Abstract
HIV CRF01_AE accounted for 84% of the recent infections among men who have sex with men (MSM) in Liaoning Province of northeastern China. CRF01_AE strains were grouped into two distinct clusters (designated clusters 1 and 2) that were also detected in other regions in China. Phylodynamics study revealed that these two CRF01_AE strains were independently introduced into the population of MSM in China in the early and mid-1990s. Our study elucidated unique features of dynamics and interrelationships of MSM epidemics in China.
TEXT
The incidence of human immunodeficiency virus (HIV) in the Western world remains high among men who have sex with men (MSM). More than half of new HIV infections occur among MSM in both the United States of America and the United Kingdom (7, 11–13, 20, 21). Recently, a high HIV prevalence among MSM has also been reported in several Asian countries (1, 23, 24, 27). In China, approximately 780,000 people were living with HIV/AIDS as of the end of 2011, and the proportion of MSM has increased to 17.4% from 14.7% in 2009 (4). Moreover, in Liaoning (LN) Province, the northeastern economic and cultural center of China, the incidence of HIV infections through sexual contact has increased rapidly, in spite of the HIV prevalence in the general population remaining low (<0.1%) (4). In the past 2 years, more than 49% of reported cases occurred through homosexual contact (Liaoning Center for Disease Control, unpublished data). Researchers who performed a recent prospective cohort study reported that the HIV incidence among MSM in Liaoning Province reached 7.1/100 person-years (15), well above the national average (17). Consequently, Liaoning might already be one of the important hot spots of HIV spread in China.
It is well known that subtype B′, CRF07_BC, CRF08_BC, and CRF01_AE are the major HIV-1 genotypes associated with particular risk populations in China (2, 25, 26, 31). Reports about China in recent years showed that multiple HIV-1 subtypes, including subtype B, CRF01_AE, and CRF07_BC, were coepidemic in the population of MSM (18, 32, 33). CRF01_AE, which was the dominant strain in the sexual-risk population (34), seems to have played a more and more important role within the population of MSM. The percentage of CRF01_AE has been increasing recently and has exceeded that of subtype B, the previously dominant strain, in different cities (10, 14, 18, 31, 32). In addition, Chen et al. reported that the time of the epidemic of the most recent common ancestor (tMRCA) of CRF01_AE in Hong Kong was in the mid-1990s (3). However, thus far, the origins and epidemic history of the HIV-1 CRF01_AE strain in Mainland China have not been explored in greater detail. To understand the latest evolution of the HIV epidemic and the time scale and regional transmission networks of CRF01_AE among Liaoning MSM, we investigated nearly full-length or partial HIV genomes from 43 newly HIV-1-infected cases from Liaoning.
A total of 43 subjects newly infected with HIV-1 were identified in MSM from Liaoning between December 2008 and September 2010, through following up a large-scale prospective HIV-negative MSM cohort (over 1,700 homosexuals) and HIV screening. All study subjects signed informed consent forms for the collection of blood samples and subsequent analyses and completed an administered epidemiological questionnaire. This study was approved by the Medical Research Ethics Committee of No.1 Hospital of China Medical University. All new infections were defined based on the detection of HIV-1-specific RNA, antigen, and antibody in plasma, according to the system described by Fiebig et al. (8).
The 5-kb 3′ or 5′ half of viral genomes was amplified from RNA in plasma using reverse transcriptase PCR (RT-PCR). Single-genome amplification (SGA) and sequencing of HIV-1 DNA were performed in order to acquire a single virus sequence from quasispecies (16). A total of 39 HIV-1 RNA genomes of nearly full length (HXB2 nucleotide sequence numbering; 769 to 9,384 nucleotides [nt]) and 4 3′-half genomes (HXB2; 4,674 to 9,384 nt) from all newly infected Liaoning MSM (n = 43) were successfully amplified and sequenced in the present study. The sequences were aligned using CLUSTAL X2.0 software and then manually edited. Phylogenetic analyses were performed using the maximum-likelihood method implemented in the MEGA 4.0 program (28). The topology of trees was tested by bootstrap analysis performed with 1,000 replicates. Maximum clade credibility (MCC) trees were constructed using MCMC (Markov Chain Monte Carlo) inference implemented in the BEAST v1.6 package using the relaxed log-normal molecular clock (6).
The distribution of HIV-1 genotypes determined based on maximum-likelihood tree analyses was as follows (Fig. 1): CRF01_AE, 83.7% (36 of 43); subtype B (U.S. and European origin), 7.0% (3 of 43); CRF07_BC, 7.0% (3 of 43); subtype B/CRF01_AE recombinant, 2.3% (1/43). The percentage of CRF01_AE in acute HIV infections is consistent with our previously published data on chronic HIV infections between 2000 and 2008, which showed that the proportions of CRF01_AE and subtype B/B′ (U.S. and European origin/Thai origin) were 81.3% and 16%, respectively (14). The predominant role of CRF01_AE in Liaoning was consistent with but more distinct than the increasing trend of CRF01_AE virus transmission among MSM in other regions around China. For instance, among Beijing MSM, the proportion of CRF01_AE increased from 3.7% in 2005 to more than 50% in 2009, whereas the proportion of subtype B decreased from more than 90% to less than 20% over the same period (9, 32). Similarly, a study of Shijiazhuang MSM showed that CRF01_AE accounted for 52.9% of infections (35.3% for subtype B) between 2008 and 2009 (18). This condition of the predominance of CRF01_AE is distinct from the MSM epidemic in the Western world, where subtype B of U.S. and European origin predominates (5, 30), and may be related to a recent upsurge of the HIV epidemic in southeastern Asia (29).
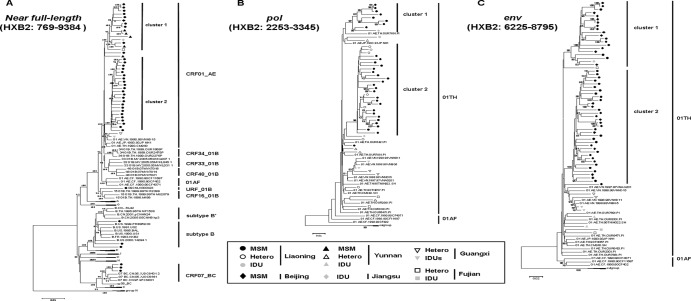
Fig 1.
Phylogenetic tree analysis of nucleotide sequences from newly infected MSM in Liaoning identifies two distinct CRF01_AE clusters. Data represent maximum-likelihood tree analysis of a total of 39 nearly full-length nucleotide sequences (HXB2; 769 to 9,384 nt) (A), 32 pol fragments (HXB2; 2,253 to 3,345 nt) (B), and 36 env fragments (HXB2; 6,225 to 8,795 nt) (C) of CRF01_AE from newly infected Liaoning MSM (solid circles) performed with HIV-1 subtype/CRF reference sequences (http://www.hiv.lanl.gov/content/index) relevant to this study and 1,000 bootstrap replicates. The HIV-1 group N sequences were used as an outgroup. Bootstrap values greater than 80 are indicated on the corresponding nodes. Each HIV-1 subtype/CRF designation is indicated on the right side of the tree. Two distinct clusters of CRF01_AE strains (clusters 1 and 2) identified in Liaoning MSM are indicated. Besides Liaoning MSM, the nearly full-length nucleotide sequences from various risk populations in the rest of China are included (see inset).
Two clusters designated clusters 1 and 2 were observed among CRF01_AE sequences in phylogenetic tree analysis based on nearly full-length sequences (HXB2; 769 to 9,384 nt). Among 36 CRF01_AE strains identified in the present study, 9 (25.0%) and 26 (72.2%) belonged to clusters 1 and 2, respectively, and 1 other CRF01_AE strain, located outside these two clusters, was the descendant of Thailand strain CRF01_AE (Fig. 1). Note that most sequences we previously identified from MSM in Beijing and Yunnan and reference sequences from intravenous drug users (IDUs) in Jiangsu and heterosexuals in Fujian downloaded from an HIV sequence database clustered together to form cluster 1 with a root corresponding to sequences from Liaoning MSM (Fig. 1), which indicates that at least the strains in cluster 1 from northeastern China have close evolutionary associations with southern and southeastern strains. On the other hand, cluster 2 appears to be unique to Liaoning MSM grouped with Liaoning heterosexuals (Fig. 1B), suggesting a single transmission pattern and a possible transmission relationship between homosexuals and heterosexuals of Liaoning. However, results of our ongoing, large-scale, molecular epidemiologic survey suggest that, although cluster 2 CRF01_AE had spread less widely than cluster 1, it can be detected among a few MSM in other regions in China (X. Han et al., unpublished data). In addition, a phylogenetic tree corresponding to the env (HXB2; 6,225 to 8,795 nucleotides) region constructed using all previous published nucleotide sequences from Beijing MSM available (n = 19) in the database revealed that the strains of CRF01_AE virus in Beijing MSM belonged to either cluster 1 (n = 10) or cluster 2 (n = 9) (Fig. 1C), indicating that these two clusters represented the predominant CRF01_AE strains circulating among MSM, at least in northeastern China. Liaoning, the northeast economic and cultural center of China, is geographically close to Beijing, which is only 700 kilometers distant (4 h by train; 1 h by plane). MSM traveling between Liaoning and Beijing could meet conveniently, which might cause the exchange of HIV strains between Liaoning and Beijing.
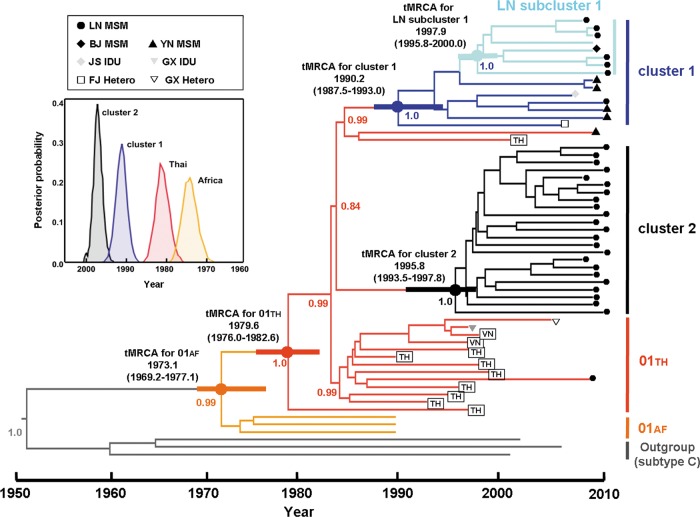
To identify the origin and the temporal spread of these two distinct CRF01_AE clusters circulating among MSM, we estimated the dates of tMRCA and the evolutionary rate, using the BEAST package, based on a data set of nearly full-length nucleotide sequences of CRF01_AE strains from Liaoning MSM (n = 32) determined in the present study in addition to 6 nearly full-length sequences of CRF01_AE strains identified between 2009 and 2010 from MSM in other provinces (our previous unpublished data) and 19 additional CRF01_AE reference sequences with specified sampling times that were downloaded from an HIV sequence database. The results showed that the evolutionary rates were 3.31 (2.86 to 3.77) ×10−3 substitutions/site/year for GTR+γ4 relaxed clock models with a constant size model. The dates of the common ancestor of the African CRF01_AE, Thailand CRF01_AE, CRF01_AE clusters 1 and 2, and LN subcluster 1 were estimated to be 1973.1 (95% credible region, 1969.2 to 1977.1), 1979.6 (1976.0 to 1982.6), 1990.2 (1987.5 to 1993.0), 1995.8 (1993.5 to 1997.8), and 1997.9 (1995.8 to 2000.0), respectively.
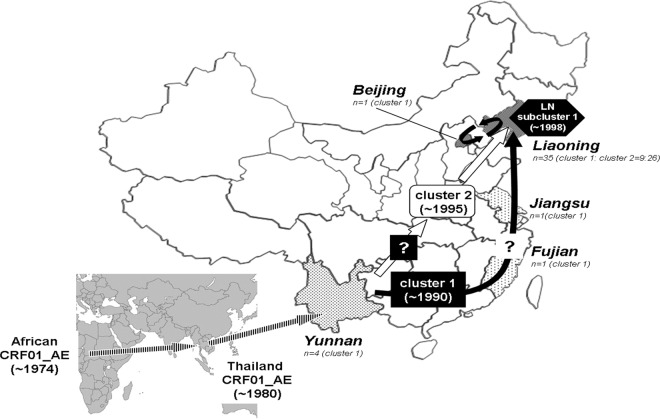
The results of temporal and spatial dynamics analyses show that the two clusters both rooted within the Thai sequences and derived from a common ancestor that existed in Thai in 1979, which was essentially similar to the previous estimations (19). Furthermore, we found these two CRF01_AE clusters were independently introduced into the Chinese population of MSM. Cluster 1 emerged earlier (∼1990) than cluster 2 (∼1996), and LN subcluster 1 from cluster 1 occurred more recently (∼1998) (Fig. 2). It is interesting that cluster 1 contained 7 additional CRF01_AE sequences from one Beijing MSM, four Yunnan MSM, one Jiangsu IDU, and one Fujian heterosexual. These sequences were located near the base of the cluster 1 branch, except for the Beijing sequence (Fig. 2), so we inferred that CRF01_AE cluster 1 may have originated in the southern or southwestern provinces and spread to North China along the southeast coast. Although the number of strains in LN subcluster 1 that derived from parental cluster 1 is lower than the number that derived from cluster 2, they appear to play an important role together with cluster 2 strains in the HIV epidemic among MSM in Liaoning. The cluster 2 strains were less widely spread than the cluster 1 strains, having been detected only in Liaoning and Beijing. The first CRF01_AE strains in China were detected among intravenous drug users (IDUs) in Yunnan and then spread to southern and southwestern China though drug injection and sexual contact (22, 35); thus, we inferred that the geographic origin of the cluster 2 strains might also have been in or near Yunnan but that the strains might disseminated directly into northeastern regions, considering the predominance of cluster 2 and the earlier estimated time of introduction into Liaoning. Taking the data together, all CRF01_AE strains collected from the population of MSM were divided into two clusters after spreading to Yunnan from Thailand. The two clusters migrated nationwide via different geographic routes. The hypothesized routes and time-space process of the dissemination of CRF01_AE strains among MSM in China and their origins are schematically illustrated in Fig. 3.
Fig 2.
Maximum clade credibility (MCC) tree representing the rooted genealogy of Liaoning CRF01_AE clusters. An MCC tree was obtained by Bayesian MCMC analysis based on nearly full-length HIV-1 nucleotide sequences (8,335 nt; HXB2, 803 to 9,181 nt) implemented in BEAST v 1.6.0. This particular tree was constructed based on a relaxed clock model in GTR+G4 combined with a constant coalescent model. HIV-1 subtype C sequences were used as outliers. The medians of tMRCAs and 95% credibility intervals (in parentheses) of CRF01_AE strains relevant to this study are as follows: African CRF01_AE ancestor, 1973.1 (1969.2 to 1977.1); Thailand CRF01_AE ancestor, 1979.6 (1976.0 to 1982.6); CRF01_AE cluster 1, 1990.2 (1987.5 to 1993.0); CRF01_AE cluster 2, 1995.8 (1993.5 to 1997.8); Liaoning (LN) subcluster 1, 1997.9 (1995.8 to 2000.0) (as determined using an GTR+γ4 relaxed clock model with a constant size model) (data not shown). The distribution of posterior probability of respective CRF01_AE clusters is illustrated in inset and shown as thick horizontal bars on the corresponding codes.
Fig 3.
Map of the hypothesized routes of migration of CRF01_AE strains circulating in Liaoning MSM. Two distinct lineages (clusters 1 and 2) of CRF01_AE strains were independently introduced into the Liaoning population of MSM, most likely through Beijing MSM (see Discussion). The estimated times of the most recent common ancestors (tMRCA) were ∼1990 and ∼1996 for clusters 1 and 2, respectively (Fig. 2). Cluster 1 is likely to have originated from CRF01_AE strains emerging in southern or southwestern provinces (see the text). Liaoning (LN) CRF01_AE subcluster 1 emerged much recently (∼1998) from parental cluster 1. The prevalence of each CRF01_AE cluster in different geographic regions in China is shown as a histogram: cluster 1 (black arrow); cluster 2 (white arrow).
In summary, using a high-risk MSM cohort, we found two distinct lineages of CRF01_AE strains among newly infected MSM in Liaoning Province, which may indicate an interrelationship of the HIV infections between MSM around China and other risk populations. Our results provide insight into our understanding of the transmission and potential public health impact of local circulating strains of HIV-1 among members of the population of MSM in China and highlight the necessity and importance of continued molecular epidemiological monitoring across China.
ACKNOWLEDGMENTS
We thank Feng Gao of the Duke Human Vaccine Institute for technical assistance in the writing of the manuscript. We are also indebted to the subjects for their participation.
The study was conceived and designed by Hong Shang and Xiaoxu Han. Data acquisition and analysis were performed by Minghui An and Xiaoxu Han. Junjie Xu, Zhenxing Chu, Manhong Jia, Hao Wu, and Lin Lu were responsible for patient recruitment. Minghui An wrote the first draft, and Minghui An, Xiaoxu Han, Yutaka Takebe, and Hong Shang contributed to the final version of the paper.
The study was supported by Mega Projects of National Science Research for the 12th Five-Year Plan (2012ZX10001-006), National Natural Science Foundation (30800969).
Footnotes
Published ahead of print 29 August 2012
REFERENCES
- 1. Brahmam GN, et al. 2008. Sexual practices, HIV and sexually transmitted infections among self-identified men who have sex with men in four high HIV prevalence states of India. AIDS 22(Suppl 5):S45–S57 [DOI] [PubMed] [Google Scholar]
- 2. Brazille P, Coutant-Perronne V, Malkin JE, Raguin G. 2000. [Agranulocytosis in acute hepatitis B in an HIV seropositive patient]. Presse Med. 29:417–418 [PubMed] [Google Scholar]
- 3. Chen JH, et al. 2009. Molecular epidemiological study of HIV-1 CRF01_AE transmission in Hong Kong. J. Acquir. Immune Defic. Syndr. 51:530–535 [DOI] [PubMed] [Google Scholar]
- 4. China Ministry of Health, World Health Organization (WHO) 2011. 2011 estimates for the HIV/AIDS epidemic in China. China Ministry of Health, Beijing, China: http://www.chinaids.org.cn/n1971/n2151/n777994.files/n777993.pdf [Google Scholar]
- 5. Cuevas M, et al. 2009. Incidence of non-B subtypes of HIV-1 in Galicia, Spain: high frequency and diversity of HIV-1 among men who have sex with men. Euro. Surveill. 14:pii=19413. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19413 [DOI] [PubMed] [Google Scholar]
- 6. Drummond AJ, Rambaut A. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214 doi:10.1186/1471-2148-7--214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fenton KA, Imrie J. 2005. Increasing rates of sexually transmitted diseases in homosexual men in Western Europe and the United States: why? Infect. Dis. Clin. North Am. 19:311–331 [DOI] [PubMed] [Google Scholar]
- 8. Fiebig EW, et al. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. AIDS 17:1871–1879 [DOI] [PubMed] [Google Scholar]
- 9. Fox J, et al. 2009. Reductions in HIV transmission risk behaviour following diagnosis of primary HIV infection: a cohort of high-risk men who have sex with men. HIV Med. 10:432–438 [DOI] [PubMed] [Google Scholar]
- 10. Guo H, et al. 2009. Rapidly increasing prevalence of HIV and syphilis and HIV-1 subtype characterization among men who have sex with men in Jiangsu, China. Sex. Transm. Dis. 36:120–125 [DOI] [PubMed] [Google Scholar]
- 11. Hall HI, An Q, Hutchinson AB, Sansom S. 2008. Estimating the lifetime risk of a diagnosis of the HIV infection in 33 states, 2004–2005. J. Acquir. Immune Defic. Syndr. 49:294–297 [DOI] [PubMed] [Google Scholar]
- 12. Hall HI, et al. 2008. Estimation of HIV incidence in the United States. JAMA 300:520–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamers FF, Downs AM. 2004. The changing face of the HIV epidemic in western Europe: what are the implications for public health policies? Lancet 364:83–94 [DOI] [PubMed] [Google Scholar]
- 14. Han X, et al. 2010. Genetic and epidemiologic characterization of HIV-1 infection In Liaoning Province, China. J. Acquir. Immune Defic. Syndr. 53(Suppl 1):S27–S33 [DOI] [PubMed] [Google Scholar]
- 15. Han X, et al. 2011. Screening acute HIV infections among Chinese men who have sex with men from voluntary counseling & testing centers. PLoS One 6:e28792 doi:10.1371/journal.pone.0028792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirchherr JL, et al. 2007. High throughput functional analysis of HIV-1 env genes without cloning. J. Virol. Methods 143:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li HM, et al. 2011. HIV incidence among men who have sex with men in China: a meta-analysis of published studies. PLoS One 6:e23431 doi:10.1371/journal.pone.0023431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li L, et al. 2011. High genetic diversity of HIV-1 was found in men who have sex with men in Shijiazhuang, China. Infect. Genet. Evol. 11:1487–1492 [DOI] [PubMed] [Google Scholar]
- 19. Liao H, et al. 2009. Phylodynamic analysis of the dissemination of HIV-1 CRF01_AE in Vietnam. Virology 391:51–56 [DOI] [PubMed] [Google Scholar]
- 20. McCutchan FE. 2006. Global epidemiology of HIV. J. Med. Virol. 78(Suppl 1):S7–S12 [DOI] [PubMed] [Google Scholar]
- 21. Miailhes P, Pradat P, Trepo C. 2009. Influence of aminotransferase values on liver stiffness measurement: the case of HIV-infected patients with acute viral hepatitis B or C. HIV Med. 10:62–63 [DOI] [PubMed] [Google Scholar]
- 22. Ministry of Health, China, UNAIDS, WHO 2006. 2005 update on the HIV/AIDS epidemic and response in China. Ministry of Health China, Beijing, China: http://data.unaids.org/publications/External-Documents/rp_2005chinaestimation_25jan06_en.pdf [Google Scholar]
- 23. National AIDS Commission 2008. Country report on the follow-up to the Declaration of Commitment on HIV/AIDS. National AIDs Commission, Jakarta, Indonesia: http://data.unaids.org/pub/Report/2008/indonesia_2008_country_progress_report_en.pdf [Google Scholar]
- 24. National AIDS Programme 2009. Report of the HIV Sentinel Sero-Surveilance Survey 2008—Myanmar. National AIDS Programme, Yangon, Myanmar [Google Scholar]
- 25. Ou C, Takebe Y, Weniger B. 1993. Independent introduction of two major HIV-1 genotypes into distinct high-risk populations in Thailand. Lancet 341:1171–1174 [DOI] [PubMed] [Google Scholar]
- 26. Shao Y, Zheng X, Tian C. 2001. HIV/AIDS: perspective on China. AIDS Patient Care STDS 15:431–432 [DOI] [PubMed] [Google Scholar]
- 27. Sheridan S, et al. 2009. HIV prevalence and risk behaviour among men who have sex with men in Vientiane Capital, Lao People's Democratic Republic, 2007. AIDS 23:409–414 [DOI] [PubMed] [Google Scholar]
- 28. Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 29. van Griensven F, de Lind van Wijngaarden JW, Baral S, Grulich A. 2009. The global epidemic of HIV infection among men who have sex with men. Curr. Opin. HIV AIDS 4:300–307 [DOI] [PubMed] [Google Scholar]
- 30. Walker PR, Pybus OG, Rambaut A, Holmes EC. 2005. Comparative population dynamics of HIV-1 subtypes B and C: subtype-specific differences in patterns of epidemic growth. Infect. Genet. Evol. 5:199–208 [DOI] [PubMed] [Google Scholar]
- 31. Wang W, et al. 2008. Identification of subtype B, multiple circulating recombinant forms and unique recombinants of HIV type 1 in an MSM cohort in China. AIDS Res. Hum. Retroviruses 24:1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang W, et al. 2011. The dynamic face of HIV-1 subtypes among men who have sex with men in Beijing, China. Curr. HIV Res. 9:136–139 [DOI] [PubMed] [Google Scholar]
- 33. Zhang X, et al. 2007. Characterization of HIV-1 subtypes and viral antiretroviral drug resistance in men who have sex with men in Beijing, China. AIDS 21(Suppl 8):S59–S65 [DOI] [PubMed] [Google Scholar]
- 34. Zhang Y, et al. 2006. Dominance of HIV-1 subtype CRF01_AE in sexually acquired cases leads to a new epidemic in Yunnan province of China. PLoS Med. 3:e443 doi:10.1371/journal.pmed.0030443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng X, et al. 1994. Injecting drug use and HIV infection in southwest China. AIDS 8:1141–1147 [DOI] [PubMed] [Google Scholar]