Abstract
Human postmortem studies of natural dengue virus (DENV) infection have reported systemically distributed viral antigen. Although it is widely accepted that DENV infects mononuclear phagocytes, the sequence in which specific tissues and cell types are targeted remains uncharacterized. We previously reported that mice lacking alpha/beta and gamma interferon receptors permit high levels of DENV replication and show signs of systemic disease (T. R. Prestwood et al., J. Virol. 82:8411–8421, 2008). Here we demonstrate that within 6 h, DENV traffics to and replicates in both CD169+ and SIGN-R1+ macrophages of the splenic marginal zone or draining lymph node, respectively, following intravenous or intrafootpad inoculation. Subsequently, high levels of replication are detected in F4/80+ splenic red pulp macrophages and in the bone marrow, lymph nodes, and Peyer's patches. Intravenously inoculated mice begin to succumb to dengue disease 72 h after infection, at which time viral replication occurs systemically, except in lymphoid tissues. In particular, high levels of replication occur in CD68+ macrophages of the kidneys, heart, thymus, and gastrointestinal tract. Over the course of infection, proportionately large quantities of DENV traffic to the liver and spleen. However, late during infection, viral trafficking to the spleen decreases, while trafficking to the liver, thymus, and kidneys increases. The present study demonstrates that macrophage populations, initially in the spleen and other lymphoid tissues and later in nonlymphoid tissues, are major targets of DENV infection in vivo.
INTRODUCTION
The four serotypes of dengue virus (DENV1 to DENV4) belong to the Flaviviridae family and cause a mosquito-borne febrile illness in more than 50 million people per year. In nearly 500,000 cases per year, individuals develop severe hemorrhage and sometimes shock, resulting in more than 25,000 deaths annually (12). The 10.7-kb positive-stranded RNA genome of DENV encodes a polyprotein that is cleaved into 3 structural proteins, the envelope (E), the premembrane/membrane (prM/M), and the capsid (C), and 7 nonstructural (NS) proteins, NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. During replication, all DENV proteins and both positive- and negative-sense DENV RNAs are produced intracellularly, while only positive-sense RNA, the structural proteins, and NS1 are known to be secreted at high levels. Thus, based on current knowledge, NS proteins, with the exception of NS1, are restricted predominantly to cells in which DENV replicates.
The cellular tropism of DENV has been investigated in humans (2, 5, 6, 8, 13, 15, 16, 25, 29) and mice (3, 9, 20, 38). In human studies, DENV antigen has been reported in the skin, liver, brain, kidney, spleen, lymph nodes, lungs, stomach, and intestines (2, 5, 6, 8, 13, 15, 16, 25, 29). These studies have mainly relied upon detection of secreted DENV antigens, which may actually localize elsewhere, complicating the interpretation of results. Recently two studies detected DENV NS antigen in the lymph nodes, spleen, liver, and bone marrow cells of mice (2, 9). Infected cell types have not been identified by costaining for specific marker expression except in a study by Fink et al. (9). Nevertheless, these studies have consistently reported the major targets of DENV to be mononuclear phagocytes. The exact cell populations targeted and the sequence of infection at the tissue and cellular levels, however, remain unclear.
Previously we reported that 129/Sv mice lacking type I and II interferon receptors (AG129 mice) are susceptible to lethal disease when challenged with a mouse-passaged DENV2 strain (28, 32, 38). In this experimental setup, the kinetics of DENV dissemination was followed by quantitative reverse transcription-PCR (qRT-PCR) (28, 38). Here we used fluorescent immunohistochemistry (FIHC) to characterize the sequence in which specific tissues and cell types are targeted by analyzing nearly every major tissue for DENV NS3 at several time points over the course of infection and by determining the precise identity of NS3-expressing cells through costaining with cell type-specific markers. Additionally, we examined viral trafficking using DENV labeled with the fluorescent lipophilic dye 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD) to quantify DENV extravasation at several time points over the course of infection. The present study characterizes the kinetics of DENV infection and trafficking at the tissue and cellular levels in vivo.
MATERIALS AND METHODS
Mice.
129/Sv mice deficient in the alpha/beta interferon (IFN-α/β) receptor (A129) or the IFN-α/β receptor and the IFN-γ receptor (AG129) were housed under specific pathogen-free conditions. All experiments were approved by the Animal Care Committee at the La Jolla Institute for Allergy and Immunology (LIAI). Mice between 5 and 6 weeks of age were inoculated intravenously (i.v.) in the lateral tail vein with 1.5 × 1011 genomic equivalents (GE) (3 × 106 PFU) or 5 × 108 GE (1 × 104 PFU) in 200 μl or intrafootpad (i.f.) with 1.5 × 1011 GE in 30 μl of the DENV2 strain E124/128-IC diluted in phosphate-buffered saline (PBS) (Invitrogen) containing 10% fetal bovine serum (FBS) (Gemini Bio-Products). For DiD-labeled virus experiments, mice were additionally inoculated i.v. 2 h before harvesting tissues.
Cell cultures and viral stocks.
C6/36 cells were obtained from the American Type Culture Collection and cultured at 28°C in Leibovitz's L-15 medium (Gibco) supplemented with penicillin, streptomycin, HEPES, and 10% FBS. The DENV2 strain E124/128-IC (from pE124/128-IC) was generated as described previously (28). Briefly, an infectious cDNA clone of PL046 (pPL046-IC) was previously constructed, and site-directed mutagenesis was performed to introduce the two mutations identified in the DENV2 strain D2S10. The plasmid was linearized with XbaI and gel purified using a QIAquick gel extraction kit (Qiagen). Infectious RNA was generated by in vitro transcription with a RiboMax large-scale RNA production system (T7; Promega) and transfected into BHK-21 cells with Lipofectamine (Invitrogen). The entire genome of the viral stocks was confirmed by sequencing. The virus was amplified twice in C6/36 cells and purified using a sucrose density gradient, as described previously (28). GE were quantified by qRT-PCR.
DiD-labeled DENV stocks.
Solid 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD) (Invitrogen) was reconstituted at 20 mg/ml in dimethyl sulfoxide (DMSO) and then diluted 1:10 in 100% ethanol. Twice-amplified stocks of the DENV2 strain E124/128-IC were used to grow stocks for DiD labeling. The third amplification was performed in CELLine AD 1000 adherent cell chambers (Wilson Wolf), and the virus in the supernatant was concentrated by ultracentrifugation, resuspended, and purified over a sucrose density gradient. After dialysis against PBS, the virus was incubated with DiD at a 1:38,000 molar ratio of virus to DiD for 1 h at room temperature. After incubation, the virus was repurified over a sucrose density gradient, which yielded a distinct band of labeled virus. The virus was dialyzed against PBS, aliquoted, and stored at −80°C. Infectivity of the DiD-labeled virus was reduced to 44% of the unlabeled virus when normalized to GE.
Immunohistochemistry.
Tissues were embedded in Tissue-Tek O.C.T. compound (Sakura). Sections (6 μm) were cut and stored at −80°C. Frozen sections were thawed and fixed for 10 min in acetone at 25°C, followed by 8 min on ice in 1% paraformaldehyde in 100 mM dibasic sodium phosphate (pH 7.4) containing 60 mM lysine and 7 mM sodium periodate.
To stain for DENV replication, tissue sections were blocked with an avidin/biotin-blocking kit (Vector), 5% normal goat serum (Caltag Laboratories), and 5% mouse serum in PBS. Sections were stained overnight with affinity-purified rabbit polyclonal anti-DENV NS3 (a generous gift from the Novartis Institute for Tropical Diseases, Singapore) and purified mouse anti-DENV prM/M (clone 2H2) labeled with EZ-Link NHS biotin (Pierce). After being washed, sections were stained with DyLight 649-labeled goat anti-rabbit IgG (Jackson ImmunoResearch) and phycoerythrin (PE)-labeled streptavidin (eBioscience).
NS3 staining with DiD visualization.
For DiD and NS3 staining experiments, sections were prepared, fixed, and blocked as stated above and then stained overnight with purified rabbit polyclonal anti-DENV NS3. After being washed, sections were stained with DyLight 549-labeled goat anti-rabbit IgG (Jackson ImmunoResearch).
Marginal zone macrophage staining with DiD visualization.
Sections were prepared, fixed, and blocked as stated above and then stained with rat anti-mouse CD169 (clone MOMA-1; BMA Biomedicals), biotinylated rat anti-mouse SIGN-R1 (clone ER-TR9; BMA Biomedicals), and rat anti-mouse MARCO (clone ED31; AbD Serotec). After being washed, sections were stained with biotinylated goat anti-rat IgG (BD) followed by additional washing and staining of the sections with PE-labeled streptavidin (BD).
Identification of infected splenocytes.
Sections were prepared, fixed, and blocked as described above. For marginal zone macrophage markers, sections were stained overnight with rat anti-mouse CD169 (clone MOMA-1), biotinylated rat anti-mouse SIGN-R1 (clone ER-TR9), and purified rabbit polyclonal anti-DENV NS3. After being washed, sections were stained with PE-conjugated mouse anti-rat IgG2a (BD), fluorescein isothiocyanate (FITC)-conjugated streptavidin (BD), and DyLight 649-labeled goat anti-rabbit IgG. Finally, after additional washing, the sections were stained with AF488-conjugated goat anti-FITC (Invitrogen).
For CD11b and CD11c, sections were stained overnight with FITC-labeled Armenian hamster anti-mouse CD11c (clone HL3; BD), PE-labeled rat anti-mouse CD11b (clone M1/70; BD), and purified rabbit polyclonal anti-DENV NS3. After being washed, sections were stained with PE-conjugated goat anti-rat IgG, AF488-conjugated goat anti-FITC, and DyLight 649-labeled goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories).
Lastly, for pan-macrophage and red pulp macrophage markers, sections were stained overnight with FITC-labeled rat anti-F4/80 (eBioscience), biotinylated rat anti-mouse CD68 (clone FA-11; AbD Serotec), and purified rabbit polyclonal anti-DENV NS3. After being washed, sections were stained with PE-conjugated streptavidin, AF488-conjugated goat anti-FITC, and DyLight 649-labeled goat anti-rabbit IgG.
Identification of infected cells in lymph nodes.
Sections were prepared, fixed, and blocked as stated above. For MARCO and SIGN-R1 staining, sections were stained overnight with purified rat anti-MARCO (clone ED31; AbD Serotec), biotinylated rat anti-mouse SIGN-R1 (clone ER-TR9), and purified rabbit polyclonal anti-DENV NS3. After being washed, sections were stained with PE-conjugated streptavidin (BD), FITC-conjugated mouse anti-rat IgG1 (BD), and DyLight 649-labeled goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories).
For CD68 and CD169 staining, sections were stained overnight with biotinylated rat anti-mouse CD68 (clone FA-11; AbD Serotec), purified rat anti-mouse CD169 (clone MOMA-1), and purified rabbit polyclonal anti-DENV NS3. After being washed, sections were stained with PE-conjugated mouse anti-rat IgG2a (BD), FITC-conjugated streptavidin (BD), and DyLight 649-labeled goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories).
Comparison of infected spleen cells in A129 and AG129 mice.
Tissue sections were prepared, fixed, and blocked as stated above. For marginal zone macrophage markers, sections were stained overnight with rat anti-mouse CD169 (clone MOMA-1), biotinylated rat anti-mouse SIGN-R1 (clone ER-TR9), and purified rabbit polyclonal anti-DENV NS3. After being washed, sections were stained with PE-conjugated mouse anti-rat IgG2a and FITC-conjugated goat anti-rabbit IgG. Finally, after additional washing, the sections were stained with AF488-conjugated goat anti-FITC and allophycocyanin (APC)-labeled streptavidin (eBioscience).
For pan-macrophage and red pulp macrophage markers, sections were stained overnight with PE-labeled rat anti-F4/80 (clone BM8; BioLegend), biotinylated rat anti-mouse CD68 (clone FA-11; AbD Serotec), and purified rabbit polyclonal anti-DENV NS3. After being washed, sections were stained with FITC-conjugated goat anti-rabbit IgG. Finally, after additional washing, the sections were stained with AF488-conjugated goat anti-FITC and APC-labeled streptavidin.
Identification of infected cells in the kidney, heart, and thymus.
For cell type identification, sections were prepared, fixed, and blocked as stated above. Sections were stained overnight with FITC-labeled rat anti-mouse CD11b (clone M1/70), biotinylated rat anti-mouse CD68 (clone FA-11), and purified rabbit polyclonal anti-DENV NS3. After being washed, sections were stained with PE-conjugated streptavidin, AF488-conjugated goat anti-FITC, and DyLight 649-labeled goat anti-rabbit IgG.
Image acquisition and preparation.
Images were recorded using a Marianas deconvolution fluorescence microscope (3i) or DM6000B upright laser-scanning confocal microscope (Leica). Final preparation of all images was performed using ImageJ software (NIH), Adobe Photoshop, and Adobe Illustrator (Adobe Systems).
Quantification of DiD.
For quantitative analysis, images were processed with the noise reduction despeckle function in ImageJ. DiD intensity was then quantified using the batch>measure function for 6 to 24 nonoverlapping low-power images from 2 tissues sections for each of the 5 mice per group per time point. The data were graphed and analyzed with Prism 5.0 (GraphPad). Statistical significance was determined using the unpaired t test.
RESULTS
Early DENV replication occurs in lymphoid tissues.
We previously reported that serum levels of DENV virions decrease rapidly following intravenous (i.v.) inoculation of AG129 mice (28). However, levels of DENV RNA in the serum begin to increase between 5 and 7 h after infection, suggesting that initial viral replication in vivo occurs in less than 7 h. To screen for DENV replication patterns characteristic of lethal infection and to coordinate with our previous RNA kinetics study (28), we utilized a high dose of DENV. AG129 mice were inoculated i.v. with 1.5 × 1011 genomic equivalents (GE) of the DENV2 strain E124/128-IC. At 0 (naïve mice), 6, 12, 24, 48, and 72 h after infection, liver, heart, lung, thymus, spleen, kidney, stomach, small intestine (including Peyer's patches), large intestine, mesenteric lymph nodes (MLNs), cutaneous lymph nodes (CLNs) (brachial, axillary, and inguinal LNs were pooled because of equivalent trends), skin, blood, spinal cord, brain, and bone marrow tissues were collected.
For the analysis of viral replication, 6-μm frozen tissue sections were cut, mounted, and subsequently stained with rabbit polyclonal antibodies raised against purified NS3, in combination with biotinylated mouse anti-DENV prM/M monoclonal antibody (clone 2H2). By 6 h after infection, DENV replication was detected in the spleen (Fig. 1), at which time both NS3 and prM/M were present in the marginal zone encircling the white pulp. At 12 h after infection, NS3 expression was still present in the marginal zone of the spleen, although the circular staining pattern had extended into the red pulp and become more diffuse. At 24 h after infection, DENV NS3 was distributed throughout the red pulp. By 48 and 72 h after infection, levels of NS3 in the spleen had decreased substantially, but low levels continued to be detectable in the red pulp at the 72-h time point. Staining with the anti-DENV prM/M antibody 2H2 showed near complete colocalization with anti-NS3 staining, confirming specificity to the sites of DENV replication.
Fig 1.
Dengue virus first infects the spleen, followed by bone marrow and then lymph nodes and Peyer's patches after intravenous inoculation. Representative immunohistochemical staining of tissue sections from naïve AG129 mice and AG129 mice at 6, 12, 24, 48, and 72 h after infection (1.5 × 1011 GE of the DENV2 strain E124/128-IC i.v. on day 0). Spleens, cutaneous lymph nodes (CLNs: axillary, brachial, and inguinal lymph nodes), mesenteric lymph nodes (MLNs), Peyer's patches (PPs), and bone marrow (BM) were sectioned and stained for DENV NS3 (white when alone, green when shown with prM/M) and DENV prM/M (antibody clone 2H2, shown in red). The results are representative of 3 mice. WP labels the white pulp, LN labels the center of the lymph nodes, and PP labels the centers of the Peyer's patches.
The kinetics of DENV infection in other lymphoid tissues was similar to that observed in the spleen (Fig. 1). In the bone marrow, NS3 expression became detectable at 12 h and peaked at 24 h after infection. In the lymph nodes and Peyer's patches, infection was consistently observed 24 h after inoculation and peaked at 48 h. As in the spleen, infection in the bone marrow, lymph nodes, and Peyer's patches decreased substantially and became almost undetectable by 72 h. Collectively, these results demonstrate that systemic lymphoid tissues are targeted by DENV before nonlymphoid tissues in this model.
Late DENV replication occurs in nonlymphoid tissues.
We additionally examined DENV replication in nonlymphoid tissues, including the kidney, heart, thymus, adrenal gland, liver, lung, forestomach, stomach, jejunum, duodenum, ileum, cecum, colon, and rectum (Fig. 2A). In contrast to replication in lymphoid tissues, replication was undetectable in nonlymphoid tissues early after infection but became detectable between 24 and 48 h and continued to increase until 72 h (Fig. 2A). Notably, a high level of DENV NS3 expression was detected in the heart, thymus, and kidneys. Several of the nonlymphoid tissues we analyzed, including the brain, spinal cord, esophagus, blood, bladder, and skin, showed little or no viral replication, even 72 h after infection (data not shown). Based on these data, a model of DENV dissemination was developed (Fig. 3). These findings are in agreement with our previous kinetic study measuring DENV RNA levels in tissues (28).
Fig 2.
Dengue virus infects systemic nonlymphoid tissues late during infection. Representative immunohistochemical staining of tissue sections from AG129 mice. (A) Tissues were harvested from naïve mice and mice 48 and 72 h after infection (1.5 × 1011 GE of the DENV2 strain E124/128-IC i.v. on day 0) and stained for DENV NS3 (white) in the kidney, heart, thymus, adrenal gland, liver, lung, forestomach, stomach, duodenum, jejunum, ileum, cecum, colon, and rectum. The results are representative of 3 mice. (B) Tissues were harvested from mice 6, 24, and 72 h after infection (1.5 × 1011 GE of the DENV2 strain E124/128-IC i.f. on day 0) and stained for DENV NS3 (white) in the spleen, draining popliteal lymph node (drPLN), contralateral popliteal lymph node (clPLN), cutaneous lymph nodes (CLNs: axillary, brachial, and inguinal lymph nodes), and kidney. The results are representative of 3 mice.
Fig 3.
Schematic depicting the kinetics of DENV tissue tropism over the course of infection in mice. Model of DENV infection. DENV first infects the spleen followed by the bone marrow and then the lymph nodes and Peyer's patches (LN, PP). Infection decreases in these tissues while it increases systemically in nonlymphoid tissues, at which point mice become moribund.
DENV replication occurs in the draining popliteal lymph node following intrafootpad inoculation.
To validate our results using a more relevant route of infection than the i.v. route (34), AG129 mice were inoculated intrafootpad with 1.5 × 1011 GE of the DENV2 strain E124/128-IC. The kinetics of systemic DENV dissemination was assessed by monitoring viral RNA levels in the serum at 6, 12, 24, 48, 72, and 96 h after infection. The levels of DENV RNA were almost undetectable at 6 h but increased steadily, peaking at 72 h and decreasing by 96 h after infection (data not shown). Based on these kinetics, the spleen, draining popliteal lymph nodes (PLNs), contralateral PLNs, cutaneous lymph nodes (CLNs), and kidneys were harvested 6, 24, and 72 h after infection. Tissues were stained for the presence of DENV NS3. At 6 h after infection, NS3 expression was detected only in the draining PLN (Fig. 2B). By 24 h, NS3 expression was observed at a reduced level in the draining PLN, while it became detectable in the marginal zone of the spleen. By 72 h, NS3 expression was detected in the contralateral PLNs as well as distant CLNs, splenic red pulp, and kidneys but not the draining PLNs. These results show that the pattern of DENV replication (as determined by staining for NS3 expression) following intrafootpad inoculation mirrors the results obtained with intravenous inoculation: early replication in the draining lymphoid tissue followed by systemic dissemination to other lymphoid tissues and, lastly, replication in nonlymphoid tissues.
Intravenous DENV particles traffic to macrophages in the marginal zone of the spleen.
Based on the pattern of initial replication in the splenic marginal zone and subsequent dissemination to the red pulp, we hypothesized that the ability of the splenic marginal zone to absorb blood-borne DENV particles diminishes over the course of infection, resulting in leakage of viral particles into the red pulp. To investigate this hypothesis, mice were inoculated i.v. with 1.5 × 1011 GE of the DENV2 strain E124/128-IC, as in previous experiments, and administered an additional 5 × 1010 GE of the DENV2 strain E124/128-IC labeled with DiD, a lipophilic far-red fluorescent dye, 2 h before tissue harvest at 0 (naïve mice), 6, 12, 18, 24, 36, 48, and 72 h after the initial inoculation (Fig. 4A). When large amounts of DiD are incorporated into the lipid bilayer of the virion, it undergoes autoquenching and emits only low levels of fluorescence. However, once the lipid bilayer fuses with the host cell membrane, the probe is diluted and renders target cells highly fluorescent. In naïve mice and mice 6 h after DENV infection, the DiD signal in the spleen was restricted to the marginal zone (Fig. 4A). DiD distribution became more diffuse in the spleens of mice 12 and 18 h after infection. After 24 h, dissemination of the DENV particles was readily observed throughout the red pulp. However, at all time points after infection, the majority of the DiD accumulated in rings within the marginal zone of the spleen, indicating that DENV particles trafficked here primarily throughout infection, even at time points when most of the NS3 signal was located in the red pulp (Fig. 4A, 24 to 48 h).
Fig 4.
Blood-borne DENV particles traffic mainly to the spleen and liver. (A) Representative immunohistochemical staining of spleen, liver, thymus, and kidney from naïve AG129 mice and from AG129 mice 6, 12, 18, 24, 36, 48, and 72 h after infection (1.5 × 1011 GE of the DENV2 strain E124/128-IC i.v. on day 0 and 5 × 1010 GE DiD-labeled DENV2 strain E124/128-IC i.v. 2 h before harvest). Sections were stained for DENV NS3 (white, top row of each panel), and the DiD in the tissue was imaged (white, middle row of each panel). Merged images are shown on the bottom row of each panel (NS3 is shown as green, and DiD is shown as red). (B) Representative immunohistochemical staining of spleen from AG129 (top) or WT129 (bottom) mice 6 h after infection (5 × 1010 GE of DiD-labeled DENV2 strain E124/128-IC i.v.). Sections were stained for CD169, SIGN-R1, and MARCO (white, left), and the DiD in the tissue was imaged (white, middle). The merged image is shown on the right (DiD is shown as green, and CD169, SIGN-R1, and MARCO are shown as red). (C) Summary of results from panel A. The DiD intensity was quantified from 6 to 24 images per mouse with 5 mice per time point. Symbols and error bars represent the means ± SEMs. P values from two-tailed unpaired t tests: *, P ≤ 0.05; **, P ≤ 0.01; *** P ≤ 0.001.
Because macrophages of the marginal zone often serve to filter blood-borne viruses, we speculated that these cells were the initial targets of intravenous DENV. To investigate this, AG129 and wild-type 129/SV (WT129) mice were inoculated with 5 × 1010 GE of DiD-labeled DENV2 strain E124/128-IC, and their spleens were harvested 6 h later. The tissues were sectioned and stained with antibodies against MARCO, CD169, and SIGN-R1, which label the heterogenous pool of macrophages in the marginal zone. Nearly all of the DiD signal in both AG129 and WT129 mice (Fig. 4B) localized to the cells expressing marginal zone macrophage markers, implicating these cells as the initial targets of DENV in this model regardless of the host's immune status.
DENV trafficking to the liver, kidneys, and thymus increases over the course of infection, while trafficking to the spleen decreases.
To determine how viral trafficking and fusion change with time in the major target tissues, the spleen, liver, thymus, and kidneys were analyzed for the presence of DiD and DENV NS3 (Fig. 4A). DiD signal intensity was quantified on 6 to 24 images of the spleen, liver, kidneys, and thymus for 5 mice at 0 (naïve mice), 12, 36, and 72 h after infection. These results are summarized in Fig. 4C. Large amounts of DENV trafficked to the spleen and liver, with the amounts decreasing in the spleen and increasing in the liver over the course of infection. Similar to the liver, the kidneys and thymus had increasing levels of DiD at late time points after infection, although less virus trafficked to these tissues than to the spleen and liver. These data show that the blood-borne DENV-trafficking pattern changes during infection, favoring early dissemination to lymphoid tissues and late dissemination to nonlymphoid tissues.
DENV initially infects splenic macrophages in the marginal zone and later spreads to red pulp macrophages.
In order to identify the DENV-permissive cells in the spleen of AG129 mice, we sought to distinguish between macrophages, monocytes, and dendritic cells (DCs), all of which have been reported to be targeted by DENV in humans. Macrophages in the spleen exist as several heterogeneous, overlapping populations (23). Sialoadhesin, or CD169, is expressed by marginal metallophilic macrophages (MMMs), which reside closest to the white pulp in the innermost part of the marginal zone. SIGN-R1 (a murine homolog of DC-SIGN) and MARCO are expressed by two highly overlapping populations of macrophages in the outer part of the marginal zone, collectively termed marginal zone macrophages (MZMs). We have observed that some of the outer MARCO- and SIGN-R1-expressing MZMs also express F4/80. Additionally, we have observed a marked upregulation of MARCO expression on F4/80+ red pulp macrophages between 12 and 24 h after infection (data not shown). MARCO was therefore not used to identify splenocytes in this study. Lastly, splenic red pulp macrophages (RPMs) can be distinguished based on their high level of F4/80 expression and lack of MZM and MMM markers. Thus, to differentiate the mononuclear phagocytes potentially permissive to DENV infection, we utilized CD11c (a dendritic cell marker), CD11b (present at high levels on monocytes and neutrophils), CD169 and SIGN-R1 (present on macrophages of the marginal zone), F4/80 (present on macrophages of the red pulp), and CD68 (expressed at high levels in macrophages).
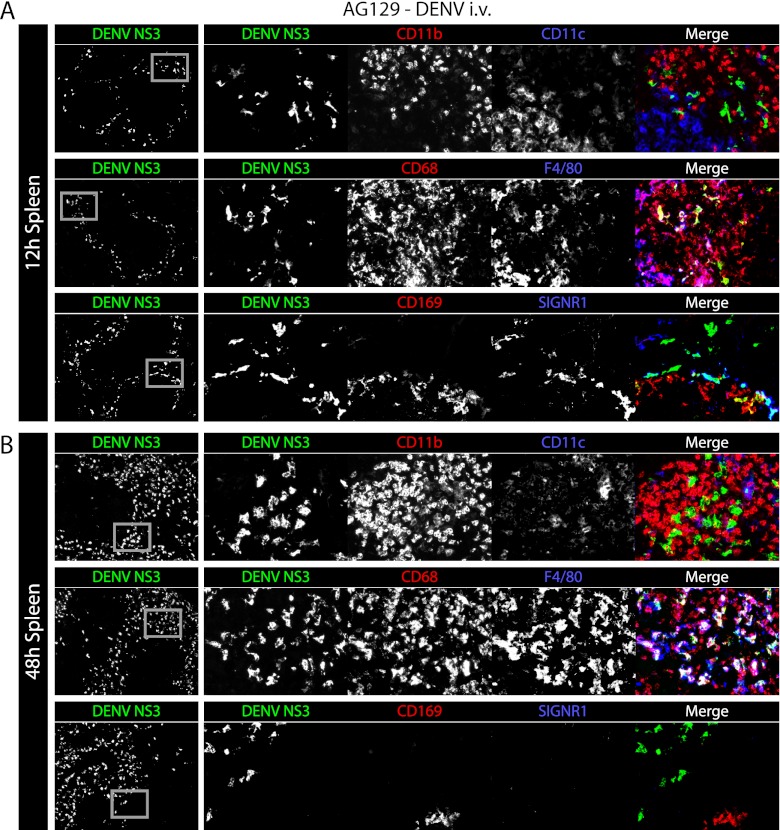
To more accurately model a natural human infection, AG129 mice were inoculated intravenously with a sublethal dose of virus (5 × 108 GE of the DENV2 strain E124/128-IC), and their spleens were harvested 12 and 48 h after infection. At this dose, replication was observed selectively in the marginal zone at 12 h. DENV NS3-expressing cells were distinct from cells expressing high levels of CD11b (CD11bhigh) or CD11c (CD11chigh) (Fig. 5A, top row). Instead, NS3 localized to the cells expressing CD68, some of which also expressed F4/80 (Fig. 5A, middle row) and most of which were also found to express CD169 and/or SIGN-R1, identifying them as MMMs and MZMs, respectively (Fig. 5A, bottom row). At 48 h, DENV NS3-expressing cells did not express high levels of CD11b, CD11c, CD169, or SIGN-R1 (Fig. 5B, top and bottom rows) but instead expressed high levels of CD68 and F4/80 (Fig. 5B, middle row). This cell marker expression and the cell localization are consistent with RPMs. Thus, in this model of primary DENV infection, virus replicates early in the spleen in the MZMs and MMMs and later in RPMs. Additionally, we did not observe DENV replication in CD11chigh dendritic cells or CD11bhigh monocytes or neutrophils.
Fig 5.
In the spleen, DENV first infects macrophages of the marginal zone and later infects macrophages of the red pulp. (A) Representative immunohistochemical staining of spleen sections from AG129 mice 12 h after infection (5 × 108 GE of the DENV2 strain E124/128-IC i.v. on day 0). Tissue sections were stained for DENV NS3 (green) and CD11b and CD11c (red and blue, respectively, top row), CD68 and F4/80 (red and blue, respectively, middle row), and CD169 and SIGN-R1 (red and blue, respectively, bottom row). Low-magnification (×5) images are shown on the left with DENV NS3 alone, while each individual channel and the merged image are shown on the right. Gray boxes on the DENV NS3 panels depict the areas of enlargement shown on the right. The results are representative of 3 mice. (B) Representative immunohistochemical staining of spleen sections from AG129 mice 48 h after infection (5 × 108 GE of the DENV2 strain E124/128-IC i.v. on day 0). Sections are stained as described for panel A. The results are representative of 3 mice.
IFN-γ signaling restricts DENV replication in macrophages of the red pulp but not in macrophages of the marginal zone.
To validate our tropism findings in mice that were less immunocompromised than AG129 mice, we next sought to determine the pattern of DENV replication in the spleens of A129 mice, which lack the IFN-α/β receptor but have functional IFN-γ signaling. Both A129 and AG129 mice were infected i.v. with 5 × 108 GE of the DENV2 strain E124/128-IC, and their spleens were harvested at 12, 24, 48, and 72 h after infection. Frozen sections of the spleens were stained for DENV NS3 together with either F4/80 and CD68 (Fig. 6A) or CD169 and SIGN-R1 (Fig. 6B). At 12 and 24 h postinoculation, A129 and AG129 mice showed an approximately equivalent infection occurring predominantly in CD169+ MMMs and SIGN-R1+ MZMs. At 48 h, however, infection in F4/80+ RPMs was strikingly reduced in A129 mice compared to those in the AG129 mice (Fig. 6A). Thus, in this experimental setup, IFN-γ receptor signaling does not affect initial DENV replication in MMMs or MZMs but is able to reduce replication in RPMs. Additionally, DENV cellular tropism in this model is unaffected by IFN-γ receptor signaling.
Fig 6.
IFN-γ receptor signaling limits DENV replication in splenic red pulp macrophages. (A) Representative immunohistochemical staining of spleen sections from AG129 or A129 mice 12, 24, 48, and 72 after infection (5 × 108 GE of the DENV2 strain E124/128-IC i.v. on day 0). Tissue sections were stained for F4/80 (red), DENV NS3 (green), and CD68 (blue). Low-magnification (×5) merged images are shown on the left as merged images only, medium-magnification (×20) images are shown in the middle as merged images only, and high-magnification (×33) images are shown on the right as the merged image followed by each individual channel (F4/80, DENV NS3, and CD68). Gray boxes on the left depict the area of enlargement shown on the immediate right. Two enlarged areas from each middle-magnification image are shown. The results are representative of 3 mice. (B) Representative immunohistochemical staining of spleen sections from AG129 or A129 mice 12, 24, 48, and 72 after infection (5 × 108 GE of the DENV2 strain E124/128-IC i.v. on day 0), with each time point represented by a row. Images are arranged as described for panel A, except tissue sections were stained for CD169 (red), DENV NS3 (green), and SIGN-R1 (blue). The results are representative of 3 mice.
DENV systemically infects CD68+ macrophages late in infection.
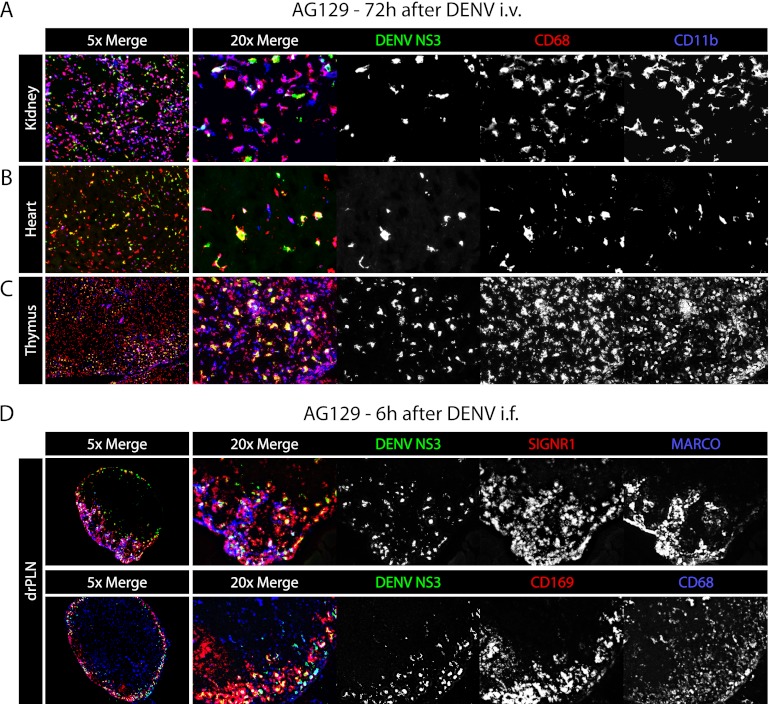
We have previously reported that in AG129 mice, DENV replicates in CD68+ lamina propria macrophages of the small intestine at late time points after infection (38). To determine whether macrophages are the cells infected at late time points in other tissues, AG129 mice were inoculated i.v. with 1.5 × 1011 GE of the DENV2 strain E124/128-IC, and 72 h later their kidneys, hearts, and thymuses were harvested and stained for CD11b and CD68. In the kidney, heart, and thymus, most of the DENV NS3 signal was present in the cells expressing CD68 and, additionally but less often, CD11b (Fig. 7A). These results confirm that macrophages in many nonlymphoid tissues are late targets of DENV infection.
Fig 7.
DENV infects macrophages of the kidney, heart, thymus, and lymph nodes. (A) Representative immunohistochemical staining of kidney, heart, and thymus sections from AG129 mice 72 h after infection (1.5 × 1011 GE of the DENV2 strain E124/128-IC i.v. on day 0). Tissue sections were stained for DENV NS3 (green), CD68 (red), and CD11b (blue). On the left are ×5-magnification images of all channels, and on the right are ×20-magnification images with each channel shown separately in white. The results are representative of 3 mice. (B) Representative immunohistochemical staining of draining popliteal lymph node sections from AG129 mice 6 h after infection (1.5 × 1011 GE of the DENV2 strain E124/128-IC i.f.). Tissue sections were stained for DENV NS3 (green), SIGN-R1 (red), and MARCO (blue) in the upper panel and DENV NS3 (green), CD169 (red), and CD68 (blue) in the lower panel. On the left are ×5-magnification images of all channels, and on the right are ×20-magnification images with each channel shown separately in white. The results are representative of 3 mice.
DENV replicates in macrophages of draining lymph nodes following intrafootpad inoculation.
Initial screening studies for DENV replication following intrafootpad inoculation revealed early replication in the draining PLNs of AG129 mice. To identify the cells in the lymph node targeted by DENV, AG129 mice were inoculated i.f. with 1.5 × 1011 GE of the DENV2 strain E124/128-IC, and their PLNs were harvested 6 h after infection. Frozen sections of the PLNs were stained for DENV NS3 together with either MARCO and SIGN-R1 (Fig. 7B, top row) or CD169 and CD68 (Fig. 7B, bottom row). As in the spleen, the cells expressing these markers were overlapping, and the cells expressing each or combinations of them were found to permit DENV replication. These results show that initial DENV infection occurs in macrophages of draining PLNs within 6 h following intrafootpad inoculation.
DISCUSSION
In this study we sought to characterize the tissues and cells targeted by DENV in vivo and the sequence in which they are infected. Following i.v. inoculation, DENV initially traffics to the spleen where it infects macrophages of the marginal zone, including MZMs and MMMs. Following i.f. inoculation, DENV initially targets macrophages of the draining popliteal LN before entering the blood and subsequently infecting the splenic marginal zone. Although blood-borne DENV continues to traffic to the marginal zone throughout the course of infection, replication in the marginal zone decreases and blood-borne DENV later disseminates into the red pulp, where it infects RPMs. The initial wave of replication in the spleen is followed by replication in the bone marrow and then lymph nodes and Peyer's patches. Finally, late during infection, when mice are becoming overtly ill, replication decreases in the lymphoid tissues and increases in the macrophages of nonlymphoid tissues, most notably in the kidneys, heart, thymus, and gastrointestinal tract.
In humans, a broad array of tissues and cell types have been reported to harbor DENV, including perivascular mononuclear cells of the skin, hepatocytes, endothelial cells, and Kupffer cells of the liver, cortical neurons, astrocytes, microglia, Purkinje's cells, and choroid plexus epithelial cells of the brain, immunoblasts, mononuclear cells, and macrophages of the kidney, immunoblasts, lymphocytes, plasma cells, and macrophages of the spleen, lymph nodes, and kidneys, endothelial cells of the spleen, alveolar macrophages of the lung, mucosal lymphocytes and lamina propria plasma cells of the stomach, and plasma and mononuclear cells of the small and large intestines (2, 5, 6, 8, 13, 15, 16, 25, 29). Importantly, because these studies detected components of mature viral particles and identified cells based on cellular morphology alone or with nonspecific markers in serial sections, it is unclear whether these studies have accurately identified the sites of viral replication. Despite these caveats, the published studies of human tissues have set a framework for understanding DENV behavior in vivo.
Due to logistical complications of studies with human tissues, small-animal studies can provide critical information to elucidate viral behavior in vivo. For example, the present study investigated the kinetics of DENV dissemination by analyzing several time points and tissues after infection at a consistent viral dose, which is nearly impossible in human studies. However, the use of nonnatural mammalian hosts, particularly mice lacking IFN receptors, indicates potential caveats for interpretation of our results. Both type I and type II IFNs have been reported to restrict the cellular and tissue tropism of flaviviruses in mice (30, 31), which may result in findings that do not represent what occurs in natural human infections. However, DENV, like other flaviviruses, possesses mechanisms to overcome or circumvent IFN responses in humans and nonhuman primates (7, 17, 18, 22, 24), and this ability appears to be species specific for a number of mosquito-borne viruses (1, 4, 24). As we are not currently able to validate our findings with human samples, it remains unclear whether other differences between mice and humans could be confounding our results. For example, there may be cell populations targeted by DENV in humans that are either not present or not infected in mice. Thus, although small-animal studies are invaluable for deducing the nature of virus replication and virus-host interactions in vivo, it is crucial that findings be evaluated in focused human studies, even if the same rigor is not possible.
Identification of the precise cellular targets of DENV in vivo has been hindered by the heterogeneity of mononuclear phagocytes present in different tissues and even within a given tissue. Macrophages of the spleen in particular are highly heterogeneous and, therefore, difficult to distinguish based on cell markers (23). Certain markers, such as CD11b, are expressed at variable levels on multiple cell populations. Moreover, the populations of cells present in a tissue, as well as cell marker expression, can change under inflammatory conditions, such as viral infections. This may help explain the inconsistent reports in the literature of individual markers on specific cell populations. For example, RPMs have been variably reported to lack or express CD11b (11, 19, 23, 37). FIHC is thus particularly useful in identifying cell populations, including monocytes/neutrophils (CD11bhigh), MZMs (SIGN-R1+), MMMs (CD169+), and RPMs (F4/80+), because it is based on anatomic location, cellular morphology, and expression of cell population-specific markers.
Tissue and cellular tropism of DENV has been investigated previously in AG129 mice by our group and by others (2, 3, 9, 20, 38). It has been reported that in the spleen, DENV infection occurs in CD11c+ DCs and in CD11bhigh F4/80+ macrophages (2, 20). In contrast to these publications, we did not find evidence that CD11c+ DCs in the spleen are major targets of DENV, and we did not find evidence of notable CD11b expression on DENV-infected F4/80+ red pulp macrophages. Instead, our findings demonstrated that MMMs, MZMs, and RPMs, but not cells expressing high levels of CD11b or CD11c, were highly permissive to DENV infection. In agreement with the present study, Balsitis et al. identified the red pulp and marginal zone as the dominant compartments for DENV infection in the spleen (2), and Fink et al. reported infection of CD169+ subcapsular sinus macrophages (SCSMs) of the draining LN following subcutaneous administration of DENV (9). In the present study, SCSMs and the equivalent CD169+ cells in the marginal zone of the spleen were infected following the i.f. and i.v. routes of administration, respectively, and all of the lymph nodes and Peyer's patches analyzed 48 h after infection contained high levels of DENV NS3.
These particular macrophage populations of the splenic marginal zone and subcapsular sinus of the lymph nodes have been shown to be important for the clearance of high-molecular-mass antigens, including viral particles from blood and lymph (21, 26, 33). These cells are believed to permit low levels of viral replication in order to amplify antigen and facilitate generation of high-affinity antibodies (14). Additionally, many viruses, including DENV, bind to DC-SIGN (36), which is the human homolog of murine SIGN-R1. This molecule is expressed at high levels on macrophages of the marginal zone and subcapsular sinus, and it acts as a pattern recognition receptor for sampling antigens in the surrounding fluid (10). These characteristics all support results that these macrophage populations, which possess molecules thought to be responsible for permitting DENV infection, are initial interaction partners and important initial targets of blood- and lymph-borne DENV.
To our knowledge, this is the first use of fluorescence-tagged viral particles for tracking the temporal and spatial patterns of DENV trafficking in vivo. The far-red fluorescent lipophilic dye DiD has been used previously for single DENV particle tracking to investigate cell entry in vitro (35). Here, DiD-labeled DENV particles were utilized in vivo to visualize the fate of the injected viral particles. In contrast to tissue section staining for DENV NS3, DiD-labeled DENV particles can be used to probe the outcome of blood-borne DENV in order to visualize trafficking at any time during infection. Our results show that high levels of DENV traffic to cells of the liver and the spleen, initially in the marginal zone and later also in the red pulp. The amount of DiD accumulating in the spleen decreases over time while it increases in the liver, kidneys, and thymus. Although kinetically not identical, this trend mirrors levels of viral replication within these organs over time, suggesting that extravasation of viral particles may dictate the sequence of cells and tissues targeted by DENV in vivo.
Our results contribute to the understanding of DENV tropism in vivo over the course of infection. We show that DENV inoculated via a peripheral route initially targets draining lymph nodes. Once DENV enters the blood, it first targets the spleen and bone marrow and later spreads to the lymph nodes and Peyer's patches and finally to nonlymphoid tissues throughout the mouse. These findings augment those in our published studies and show that DENV largely infects macrophages in a number of tissues of AG129 mice: the kidneys, heart, thymus, and gastrointestinal tract, marginal zone and red pulp of the spleen, subcapsular sinus of the lymph nodes, and sinusoidal endothelial cells of the liver (9, 38). By elucidating the pattern of DENV replication in mice, our study provides an overall framework for DENV behavior in vivo to guide subsequent investigations of DENV tropism in naturally infected humans.
ACKNOWLEDGMENTS
We thank Chris Benedict, Raphaël M. Zellweger, and Stuart T. Perry at LIAI for experimental advice. We also thank Olga Turovskaya, Sarala Joshi, Steven Lada, and Satoshi Fukuyama for technical help.
This work was supported by NIH grants U01 AI082185 to S.S. and U54 AI057157 from the Southeast Regional Center of Excellence for Emerging Infections and Biodefense to P. F. Sparling.
We declare no competing financial interests.
Footnotes
Published ahead of print 29 August 2012
REFERENCES
- 1. Ashour J, et al. 2010. Mouse STAT2 restricts early dengue virus replication. Cell Host Microbe 8:410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Balsitis SJ, et al. 2009. Tropism of dengue virus in mice and humans defined by viral nonstructural protein 3-specific immunostaining. Am. J. Trop. Med. Hyg. 80:416–424 [PubMed] [Google Scholar]
- 3. Balsitis SJ, et al. 2010. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 6:e1000790 doi:10.1371/journal.ppat.1000790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bente DA, et al. 2010. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J. Virol. 84:11089–11100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhoopat L, et al. 1996. Immunohistochemical characterization of a new monoclonal antibody reactive with dengue virus-infected cells in frozen tissue using immunoperoxidase technique. Asian Pac. J. Allergy Immunol. 14:107–113 [PubMed] [Google Scholar]
- 6. Boonpucknavig S, Boonpucknavig V, Bhamarapravati N, Nimmannitya S. 1979. Immunofluorescence study of skin rash in patients with dengue hemorrhagic fever. Arch. Pathol. Lab. Med. 103:463–466 [PubMed] [Google Scholar]
- 7. Chung KM, et al. 2006. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. U. S. A. 103:19111–19116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Couvelard A, et al. 1999. Report of a fatal case of dengue infection with hepatitis: demonstration of dengue antigens in hepatocytes and liver apoptosis. Hum. Pathol. 30:1106–1110 [DOI] [PubMed] [Google Scholar]
- 9. Fink K, et al. 2009. Depletion of macrophages in mice results in higher dengue virus titers and highlights the role of macrophages for virus control. Eur. J. Immunol. 39:2809–2821 [DOI] [PubMed] [Google Scholar]
- 10. Geijtenbeek TB, et al. 2002. Marginal zone macrophages express a murine homologue of DC-SIGN that captures blood-borne antigens in vivo. Blood 100:2908–2916 [DOI] [PubMed] [Google Scholar]
- 11. Gorgani NN, Ma Y, Clark HF. 2008. Gene signatures reflect the marked heterogeneity of tissue-resident macrophages. Immunol. Cell Biol. 86:246–254 [DOI] [PubMed] [Google Scholar]
- 12. Gubler DJ, Meltzer M. 1999. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv. Virus Res. 53:35–70 [DOI] [PubMed] [Google Scholar]
- 13. Hall WC, et al. 1991. Demonstration of yellow fever and dengue antigens in formalin-fixed paraffin-embedded human liver by immunohistochemical analysis. Am. J. Trop. Med. Hyg. 45:408–417 [DOI] [PubMed] [Google Scholar]
- 14. Honke N, et al. 2011. Enforced viral replication activates adaptive immunity and is essential for the control of a cytopathic virus. Nat. Immunol. 13:51–57 [DOI] [PubMed] [Google Scholar]
- 15. Huerre MR, et al. 2001. Liver histopathology and biological correlates in five cases of fatal dengue fever in Vietnamese children. Virchows Arch. 438:107–115 [DOI] [PubMed] [Google Scholar]
- 16. Jessie K, Fong MY, Devi S, Lam SK, Wong KT. 2004. Localization of dengue virus in naturally infected human tissues, by immunohistochemistry and in situ hybridization. J. Infect. Dis. 189:1411–1418 [DOI] [PubMed] [Google Scholar]
- 17. Jones M, et al. 2005. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 79:5414–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keller BC, et al. 2006. Resistance to alpha/beta interferon is a determinant of West Nile virus replication fitness and virulence. J. Virol. 80:9424–9434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirby AC, Beattie L, Maroof A, van Rooijen N, Kaye PM. 2009. SIGNR1-negative red pulp macrophages protect against acute streptococcal sepsis after Leishmania donovani-induced loss of marginal zone macrophages. Am. J. Pathol. 175:1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kyle JL, Beatty PR, Harris E. 2007. Dengue virus infects macrophages and dendritic cells in a mouse model of infection. J. Infect. Dis. 195:1808–1817 [DOI] [PubMed] [Google Scholar]
- 21. Lang PA, et al. 2010. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology 52:25–32 [DOI] [PubMed] [Google Scholar]
- 22. Lin RJ, Liao CL, Lin E, Lin YL. 2004. Blocking of the alpha interferon-induced Jak-Stat signaling pathway by Japanese encephalitis virus infection. J. Virol. 78:9285–9294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lloyd CM, Phillips AR, Cooper GJ, Dunbar PR. 2008. Three-colour fluorescence immunohistochemistry reveals the diversity of cells staining for macrophage markers in murine spleen and liver. J. Immunol. Methods 334:70–81 [DOI] [PubMed] [Google Scholar]
- 24. Meier KC, Gardner CL, Khoretonenko MV, Klimstra WB, Ryman KD. 2009. A mouse model for studying viscerotropic disease caused by yellow fever virus infection. PLoS Pathog. 5:e1000614 doi:10.1371/journal.ppat.1000614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miagostovich MP, et al. 1997. Retrospective study on dengue fatal cases. Clin. Neuropathol. 16:204–208 [PubMed] [Google Scholar]
- 26. Oehen S, et al. 2002. Marginal zone macrophages and immune responses against viruses. J. Immunol. 169:1453–1458 [DOI] [PubMed] [Google Scholar]
- 27. Reference deleted. [Google Scholar]
- 28. Prestwood TR, Prigozhin DM, Sharar KL, Zellweger RM, Shresta S. 2008. A mouse-passaged dengue virus strain with reduced affinity for heparan sulfate causes severe disease in mice by establishing increased systemic viral loads. J. Virol. 82:8411–8421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramos C, et al. 1998. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J. Neurovirol. 4:465–468 [DOI] [PubMed] [Google Scholar]
- 30. Ryman KD, Klimstra WB, Nguyen KB, Biron CA, Johnston RE. 2000. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 74:3366–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Samuel MA, Diamond MS. 2005. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J. Virol. 79:13350–13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. 2006. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 80:10208–10217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steiniger B, Barth P. 2000. Microanatomy and function of the spleen. Adv. Anat. Embryol. Cell Biol. 151:III–IX, 1–101 [DOI] [PubMed] [Google Scholar]
- 34. Styer LM, et al. 2007. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 3:1262–1270 doi:10.1371/journal.ppat.0030132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. van der Schaar HM, et al. 2008. Dissecting the cell entry pathway of dengue virus by single-particle tracking in living cells. PLoS Pathog. 4:e1000244 doi:10.1371/journal.ppat.1000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Kooyk Y, Geijtenbeek TB. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697–709 [DOI] [PubMed] [Google Scholar]
- 37. Witmer-Pack MD, et al. 1993. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J. Cell Sci. 104(Pt 4):1021–1029 [DOI] [PubMed] [Google Scholar]
- 38. Zellweger RM, Prestwood TR, Shresta S. 2010. Enhanced infection of liver sinusoidal endothelial cells in a mouse model of antibody-induced severe dengue disease. Cell Host Microbe 7:128–139 [DOI] [PMC free article] [PubMed] [Google Scholar]