Abstract
While Friend retrovirus-infected mice readily mount a vigorous CD8+ T cell response to the leader-gag-derived peptide GagL85–93, no GagL85–93-specific T cells were detectable in mice immunized against Friend virus (FV) with viral vectors or DNA vaccines. By exchanging one epitope-flanking amino acid or using a scaffold protein we were able to demonstrate for the first time the induction of GagL85–93-specific CD8+ T cells by genetic vaccination and show their high protective effect against FV challenge infection.
TEXT
The Gag polyprotein p65 of Friend murine leukemia virus (F-MuLV) consists of the structural proteins p15, p12, p30, and p10, which are the main components of the viral capsid. Usage of an alternative start codon upstream of the gag open reading frame adds a leader region to the Gag polyprotein, resulting in the glycosylated protein gp85gag (12). The leader region of gp85gag contains an immunodominant CD8+ T cell epitope of Friend virus (FV) (9), and in acutely FV-infected mice up to 15% of the activated CD8+ T cells are GagL85–93 specific (26). Despite the immunodominance of this epitope in natural FV infection, no induction of GagL85–93-specific CD8+ T cells could be detected after immunization of mice with adenovirus (Ad)-based vectors encoding leader-gag (3). Similarly, in mice genetically immunized by plasmid DNA encoding leader-gag, no GagL85–93-specific CD8+ T cells could be detected before FV challenge (11), and in vaccination studies with vaccinia virus-based vectors encoding different parts of p65gag or gp85gag, no difference in protection was seen whether or not the leader region was included in the vaccine (19). Thus, although the FV model has been used extensively for the development of new concepts for vaccination against retroviruses, none of the genetic vaccines utilized in the past were able to induce CD8+ T cell responses against the immunodominant GagL85–93 epitope of FV. Therefore, the goal of this study was to develop a genetic vaccine capable of inducing GagL85–93-specific immunity.
Two factors critically influence if a peptide sequence can be presented as an epitope on a major histocompatibility complex (MHC): the ability to bind to a certain MHC allele and the efficiency of proteasomal degradation resulting in that peptide. Interestingly, neither property is predicted for the immunodominant GagL85–93 epitope in H-2Db mice using the prediction tools NetMHC (17) and NetChop (20). As the tyrosine flanking the GagL85–93 epitope C terminally (C + 1) in the native sequence has been described to be unfavorable for proteasomal degradation (16), inefficiency of processing might be an explanation for the lack of CD8+ T cell immunity after genetic immunization. To overcome this problem, we exchanged the C+1 tyrosine with lysine, since for the resulting protein, leader-gagC1K, proteasomal cleavage after Leu93 is predicted (20).
As an alternative approach, we constructed an adenoviral vector encoding a fusion protein of the murine cellular protein thioredoxin (Txn) and the GagL85–93 peptide, TxnGagL. Thioredoxin is a cellular protein involved in redox processes, and, while being a nonantigenic self-protein, it has been ascribed immunostimulatory and chemotactic properties, making it an attractive protein scaffold for immunization (5, 6).
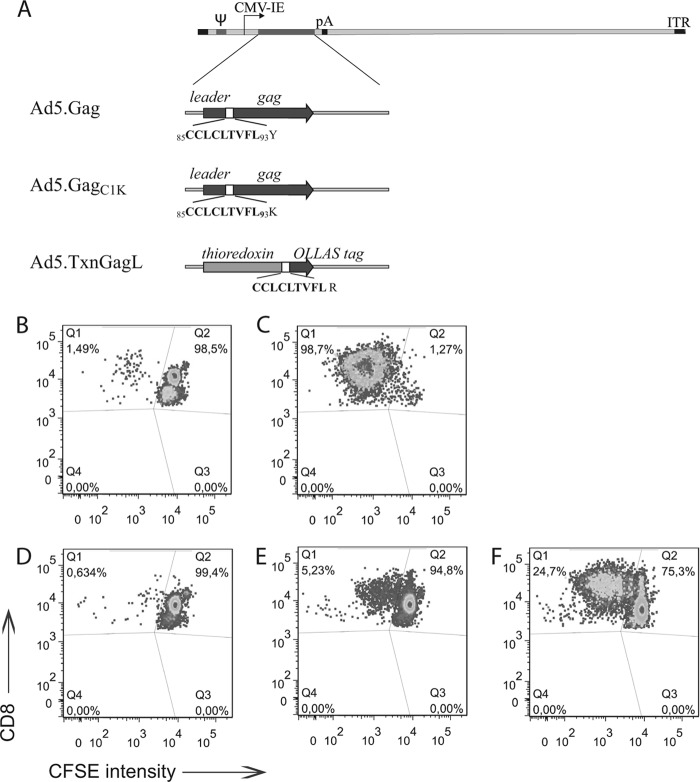
Adenoviral vectors encoding leader-gagC1K or TxnGagL (Fig. 1A) were constructed as described before (14); transgene expression and sequence identity were verified (data not shown). To analyze whether expression of the new transgenes leads to processing of the GagL85–93 epitope, an in vitro proliferation assay was performed using GagL85–93-specific CD8+ T cells from T cell receptor-transgenic mice (22) and vector-transduced bone marrow-derived dendritic cells (DC). While no proliferation of the CD8+ T cells was detectable when DC were transduced with Ad5.Gag (3), a vector encoding the native leader-gag sequence (Fig. 1A), proliferation was observed after transduction of DC with either Ad5.GagC1K or Ad5.TxnGagL (Fig. 1D to F), indicating efficient processing of the GagL85–93 epitope from the engineered transgenes.
Fig 1.
Vector design and in vitro processing of the Ad-encoded GagL epitope. (A) Schematic presentation of the employed adenoviral vectors. The location of the GagL85–93 epitope CCLCLTVFL is shown, and the C+1 flanking amino acids in the native and modified sequences are indicated. ITR, inverted terminal repeat; Ψ, packaging signal; CMV-IE, cytomegalovirus immediate early promoter; pA, polyadenylation signal. (B to F) For the analysis of antigen processing, an in vitro proliferation assay was performed using carboxyfluorescein succinimidyl ester (CFSE)-stained, naive CD8+ T cell receptor (TCR)-transgenic T cells specific for the GagL85–93 epitope and bone marrow-derived dendritic cells (DC) transduced with the adenoviral construct Ad5.Gag (D), Ad5.GagC1K (E), or Ad5.TxnGagL (F) at a multiplicity of infection of 1,000. Nontransduced DC served as the negative control (B), and DC loaded with a modified GagL85–93 peptide (where the cysteine residues are replaced by α-aminobutyric acid) served as the positive control (C). CFSE intensity of CD8+ T cells was analyzed after 3 days of coincubation with the respective DC. Plots are representative for three independent experiments.
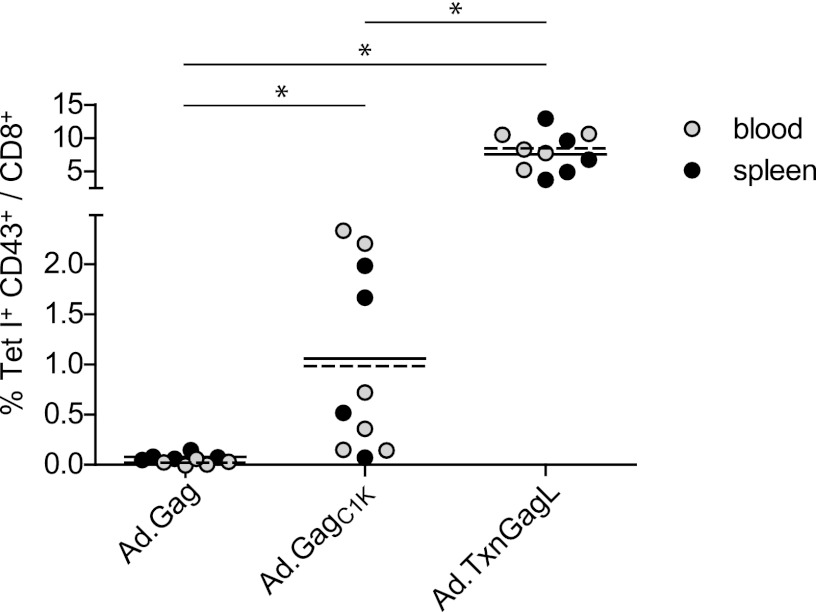
To analyze the induction of CD8+ T cell responses by the new vaccine vectors, highly FV-susceptible CB6F1 hybrid mice (H-2b/d, FV2r/s, Rfv3r/s) were immunized twice in a 3-week interval with 109 particles of the adenoviral vectors using a heterologous prime-boost combination of Ad5-based vectors and fiber-chimeric Ad5F35 (21) vectors as described before (3). Immune responses were analyzed 2 weeks after the second immunization using MHC class I (MHC-I) tetramers containing a modified GagL85–93 epitope peptide (25) for staining of spleen or blood cells. As reported before (3), immunization with Ad.Gag did not lead to the induction of GagL85–93-specific CD8+ T cells, yet when mice were immunized with Ad.GagC1K or Ad.TxnGagL, GagL85–93-specific CD8+ T cells were readily detectable by tetramer staining in most mice (Fig. 2). Especially high levels of GagL85–93-specific CD8+ T cells were found in mice immunized with Ad.TxnGagL; the levels were significantly higher than those in mice immunized with Ad.GagC1K (P < 0.05).
Fig 2.
Vaccine-induced CD8+ T cell response. CB6F1 mice were immunized twice in a 3-week interval using 109 viral particles (vp) of adenoviral vectors carrying the indicated transgenes; Ad5-based vectors were used for prime immunization, and Ad5F35 vectors were used for boost immunization. Two weeks after the second immunization, the induction of MHC-I tetramer (Tet I)-positive CD8+ T cells was analyzed either in spleen cells or blood cells. Shown are percentages of Tet I+ CD43+ cells. Lines indicate mean values for spleen (solid lines) and blood cells (dashed lines), respectively. Statistically significant differences are indicated by asterisks (P < 0.05; analysis of variance [ANOVA] on ranks, Student-Newman-Keuls test). Data for blood cells were combined from two independent experiments; data for spleen cells were obtained from a single additional experiment. Spleen cells and blood cells were collected from different mice immunized in two independent experiments.
To possibly augment vaccine-induced CD8+ T cell responses, we coapplied vectors encoding interleukin-12 (IL-12), IL-15, IL-18, IL-21, or granulocyte-macrophage colony-stimulating factor (GM-CSF) together with Ad.GagC1K, since these cytokines have been previously reported to enhance CD8+ T cell responses to peptide or DNA vaccines (1, 2, 7). However, no significant differences in levels of GagL85–93-specific CD8+ T cells compared to those resulting from immunization with Ad.GagC1K alone were found (data not shown).
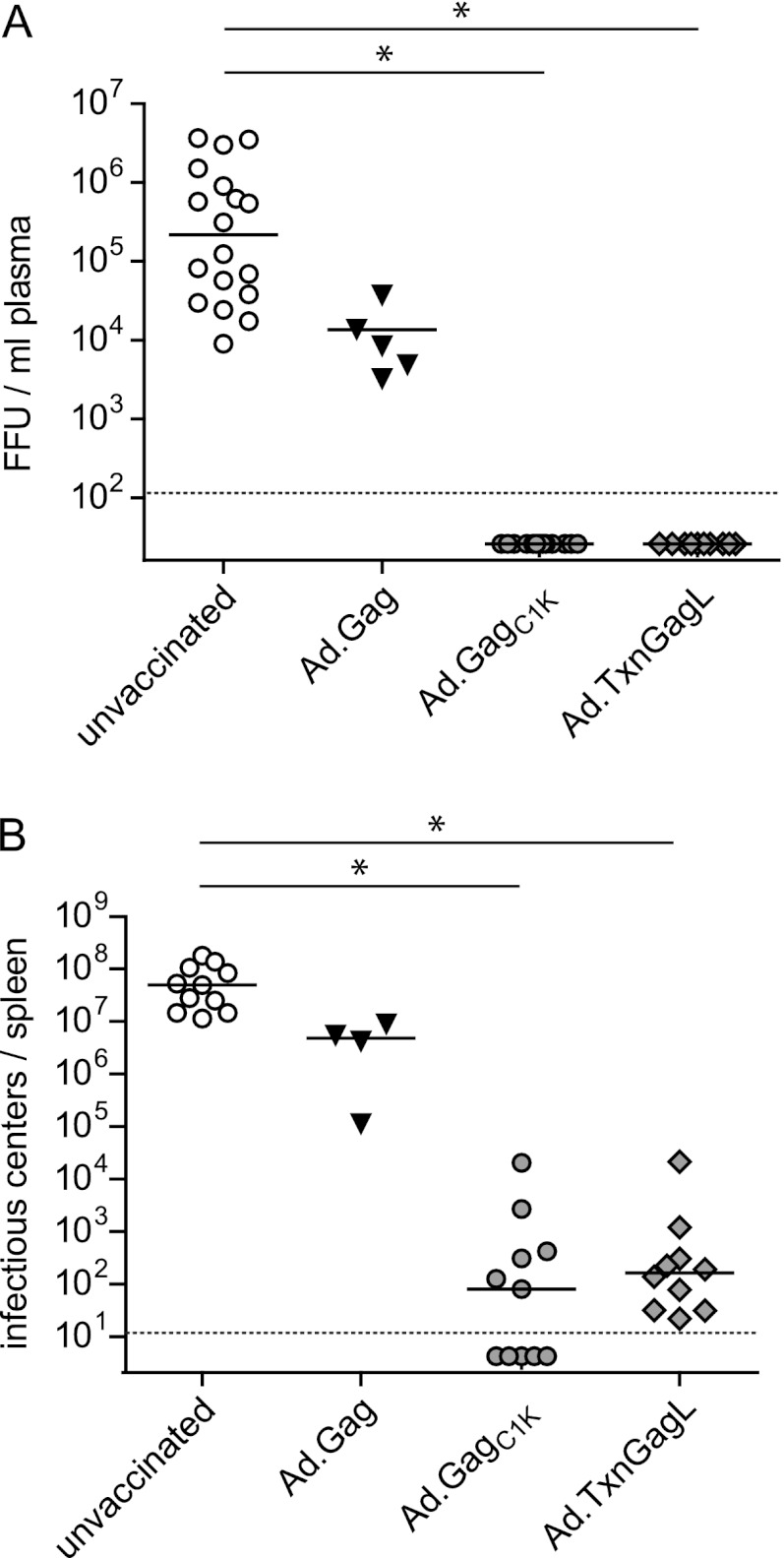
To analyze protection conferred by the new vectors, mice were challenged with 5,000 spleen focus-forming units of FV 3 weeks after boost immunization. Only mice that had received Ad.GagC1K or Ad.TxnGagL were strongly protected, with no detectable viral loads in the plasma on day 10 (Fig. 3A) and very low viral loads in the spleen on day 21 (Fig. 3B) after FV challenge. Thus, although a few mice immunized with Ad.GagC1K exhibited very low frequencies of MHC-I tetramer-positive (Tet I+) CD8+ T cells, they were still protected from high-dose FV challenge, suggesting that also these mice had vaccine-primed CD8+ T cells.
Fig 3.
Protection from high-dose FV challenge infection. CB6F1 mice were immunized twice in a 3-week interval using 109 vp of adenoviral vectors carrying the indicated transgenes; Ad5-based vectors were used for prime immunization, and Ad5F35 vectors were used for boost immunization. Three weeks after boost, mice were challenged with 5,000 spleen focus-forming units (SFFU) of FV complex. Viral load in plasma (A) was analyzed 10 days after FV challenge; viral load in spleen (B) was analyzed on day 21 after FV challenge. Solid lines indicate median values. Statistically significant differences are indicated by asterisks (P < 0.05; ANOVA on ranks, Dunn's test). Dashed lines indicate the detection limit. Data were acquired in three independent experiments.
The strong protection after vaccination with the new vectors emphasizes the importance of CD8+ T cells for protection against retroviral infection. The CD8+ T cell response induced by the TxnGagL construct is especially high and similar in strength to the CD8+ T cell response seen in acutely FV infected mice (26). Also in this construct, the epitope-flanking amino acid is modified compared to the native sequence (C + 1: R), evidently allowing for efficient processing. Thioredoxin has been ascribed immunostimulatory properties (5, 6), and bacterial Txn has been used before as a peptide carrier for antibody induction (8, 24); the high frequency of GagL85–93-specific CD8+ T cells in Ad.TxnGagL-immunized mice indicates that its immunogenicity may be beneficial for T cell induction as well.
The reasons for the apparent discrepancy in processing of the leader-gag protein expressed from F-MuLV or a genetic vaccine are not completely understood. As this finding might have possible implications for the use of adenoviral vectors for vaccine development in general, it shall be thoroughly investigated. Possible mechanisms are an involvement of other F-MuLV proteins in processing and differences in the induction of the immunoproteasome, although it is known that nonreplicating adenoviral vectors are very immunogenic and induce immunoproteasome formation (13, 18). More likely, it may be competition between the GagL85–93 epitope and adenovirus-derived epitopes that hampers GagL85–93-specific T cell induction. The GagL85–93 epitope is the immunodominant epitope of FV, where CD8+ T cell responses to other epitopes are very weak or undetectable. In the context of an adenoviral vector, however, the GagL85–93 epitope has to compete with strong epitopes from adenoviral proteins. While the unfavorable C + 1 amino acid may not play a significant role in the barely competitive environment of FV, it may be a strong disadvantage when the epitope is competing with adenoviral epitopes, and this may explain the subdominance of the GagL85-93 epitope. Enhancing processing of GagL85-93 through the amino acid substitution in the GagC1K construct apparently improves its rank in the immunodominance hierarchy. Similar suppression of immune responses to transgene epitopes by adenovirus epitopes has recently been described (15). Epitope competition is also a possible explanation for the considerably higher levels of GagL85-93-specific CD8+ T cells induced by the TxnGagL vaccine. The TxnGagL fusion protein is likely secreted from vector-transduced cells, as has been reported for Txn before (23). Secreted protein could then be taken up by nontransduced antigen-presenting cells, and the GagL85-93 epitope would be processed and presented in the absence of adenovirus-derived epitopes.
The ability to induce CD8+ T cell responses by genetic vaccination is an important prerequisite to further advance immunization strategies in the FV model. It is known that for complete protection from retrovirus infection, complex immune responses are necessary, comprising CD4+ and CD8+ T cells as well as neutralizing antibodies (10). In the past we reported on a new adenovirus-based vector type that displays the encoded antigen on the capsid and thereby induces strong CD4+ T cell and antibody responses (4). The combination of this vector with the cytotoxic T lymphocyte (CTL)-inducing vectors described here shall be evaluated to further improve vaccination efficiency, with sterile immunity against a retrovirus infection mediated by a genetic vaccine probably being within reach.
ACKNOWLEDGMENTS
This work was supported by a grant to P.G. from the internal research promotion program IFORES of the Medical Faculty of the University Duisburg-Essen, by a grant from the Mercator Research Center Ruhr to U.D., and by grants from the Deutsche Forschungsgemeinschaft (DFG grant TRR60 to U.D. and WI 1329/6-1 to U.D. and W.B.).
Footnotes
Published ahead of print 29 August 2012
REFERENCES
- 1. Ahlers JD, Belyakov IM, Matsui S, Berzofsky JA. 2001. Mechanisms of cytokine synergy essential for vaccine protection against viral challenge. Int. Immunol. 13:897–908 [DOI] [PubMed] [Google Scholar]
- 2. Ahlers JD, Dunlop N, Alling DW, Nara PL, Berzofsky JA. 1997. Cytokine-in-adjuvant steering of the immune response phenotype to HIV-1 vaccine constructs: granulocyte-macrophage colony-stimulating factor and TNF-alpha synergize with IL-12 to enhance induction of cytotoxic T lymphocytes. J. Immunol. 158:3947–3958 [PubMed] [Google Scholar]
- 3. Bayer W, Schimmer S, Hoffmann D, Dittmer U, Wildner O. 2008. Evaluation of the Friend virus model for the development of improved adenovirus-vectored anti-retroviral vaccination strategies. Vaccine 26:716–726 [DOI] [PubMed] [Google Scholar]
- 4. Bayer W, et al. 2010. Vaccination with an adenoviral vector that encodes and displays a retroviral antigen induces improved neutralizing antibody and CD4+ T-cell responses and confers enhanced protection. J. Virol. 84:1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertini R, et al. 1999. Thioredoxin, a redox enzyme released in infection and inflammation, is a unique chemoattractant for neutrophils, monocytes, and T cells. J. Exp. Med. 189:1783–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blum H, Rollinghoff M, Gessner A. 1996. Expression and co-cytokine function of murine thioredoxin/adult T cell leukaemia-derived factor (ADF). Cytokine 8:6–13 [DOI] [PubMed] [Google Scholar]
- 7. Bolesta E, et al. 2006. Increased level and longevity of protective immune responses induced by DNA vaccine expressing the HIV-1 Env glycoprotein when combined with IL-21 and IL-15 gene delivery. J. Immunol. 177:177–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chakraborty K, et al. 2006. Design of immunogens that present the crown of the HIV-1 V3 loop in a conformation competent to generate 447-52D-like antibodies. Biochem. J. 399:483–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen W, Qin H, Chesebro B, Cheever MA. 1996. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J. Virol. 70:7773–7782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dittmer U, Brooks DM, Hasenkrug KJ. 1999. Requirement for multiple lymphocyte subsets in protection by a live attenuated vaccine against retroviral infection. Nat. Med. 5:189–193 [DOI] [PubMed] [Google Scholar]
- 11. Dittmer U, Werner T, Kraft AR. 2008. Co-immunization of mice with a retroviral DNA vaccine and GITRL-encoding plasmid augments vaccine-induced protection against retrovirus infection. Viral Immunol. 21:459–467 [DOI] [PubMed] [Google Scholar]
- 12. Evans LH, Dresler S, Kabat D. 1977. Synthesis and glycosylation of polyprotein precursors to the internal core proteins of Friend murine leukemia virus. J. Virol. 24:865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hartman ZC, et al. 2007. Adenovirus infection triggers a rapid, MyD88-regulated transcriptome response critical to acute-phase and adaptive immune responses in vivo. J. Virol. 81:1796–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. He TC, et al. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. U. S. A. 95:2509–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kron MW, et al. 2011. High-capacity adenoviral vectors circumvent the limitations of DeltaE1 and DeltaE1/DeltaE3 adenovirus vectors to induce multispecific transgene product-directed CD8 T-cell responses. J. Gene Med. 13:648–657 [DOI] [PubMed] [Google Scholar]
- 16. Livingston BD, et al. 2001. Optimization of epitope processing enhances immunogenicity of multiepitope DNA vaccines. Vaccine 19:4652–4660 [DOI] [PubMed] [Google Scholar]
- 17. Lundegaard C, et al. 2008. NetMHC-3.0: accurate web accessible predictions of human, mouse and monkey MHC class I affinities for peptides of length 8–11. Nucleic Acids Res. 36:W509–W512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCaffrey AP, et al. 2008. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol. Ther. 16:931–941 [DOI] [PubMed] [Google Scholar]
- 19. Miyazawa M, Nishio J, Chesebro B. 1992. Protection against Friend retrovirus-induced leukemia by recombinant vaccinia viruses expressing the gag gene. J. Virol. 66:4497–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nielsen M, Lundegaard C, Lund O, Kesmir C. 2005. The role of the proteasome in generating cytotoxic T cell epitopes: insights obtained from improved predictions of proteasomal cleavage. Immunogenetics 57:33–41 [DOI] [PubMed] [Google Scholar]
- 21. Nilsson M, et al. 2004. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J. Gene Med. 6:631–641 [DOI] [PubMed] [Google Scholar]
- 22. Ohlen C, et al. 2002. CD8+ T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J. Exp. Med. 195:1407–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rubartelli A, Bajetto A, Allavena G, Wollman E, Sitia R. 1992. Secretion of thioredoxin by normal and neoplastic cells through a leaderless secretory pathway. J. Biol. Chem. 267:24161–24164 [PubMed] [Google Scholar]
- 24. Rubio I, et al. 2009. Potent anti-HPV immune responses induced by tandem repeats of the HPV16 L2 (20–38) peptide displayed on bacterial thioredoxin. Vaccine 27:1949–1956 [DOI] [PubMed] [Google Scholar]
- 25. Schepers K, et al. 2002. Differential kinetics of antigen-specific CD4+ and CD8+ T cell responses in the regression of retrovirus-induced sarcomas. J. Immunol. 169:3191–3199 [DOI] [PubMed] [Google Scholar]
- 26. Zelinskyy G, Kraft AR, Schimmer S, Arndt T, Dittmer U. 2006. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur. J. Immunol. 36:2658–2670 [DOI] [PubMed] [Google Scholar]