Abstract
Zinc finger antiviral protein (ZAP) is a host factor that specifically inhibits the replication of certain viruses by binding to specific viral mRNAs and repressing mRNA expression. Here we report that ZAP inhibits expression of murine gammaherpesvirus 68 (MHV-68) M2, which plays important roles in establishment and maintenance of viral latency. Downregulation of endogenous ZAP in cells harboring latent MHV-68 promoted lytic replication of the virus. These results suggest that ZAP inhibits M2 expression and regulates the maintenance of MHV-68 latency.
TEXT
Zinc finger antiviral protein (ZAP) was initially identified as a host factor that inhibits the replication of murine leukemia virus (MLV) (5). In addition to MLV, ZAP inhibits the replication of HIV-1 (27), Ebola virus, Marburg virus (19), and certain alphaviruses, such as Sindbis virus (SINV) (2). ZAP is not a universal antiviral factor since some viruses, including herpes simplex virus 1 and yellow fever virus, grow normally in ZAP-expressing cells (2). ZAP binds directly to specific viral mRNAs and recruits the cellular mRNA degradation machinery to degrade the target mRNA (7, 8, 27, 28). It has been suggested that ZAP also represses the translation of target mRNA (27). Whether a virus is sensitive to ZAP seems to be determined by the presence of ZAP-responsive element (ZRE) in the viral mRNA. No obvious common motifs or conserved sequences have been identified in the known ZREs, except that they are all more than 500 nucleotides (nt) long.
Murine gammaherpesvirus 68 (MHV-68) is a member of the Gammaherpesvirinae subfamily, whose replication cycle is composed of the latent phase and lytic phase (22). Only a small number of genes are expressed in the latent phase (17), including the M2 gene, which plays important roles in the establishment and maintenance of latency (9, 11, 15, 21, 24). In cell culture, latent MHV-68 can be reactivated into lytic replication by various means, such as treatment of cells with 12-O-tetradecanoylphorbol-13-acetate (TPA). While the mechanisms for the establishment and maintenance of viral latency are not completely understood, several host factors have been reported to be involved in these processes (3, 6, 12, 14, 16).
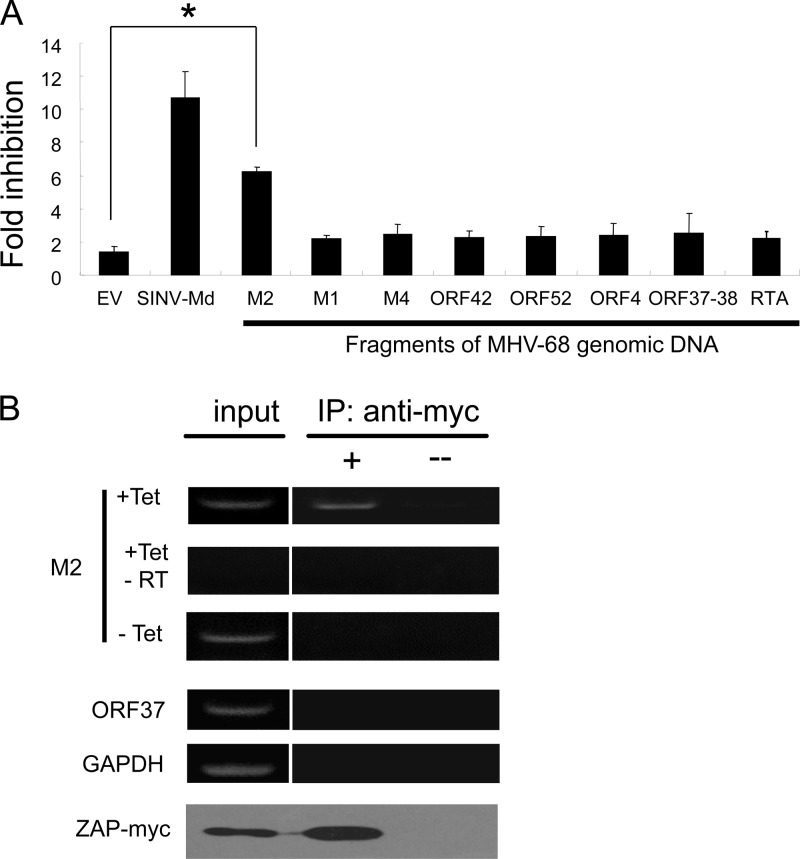
To determine whether any MHV-68 mRNAs can be targeted by ZAP, genomic DNA fragments covering known MHV-68 open reading frames (ORFs) were PCR amplified from MHV-68 bacterial artificial chromosome (BAC) DNA (25) and individually cloned into the reporter pGL3-Luc-linker (7) downstream of the firefly luciferase coding sequence. The reporters were transfected into 293Trex-rZAP cells, which express myc-tagged ZAP in a tetracycline-inducible manner (7), along with pRL-TK (Promega), a Renilla luciferase reporter that is insensitive to ZAP (7) to serve as a control for transfection efficiency and sample handling. The luciferase activities were measured at 48 h posttransfection using a dual-luciferase assay (Promega). Sensitivity of the reporters to ZAP is indicated by fold inhibition, which is calculated as normalized luciferase activity in mock-treated cells divided by that in tetracycline-treated cells. A fragment derived from SINV that has been shown to be sensitive to ZAP (7) was used as a positive control.
Out of 37 MHV-68 fragments tested (see the supplemental material), only a fragment containing the M2 locus conferred significant sensitivity to the reporter (Fig. 1A) (data not shown). We previously reported that ZAP binds directly to its target RNA (7). To substantiate that M2 is the target of ZAP, we analyzed whether M2 mRNA is associated with ZAP in MHV-68-infected ZAP-expressing cells. 293TRex-rZAP cells were infected with MHV-68 for 1 h at a multiplicity of infection (MOI) of 0.5 PFU per cell, followed by treatment with tetracycline to induce ZAP expression. At 72 h postinfection, cells were lysed and ZAP was immunoprecipitated using anti-myc antibody. The associated RNA was extracted, reverse transcribed using oligo(dT) as a primer, and detected by PCR (for primer sequences, see the supplemental material). As expected, immunoprecipitation of ZAP coprecipitated M2 mRNA (Fig. 1B). In contrast, immunoprecipitation of ZAP failed to coprecipitate MHV-68 ORF37 mRNA or cellular GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA (Fig. 1B). The identity of mature M2 mRNA was confirmed by sequencing analysis of the PCR product (data not shown).
Fig 1.
Identification of MHV-68 M2 as a target of ZAP. (A) DNA fragments of MHV-68 indicated were individually cloned into pGL3-Luc-linker. The plasmids were transfected into 293TRex-rZAP cells. Cells were mock treated or treated with tetracycline to induce ZAP expression. Fold inhibition is calculated as luciferase activity in mock-treated cells divided by that in tetracycline-treated cells. The data presented are means ± standard deviations (SD) from three independent experiments. *, P < 0.05. EV, empty vector; SINV-Md, pGL3-Luc-linker reporter containing fragment Md from SINV. (B) 293TRex-rZAP cells were infected with MHV-68 and mock treated (− Tet) or treated with tetracycline (+ Tet). Cell lysates were incubated with protein G beads with (+) or without (−) anti-myc antibody to precipitate ZAP. The RNA that coprecipitated with ZAP was detected by reverse transcription-PCR (RT-PCR), and the expression and immunoprecipitation (IP) of ZAP were analyzed by Western blotting. −RT, no reverse transcriptase was added in the reverse transcription reaction.
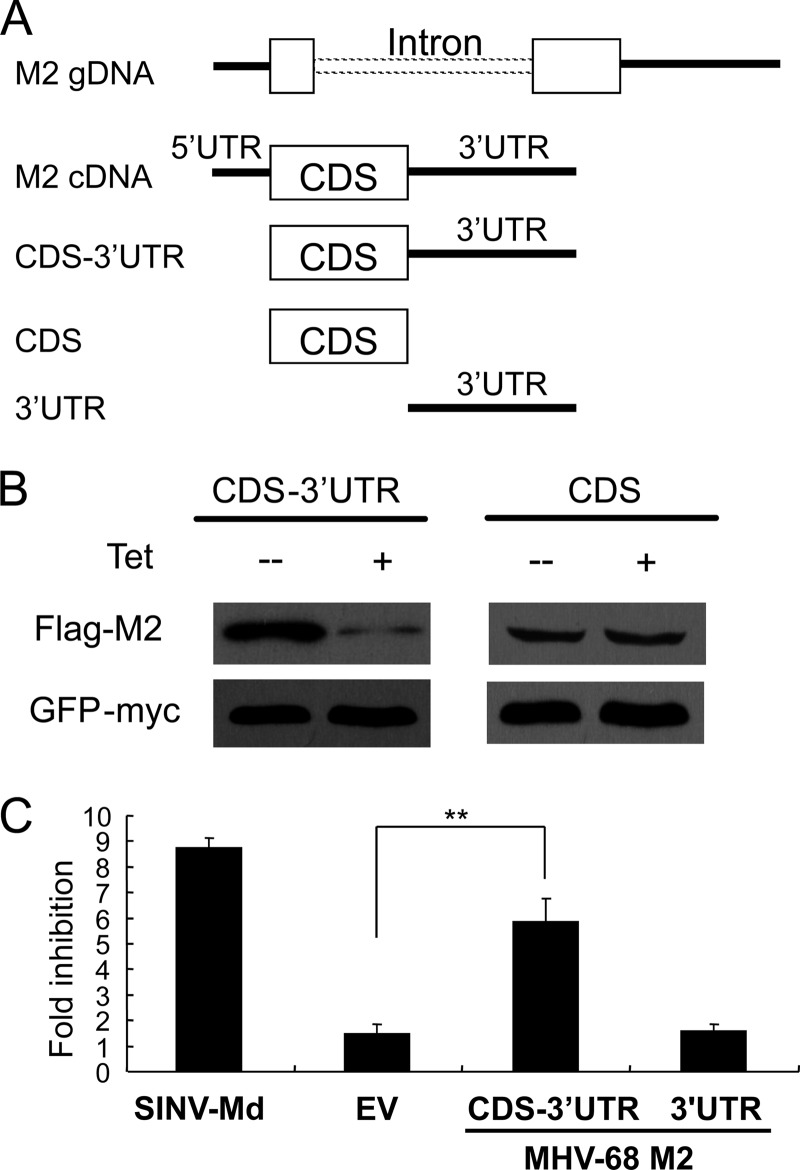
We further analyzed whether ZAP inhibits the expression of M2. Mature M2 mRNA is composed of a 5′ untranslated region (5′ UTR) of 110 nt, a coding sequence (CDS) of 597 nt and a 3′ UTR of 656 nt (4). The coding sequence plus the 5′ UTR (5′ UTR-CDS) or the 3′ UTR (CDS-3′ UTR) was cloned into a protein expression vector pCMV-HF (8) and transfected into 293Trex-rZAP cells. In ZAP-expressing cells, M2 expression from the CDS-3′ UTR was dramatically reduced (Fig. 2B, left panel), while M2 expression from 5′ UTR-CDS was not affected (data not shown). Furthermore, when the CDS alone was cloned into the vector, M2 expression was little affected (Fig. 2B, right panel), suggesting that the 3′ UTR is required for the inhibition. To determine whether the 3′ UTR of M2 is sufficient to be responsive to ZAP, it was cloned into pGL3-luc-linker. While CDS-3′ UTR rendered the reporter responsive to ZAP, the 3′ UTR alone failed to do so (Fig. 2C). These results indicate that ZAP inhibits M2 expression in a manner dependent on both the CDS and the 3′ UTR.
Fig 2.
ZAP inhibits M2 expression. (A) Schematic representation of M2 gene segments. gDNA, genomic DNA. (B) The CDS alone or CDS-3′ UTR of M2 was cloned into a protein expression vector, followed by transfection into 293TRex-rZAP cells. At 6 h posttransfection, cells were treated with tetracycline to induce ZAP expression. At 54 h posttransfection, cells were lysed and the expression of Flag-tagged M2 was detected by Western blotting. (C) The 3′ UTR of M2 was cloned into pGL3-Luc-linker. The sensitivity of the reporter was assayed as described in the legend to Fig. 1A. Data presented are means ± SD from three independent experiments. **, P < 0.01. EV, empty vector.
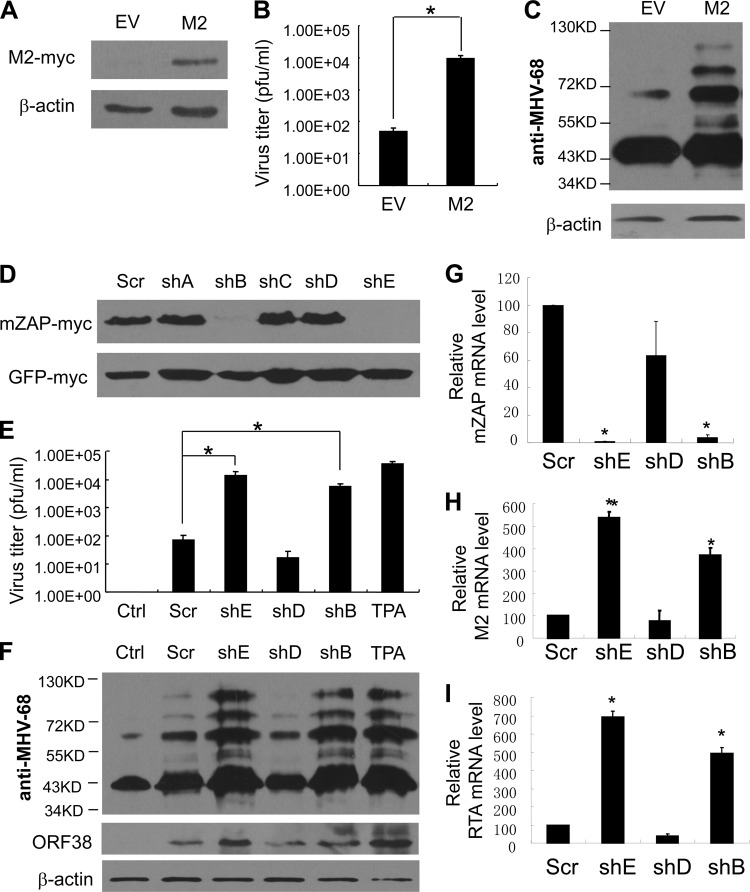
It has been reported that overexpression of M2 is sufficient to induce MHV-68 reactivation in cultured cells (13). We speculated that ZAP might affect MHV-68 latency by inhibiting M2 expression. To test this idea, we used S11E cells, a cell line harboring latently infected MHV-68 derived from a B-cell lymphoma in an MHV-68-infected mouse (23). The coding sequence of M2 was cloned into retroviral vector pBabe-puro (18) to generate vesicular stomatitis virus glycoprotein G (VSV-G)-pseudotyped M2-expressing retrovirus vector by transient transfection of HEK293T cells with the retroviral vector, pVSV-G, and pHIT60 (27), which was then used to transduce S11E cells at an MOI of 1. MHV-68 reactivation was monitored by measuring viral titers in the supernatants and by detection of the expression of lytic replication-associated viral antigens using rabbit polyclonal antisera, which were generated by immunizing rabbits with the lysate of MHV-68-infected rabbit cells (a generous gift from Ren Sun, UCLA). Consistent with previous reports, overexpression of M2 induced MHV-68 reactivation in S11E cells (Fig. 3A to 3C). To determine whether ZAP expression affects MHV-68 latency, endogenous ZAP was downregulated by RNA interference (RNAi). Five short hairpin RNAs (shRNAs) directed against mouse ZAP (mZAP) were designed (for the target sequences, see the supplemental material) and cloned into pSuper-retro (OligoEngine). The abilities of these shRNAs to downregulate mZAP expression were tested by Western analysis following cotransfection of the shRNA-expressing vectors with an mZAP-expressing construct into HEK293 cells. Two shRNAs (shB and shE) efficiently downregulated mZAP expression (Fig. 3D). S11E cells were transduced with a VSV-G-pseudotyped retrovirus vector expressing shB, shE, or shD at an MOI of 1. TPA treatment was used as a positive control for viral reactivation. Compared with untreated cells, transduction of S11E cells with the retrovirus vectors generally led to detectable amounts of MHV-68 in the supernatants, for reasons to be determined. Nonetheless, expression of shB or shE significantly increased MHV-68 titers in the supernatants compared with that of the control shRNA (Fig. 3E). In line with these results, transduction of S11E cells with the shB- or shE-expressing retrovirus vector resulted in increased expression of multiple lytic replication-associated viral antigens (Fig. 3F). In addition, the relative expression levels of a lytic replication-specific protein, ORF38, correlated well with the viral titers in the supernatants, as judged by Western blotting using a rabbit polyclonal antibody against ORF38 (Fig. 3F). To confirm that endogenous mZAP expression was downregulated by the shRNAs and that M2 expression was thus increased in S11E cells, the mRNA levels of mZAP and M2 were measured by real-time PCR using GAPDH mRNA as an internal control (for primer sequences, see the supplemental material). As expected, expression of shB or shE significantly reduced mZAP mRNA levels (Fig. 3G) and increased M2 mRNA levels (Fig. 3H). Since the virally encoded reactivation and transcription activator (RTA) is necessary and sufficient to trigger MHV-68 reactivation into lytic replication in latently infected cells (1, 10, 20, 26), the mRNA levels of RTA were also measured by real-time-PCR using GAPDH mRNA as an internal control (for primer sequences, see the supplemental material). The data show that downregulation of ZAP in S11E cells led to increased levels of RTA (Fig. 3I). Since results from the luciferase reporter assay suggest that RTA is not a target of ZAP (Fig. 1A), it is likely that the increased expression of RTA is an indirect consequence of ZAP downregulation.
Fig 3.
Downregulation of ZAP expression promotes MHV-68 reactivation. (A) S11E cells were transduced with a retrovirus vector expressing myc-tagged M2. The expression of M2 was detected by Western blotting. (B and C) MHV-68 titers in the supernatants of transduced S11E cells were measured at 30 h postransduction (B), and expression of viral lytic replication-associated antigens was detected by Western blotting (C). *, P < 0.05. EV, empty vector. (D) shRNAs against mZAP were cotransfected into HEK293 cells with a construct expressing myc-tagged mZAP along with a construct expressing myc-tagged green fluorescent protein (GFP) to serve as a control. At 48 h posttransfection, mZAP expression levels were measured by Western blotting. (E to I) S11E cells were transduced with retrovirus vectors expressing a scrambled control shRNA or shRNAs against mZAP at an MOI of 1 or were treated with TPA as a positive control. At 30 h postransduction, the supernatants were collected, viral titers were measured (E), and expression of viral replication-associated antigens in the cells was detected by Western blotting (F). The mRNA levels of mZAP (G), M2 (H), and RTA (I) in the S11E cells were measured by real-time PCR. The viral titer data presented are means ± SD of three independent experiments. *, P < 0.05. EV, empty vector; Ctrl, untreated; Scr, scrambled shRNA; TPA, 12-O-tetradecanoylphorbol-13-acetate.
The above results suggest that ZAP regulates MHV-68 latency by inhibiting M2 expression, although the possibility that ZAP regulates viral latency through other mechanisms cannot be excluded. Due to the lack of a comprehensive understanding of how M2 is involved in viral reactivation, how ZAP regulates MHV-68 latency in S11E cells remains unclear. Nonetheless, these results indicate that ZAP not only inhibits M2 expression in vitro but also affects MHV-68 latency in cultured cells. Whether ZAP regulates MHV-68 latency in vivo awaits further investigation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Xudong Zhao of the core facility of the Institute of Biophysics for technical support.
This work was supported by grants from the National Science Foundation (30530020 and 81028011 to G. Gao) and the Ministry of Science and Technology of China (973 Programs 2012CB910203 to G. Gao and 2011CB504805 to H. Deng).
Footnotes
Published ahead of print 5 September 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Allen RD, III, DeZalia MN, Speck SH. 2007. Identification of an Rta responsive promoter involved in driving gammaHV68 v-cyclin expression during virus replication. Virology 365:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bick MJ, et al. 2003. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J. Virol. 77:11555–11562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown HJ, et al. 2003. NF-kappaB inhibits gammaherpesvirus lytic replication. J. Virol. 77:8532–8540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. DeZalia M, Speck SH. 2008. Identification of closely spaced but distinct transcription initiation sites for the murine gammaherpesvirus 68 latency-associated M2 gene. J. Virol. 82:7411–7421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao G, Guo X, Goff SP. 2002. Inhibition of retroviral RNA production by ZAP, a CCCH-type zinc finger protein. Science 297:1703–1706 [DOI] [PubMed] [Google Scholar]
- 6. Goodwin MM, et al. 2010. Histone deacetylases and the nuclear receptor corepressor regulate lytic-latent switch gene 50 in murine gammaherpesvirus 68-infected macrophages. J. Virol. 84:12039–12047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo X, Carroll JW, MacDonald MR, Goff SP, Gao G. 2004. The zinc finger antiviral protein directly binds to specific viral mRNAs through the CCCH zinc finger motifs. J. Virol. 78:12781–12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo X, Ma J, Sun J, Gao G. 2007. The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. Proc. Natl. Acad. Sci. U. S. A. 104:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Herskowitz JH, Jacoby MA, Speck SH. 2005. The murine gammaherpesvirus 68 M2 gene is required for efficient reactivation from latently infected B cells. J. Virol. 79:2261–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hong Y, Qi J, Gong D, Han C, Deng H. 2011. Replication and transcription activator (RTA) of murine gammaherpesvirus 68 binds to an RTA-responsive element and activates the expression of ORF18. J. Virol. 85:11338–11350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacoby MA, Virgin HW, IV, Speck SH. 2002. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J. Virol. 76:1790–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krug LT, Moser JM, Dickerson SM, Speck SH. 2007. Inhibition of NF-kappaB activation in vivo impairs establishment of gammaherpesvirus latency. PLoS Pathog. 3:e11 doi:10.1371/journal.ppat.0030011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang X, Collins CM, Mendel JB, Iwakoshi NN, Speck SH. 2009. Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog. 5:e1000677 doi:10.1371/journal.ppat.1000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Loh J, Thomas DA, Revell PA, Ley TJ, Virgin HW., IV 2004. Granzymes and caspase 3 play important roles in control of gammaherpesvirus latency. J. Virol. 78:12519–12528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macrae AI, et al. 2003. Murid herpesvirus 4 strain 68 M2 protein is a B-cell-associated antigen important for latency but not lymphocytosis. J. Virol. 77:9700–9709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mandal P, et al. 2011. A gammaherpesvirus cooperates with interferon-alpha/beta-induced IRF2 to halt viral replication, control reactivation, and minimize host lethality. PLoS Pathog. 7:e1002371 doi:10.1371/journal.ppat.1002371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinez-Guzman D, et al. 2003. Transcription program of murine gammaherpesvirus 68. J. Virol. 77:10488–10503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morgenstern JP, Land H. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Muller S, et al. 2007. Inhibition of filovirus replication by the zinc finger antiviral protein. J. Virol. 81:2391–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pavlova, Virgin HW, IV, Speck SH. 2003. Disruption of gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J. Virol. 77:5731–5739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rodrigues L, Pires de Miranda M, Caloca MJ, Bustelo XR, Simas JP. 2006. Activation of Vav by the gammaherpesvirus M2 protein contributes to the establishment of viral latency in B lymphocytes. J. Virol. 80:6123–6135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stevenson PG, Efstathiou S. 2005. Immune mechanisms in murine gammaherpesvirus-68 infection. Viral Immunol. 18:445–456 [DOI] [PubMed] [Google Scholar]
- 23. Usherwood EJ, Stewart JP, Nash AA. 1996. Characterization of tumor cell lines derived from murine gammaherpesvirus-68-infected mice. J. Virol. 70:6516–6518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Virgin HW, IV, et al. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894–5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu TT, et al. 2011. Construction and characterization of an infectious murine gammaherpesivrus-68 bacterial artificial chromosome. J. Biomed. Biotechnol. 2011:926258 doi:10.1155/2011/926258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wu TT, Tong L, Rickabaugh T, Speck S, Sun R. 2001. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J. Virol. 75:9262–9273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhu Y, et al. 2011. Zinc-finger antiviral protein inhibits HIV-1 infection by selectively targeting multiply spliced viral mRNAs for degradation. Proc. Natl. Acad. Sci. U. S. A. 108:15834–15839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhu Y, Gao G. 2008. ZAP-mediated mRNA degradation. RNA Biol. 5:65–67 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.