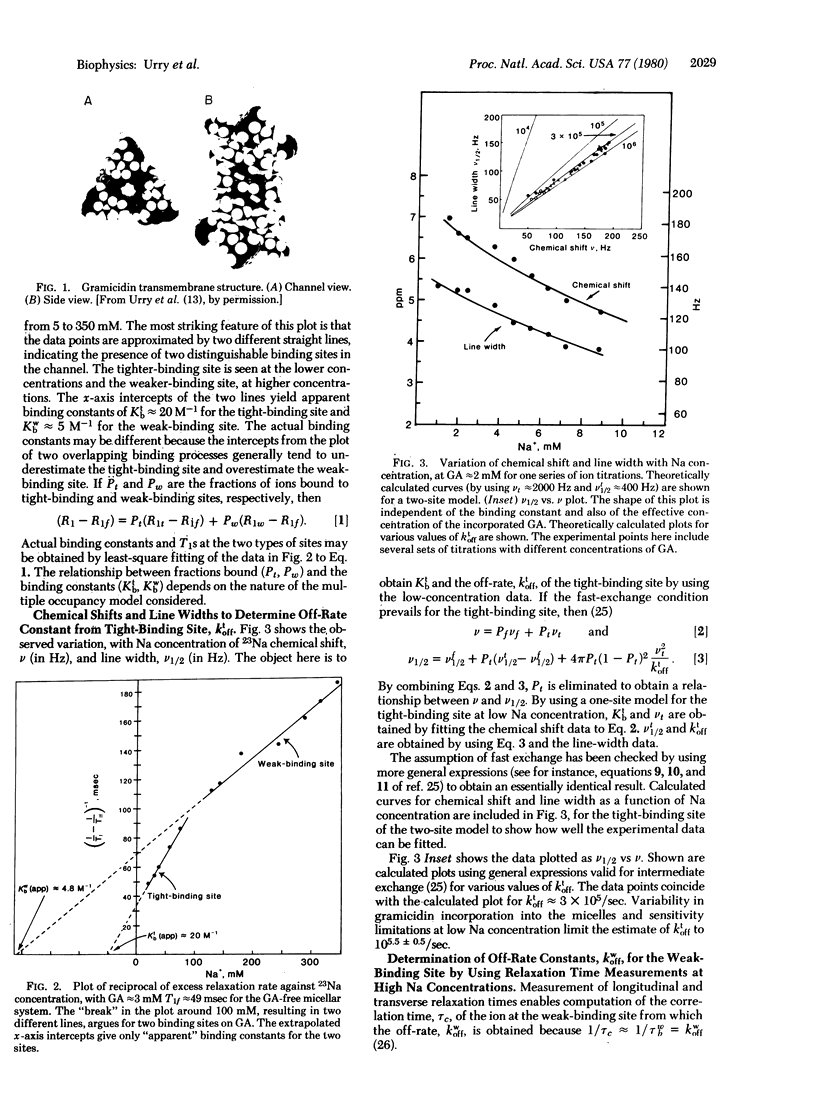
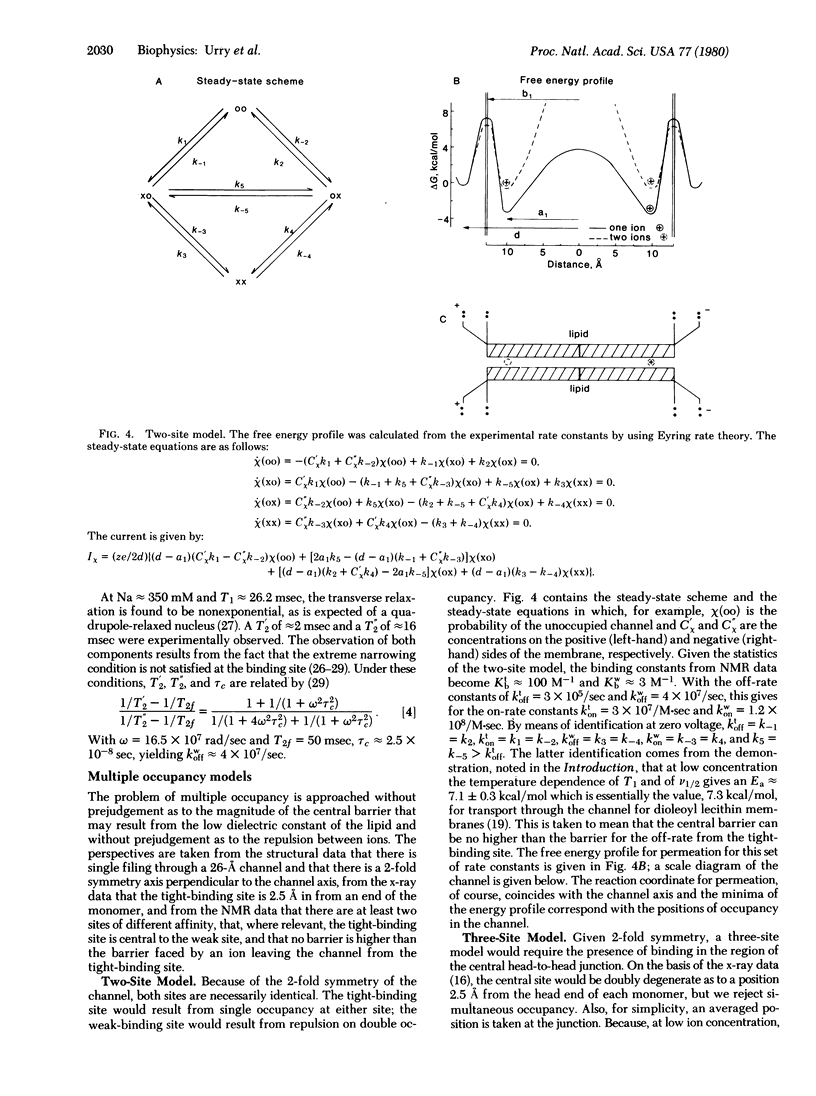

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bamberg E., Apell H. J., Alpes H. Structure of the gramicidin A channel: discrimination between the piL,D and the beta helix by electrical measurements with lipid bilayer membranes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2402–2406. doi: 10.1073/pnas.74.6.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Temperature-dependent properties of gramicidin A channels. Biochim Biophys Acta. 1974 Oct 29;367(2):127–133. doi: 10.1016/0005-2736(74)90037-6. [DOI] [PubMed] [Google Scholar]
- Bamberg E., Läuger P. Temperature-dependent properties of gramicidin A channels. Biochim Biophys Acta. 1974 Oct 29;367(2):127–133. doi: 10.1016/0005-2736(74)90037-6. [DOI] [PubMed] [Google Scholar]
- Cornélis A., Laszlo P. Sodium binding sites of gramicidin A: sodium-23 nuclear magnetic resonance study. Biochemistry. 1979 May 15;18(10):2004–2007. doi: 10.1021/bi00577a025. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Discreteness of conductance change in bimolecular lipid membranes in the presence of certain antibiotics. Nature. 1970 Jan 31;225(5231):451–453. doi: 10.1038/225451a0. [DOI] [PubMed] [Google Scholar]
- Hladky S. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. I. Studies of the unit conductance channel. Biochim Biophys Acta. 1972 Aug 9;274(2):294–312. doi: 10.1016/0005-2736(72)90178-2. [DOI] [PubMed] [Google Scholar]
- James T. L., Noggle J. H. 23Na nuclear magnetic resonance relaxation studies of sodium ion interaction with soluble RNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):644–649. doi: 10.1073/pnas.62.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeppe R. E., 2nd, Berg J. M., Hodgson K. O., Stryer L. Gramicidin A crystals contain two cation binding sites per channel. Nature. 1979 Jun 21;279(5715):723–725. doi: 10.1038/279723a0. [DOI] [PubMed] [Google Scholar]
- Krasne S., Eisenman G., Szabo G. Freezing and melting of lipid bilayers and the mode of action of nonactin, valinomycin, and gramicidin. Science. 1971 Oct 22;174(4007):412–415. doi: 10.1126/science.174.4007.412. [DOI] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- Mueller P., Rudin D. O. Development of K+-Na+ discrimination in experimental bimolecular lipid membranes by macrocyclic antibiotics. Biochem Biophys Res Commun. 1967 Feb 21;26(4):398–404. doi: 10.1016/0006-291x(67)90559-1. [DOI] [PubMed] [Google Scholar]
- Myers V. B., Haydon D. A. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972 Aug 9;274(2):313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Sandblom J., Eisenman G., Neher E. Ionic selectivity, saturation and block in gramicidin A channels: I. Theory for the electrical properties of ion selective channels having two pairs of binding sites and multiple conductance states. J Membr Biol. 1977 Mar 23;31(4):383–347. doi: 10.1007/BF01869414. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Goodall M. C., Glickson J. D., Mayers D. F. The gramicidin A transmembrane channel: characteristics of head-to-head dimerized (L,D) helices. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1907–1911. doi: 10.1073/pnas.68.8.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Long M. M., Jacobs M., Harris R. D. Conformation and molecular mechanisms of carriers and channels. Ann N Y Acad Sci. 1975 Dec 30;264:203–220. doi: 10.1111/j.1749-6632.1975.tb31484.x. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Spisni A., Khaled A. Characterization of micellar-packaged gramicidin A channels. Biochem Biophys Res Commun. 1979 Jun 13;88(3):940–949. doi: 10.1016/0006-291x(79)91499-2. [DOI] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein S., Wallace B. A., Blout E. R., Morrow J. S., Veatch W. Conformation of gramicidin A channel in phospholipid vesicles: a 13C and 19F nuclear magnetic resonance study. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4230–4234. doi: 10.1073/pnas.76.9.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]