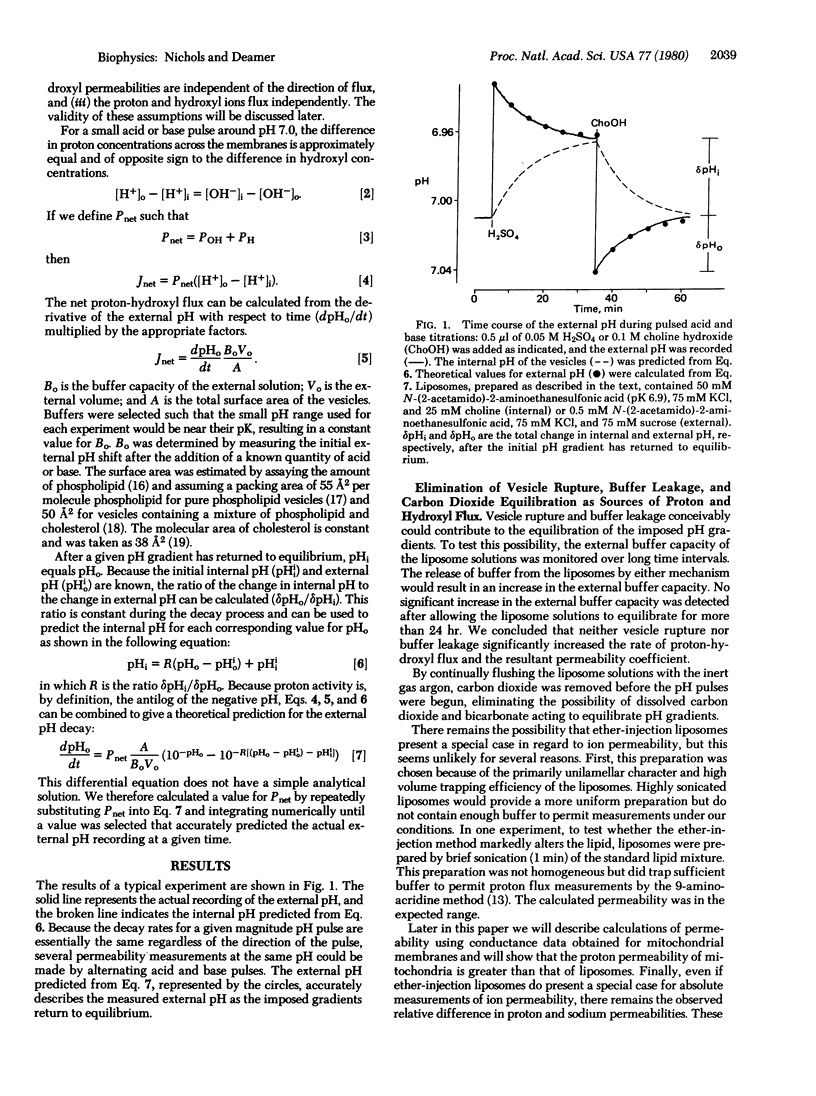
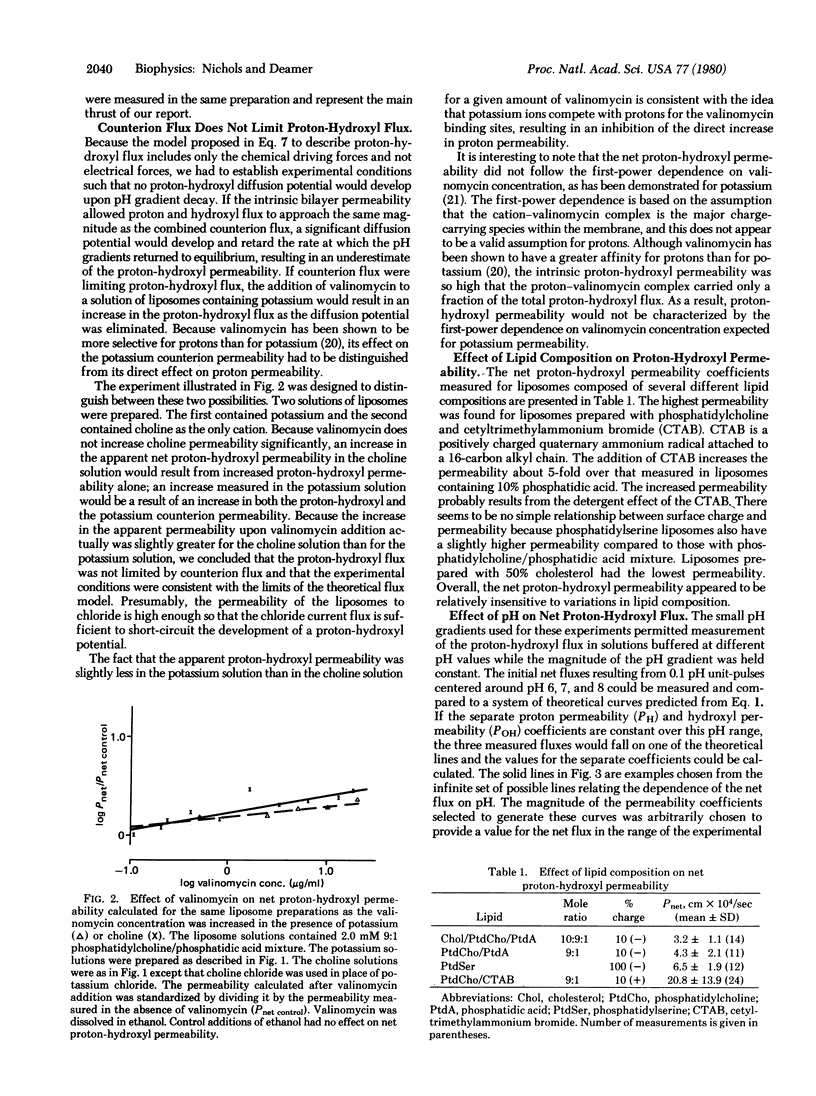
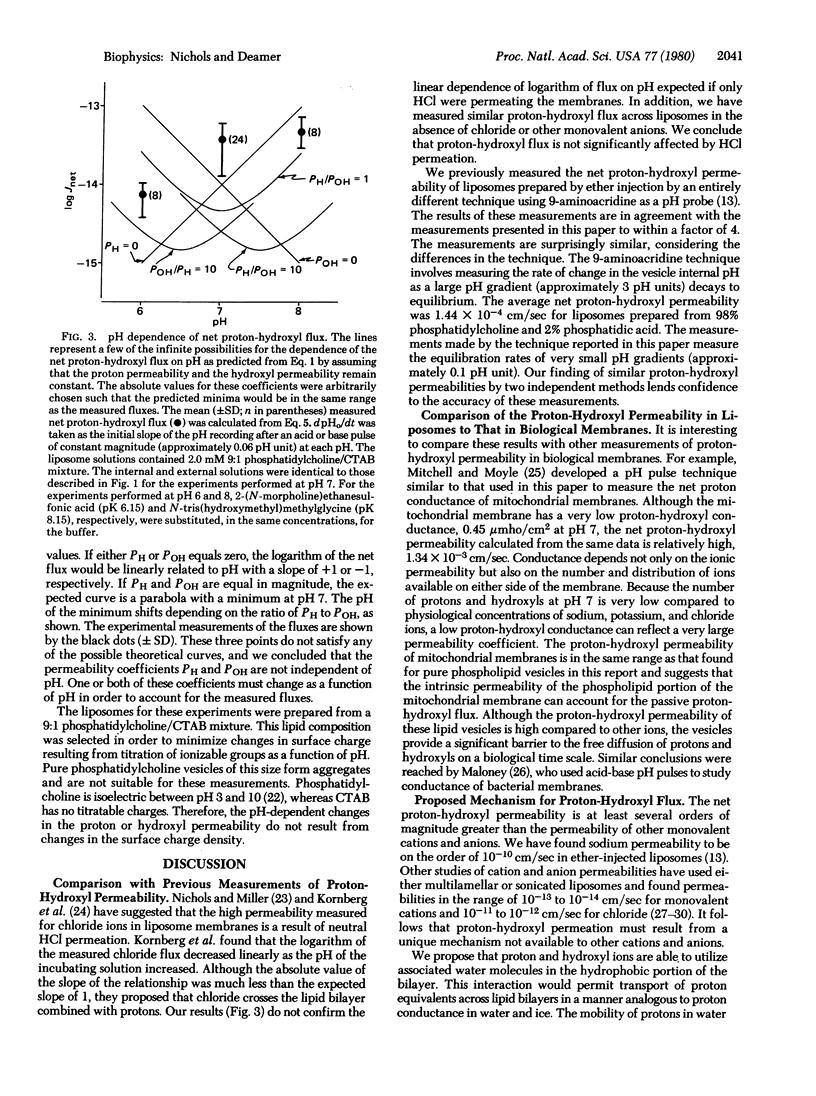
Abstract
The net proton-hydroxyl permeability of large unilamellar liposomes has been measured by an acid-base pulse titration technique and has been determined to be several orders of magnitude greater than that measured for other monovalent ions. This permeability is relatively insensitive to variations in lipid composition. Proton permeability and hydroxyl permeability vary with pH 6 to 8, and this variation can occur in the absence of alterations in surface charge density resulting from titrations of acidic and basic groups on the lipids. In order to account for the exceptionally high proton-hydroxyl permeability with respect to other monovalent ions, we propose that protons or hydroxyls or both interact with clusters of hydrogen-bonded water molecules in the lipid bilayer, such that they are transferred across the bilayer by rearrangement of hydrogen bonds in a manner similar to their transport in water and ice.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burton A. C. Cancer and altitude. Does intracellular pH regulate cell division? Eur J Cancer. 1975 May;11(5):365–371. doi: 10.1016/0014-2964(75)90065-1. [DOI] [PubMed] [Google Scholar]
- Cass A., Finkelstein A. Water permeability of thin lipid membranes. J Gen Physiol. 1967 Jul;50(6):1765–1784. doi: 10.1085/jgp.50.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deamer D. W. Preparation and properties of ether-injection liposomes. Ann N Y Acad Sci. 1978;308:250–258. doi: 10.1111/j.1749-6632.1978.tb22027.x. [DOI] [PubMed] [Google Scholar]
- Deamer D., Bangham A. D. Large volume liposomes by an ether vaporization method. Biochim Biophys Acta. 1976 Sep 7;443(3):629–634. doi: 10.1016/0005-2736(76)90483-1. [DOI] [PubMed] [Google Scholar]
- Gerson D. F., Burton A. C. The relation of cycling of intracellular pH to mitosis in the acellular slime mould Physarum polycephalum. J Cell Physiol. 1977 May;91(2):297–303. doi: 10.1002/jcp.1040910214. [DOI] [PubMed] [Google Scholar]
- Gillies R. J., Deamer D. W. Intracellular pH changes during the cell cycle in Tetrahymena. J Cell Physiol. 1979 Jul;100(1):23–31. doi: 10.1002/jcp.1041000103. [DOI] [PubMed] [Google Scholar]
- Hanai T., Haydon D. A. The permeability to water of bimolecular lipid membranes. J Theor Biol. 1966 Aug;11(3):370–382. doi: 10.1016/0022-5193(66)90099-3. [DOI] [PubMed] [Google Scholar]
- Hauser H., Oldani D., Phillips M. C. Mechanism of ion escape from phosphatidylcholine and phosphatidylserine single bilayer vesicles. Biochemistry. 1973 Oct 23;12(22):4507–4517. doi: 10.1021/bi00746a032. [DOI] [PubMed] [Google Scholar]
- Henning R. pH gradient across the lysosomal membrane generated by selective cation permeability and Donnan equilibrium. Biochim Biophys Acta. 1975 Aug 20;401(2):307–316. doi: 10.1016/0005-2736(75)90314-4. [DOI] [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Ion permeability of isolated chromaffin granules. J Gen Physiol. 1976 Dec;68(6):601–631. doi: 10.1085/jgp.68.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. M., Bangham A. D. Potassium permeability of single compartment liposomes with and without valinomycin. Biochim Biophys Acta. 1969 Oct 14;193(1):82–91. doi: 10.1016/0005-2736(69)90061-3. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., McNamee M. G., McConnell H. M. Measurement of transmembrane potentials in phospholipid vesicles. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1508–1513. doi: 10.1073/pnas.69.6.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C. Membrane H+ conductance of Streptococcus lactis. J Bacteriol. 1979 Oct;140(1):197–205. doi: 10.1128/jb.140.1.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Acid-base titration across the membrane system of rat-liver mitochondria. Catalysis by uncouplers. Biochem J. 1967 Aug;104(2):588–600. doi: 10.1042/bj1040588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Morowitz H. J. Molecular mechanisms for proton transport in membranes. Proc Natl Acad Sci U S A. 1978 Jan;75(1):298–302. doi: 10.1073/pnas.75.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P., Miller N. Chloride diffusion from liposomes. Biochim Biophys Acta. 1974 Jul 31;356(2):184–198. doi: 10.1016/0005-2736(74)90282-x. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Nir S., Oki S. Permeability properties of phospholipid membranes: effect of cholesterol and temperature. Biochim Biophys Acta. 1972 Jun 20;266(3):561–583. doi: 10.1016/0006-3002(72)90001-7. [DOI] [PubMed] [Google Scholar]
- Racker E. Bioenergetics and the problem of tumor growth. Am Sci. 1972 Jan-Feb;60(1):56–63. [PubMed] [Google Scholar]
- Raheja R. K., Kaur C., Singh A., Bhatia I. S. New colorimetric method for the quantitative estimation of phospholipids without acid digestion. J Lipid Res. 1973 Nov;14(6):695–697. [PubMed] [Google Scholar]
- Rand R. P., Luzzati V. X-ray diffraction study in water of lipids extracted from human erythrocytes: the position of cholesterol in the lipid lamellae. Biophys J. 1968 Jan;8(1):125–137. doi: 10.1016/S0006-3495(68)86479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toll L., Howard B. D. Role of Mg2+-ATPase and a pH gradient in the storage of catecholamines in synaptic vesicles. Biochemistry. 1978 Jun 27;17(13):2517–2523. doi: 10.1021/bi00606a010. [DOI] [PubMed] [Google Scholar]
- van Dijck P. W., de Kruijff B., Verkleij A. J., van Deenen L. L., de Gier J. Comparative studies on the effects of pH and Ca2+ on bilayers of various negatively charged phospholipids and their mixtures with phosphatidylcholine. Biochim Biophys Acta. 1978 Sep 11;512(1):84–96. doi: 10.1016/0005-2736(78)90219-5. [DOI] [PubMed] [Google Scholar]



