Abstract
Single, double, and triple null combinations of Arabidopsis mutants lacking the photoreceptors phytochrome (phy) A (phyA-201), phyB (phyB-5), and cryptochrome (cry) 1 (hy4-2.23n) were examined for de-etiolation responses in high-fluence red, far-red, blue, and broad-spectrum white light. Cotyledon unhooking, unfolding, and expansion, hypocotyl growth, and the accumulation of chlorophylls and anthocyanin in 5-d-old seedlings were measured under each light condition and in the dark. phyA was the major photoreceptor/effector for most far-red-light responses, although phyB and cry1 modulated anthocyanin accumulation in a phyA-dependent manner. phyB was the major photoreceptor in red light, although cry1 acted as a phyA/phyB-dependent modulator of chlorophyll accumulation under these conditions. All three photoreceptors contributed to most blue light deetiolation responses, either redundantly or additively; however, phyB acted as a modulator of cotyledon expansion dependent on the presence of cry1. As reported previously, flowering time in long days was promoted by phyA and inhibited by phyB, with each suppressing the other's effect. In addition to the effector/modulator relationships described above, measurements of hypocotyls from blue-light-grown seedlings demonstrated phytochrome activity in blue light and cry1 activity in a phyAphyB mutant background.
Because of their sessile nature, plants have evolved multiple photoreceptor systems for perceiving the quality and quantity of light in their surrounding environment. This information drives morphological and physiological adaptations necessary for survival. Photobiological analyses have defined at least three photoreceptor systems: the red/ far-red-absorbing phytochromes, the blue/UV-A-absorbing cryptochromes, and unknown UV-B photoreceptors (Quail et al., 1995; Fankhauser and Chory, 1997).
Action spectra have shown that individual classes of photoreceptors can independently affect certain developmental responses, although their effect can be modulated by other photoreceptor systems (Mohr, 1994). For example, phototropism in many plants is directly controlled by cryptochromes and not by phytochromes. However, a pretreatment with omnilateral red light will enhance the phototropic response to a subsequent unilateral blue-light treatment. These results demonstrate that phytochromes can act as preprogrammed amplifiers of certain cryptochrome-regulated responses (Shropshire and Mohr, 1970; Woitzik and Mohr, 1988). Conversely, cryptochromes have been shown to amplify phytochrome-mediated responses. For example, phytochrome's induction of the accumulation of glyceraldehyde-3-phosphate dehydrogenase in milo seedlings can be amplified by cryptochromes (Oelmüller and Mohr, 1984, 1985b). Anthocyanin accumulation in milo is driven by phytochrome(s), although this response requires a blue-light stimulus of cryptochrome(s) (Drumm and Mohr, 1978; Drumm-Herrel and Mohr, 1981; Oelmüller and Mohr, 1985a). Photobiological analyses demonstrate that under certain conditions there is interplay between the cryptochromes and the phytochromes.
In Arabidopsis many de-etiolation events such as hypocotyl growth inhibition and cotyledon expansion are controlled by blue, red, and far-red light. Simple genetic screens for Arabidopsis seedlings with long hypocotyls and less-developed cotyledons in high-fluence light have identified null mutants lacking three of these photoreceptors. phyA mutants lacking phyA were identified as having long hypocotyls in continuous far-red light (Nagatani et al., 1993; Parks and Quail, 1993; Whitelam et al., 1993). phyB (previously hy3) mutants, which lack phyB (Reed et al., 1993), and cry1 (previously hy4) mutants, which lack cry1 (Ahmad and Cashmore, 1993), were identified as having long hypocotyls in continuous white light (Koornneef et al., 1980). Analysis in monochromatic light showed that the phyB mutant was primarily deficient in its response to red light and that the cry1 mutant was primarily deficient in its response to blue and UV-A light (Koornneef et al., 1980).
Photoreceptor null mutants in Arabidopsis have defined the role that each of these photoreceptors plays in plant development and the interactions between the photoreceptors. For instance, analysis of the phyAphyB double mutant has shown co-action between the two photoreceptors during seedling development in hypocotyl and cotyledon growth, as well as CAB gene induction and chlorophyll accumulation (Reed et al., 1994; Casal and Boccalandro, 1995). Both phyA and phyB control seed germination, although the mechanisms are physiologically distinct, with phyB controlling a photoreversible low-fluence response and phyA controlling a nonphotoreversible low-fluence response (Shinomura et al., 1996). Reed et al. (1994) also examined seed germination with respect to phyA and phyB and found that a phyB mutation partially suppresses a phyA mutation and vice versa, indicating a possible interaction between the two pathways.
Interactions between phyB and cry1 have also been examined for hypocotyl growth and cotyledon unfolding. In these experiments seedlings were exposed to orange light, which activates phytochromes, and then compared with seedlings that were given an additional blue-light treatment. Under these growth conditions the wild type and the phyA mutant responded to the addition of blue light, but the phyB and cry1 mutants did not. These results demonstrate a lack of interaction between phyA and cry1 (Casal and Boccalandro, 1995). In contrast, Ahmad and Cashmore (1997) proposed that there is a functional dependence of cry1 on phyA or phyB in the hypocotyl-growth response.
In this study we tested the model proposed by Ahmad and Cashmore (1997) by comparing the phyAphyBcry1 triple mutant with the phyAphyB double mutant and with other double-mutant combinations, single mutants, and the wild type. For the purpose of testing this model, we have examined each of these mutants in blue light to determine if the triple mutant is more severe than the phyAphyB double mutant, thus demonstrating activity of cry1 in a phyAphyB mutant background. We also wanted to determine if either of the phytochrome null mutants conferred an altered growth response in blue light, which would explain the obvious mutant phenotype described in phyAphyB. In addition, we have measured a variety of seedling-developmental responses for each of the mutant combinations in red, far-red, blue, and broad-spectrum white light, and examined the interplay between these three photoreceptors during flowering in long-day conditions. These measurements allowed us to identify genetic interactions between the three photoreceptors for specific growth responses in varying light conditions.
The results of this study demonstrate that (a) phyA and phyB act as blue-light photoreceptors, (b) in blue light cry1 has biological activity in a phyAphyB null mutant background, (c) phyB acts as a modulator of cry1-mediated cotyledon expansion in blue light, (d) cry1 acts as a modulator of the effects of phyA and phyB on red-light-mediated chlorophyll accumulation, and (e) phyB and cry1 act as modulators of phyA-mediated anthocyanin accumulation in far-red light.
MATERIALS AND METHODS
Isolation of Double and Triple Mutants
Each of the mutants studied (phyA-201, phyB-5, cry1/hy4-2.23n) was a null allele in the La-er background. The phyAphyB double mutant was originally described by Reed et al. (1994). The phyBcry1 double mutant was identified by crossing phyB with cry1, allowing the F1 plant to self-fertilize, and isolating long plants from an F2 population grown for 3 d in blue light followed by 2 d in red light. The phyAcry1 double mutant was identified by crossing phyA with cry1, allowing the F1 plants to self-fertilize, and isolating long plants from an F2 population grown for 3 d in far-red light followed by 2 d in blue light. The phyAphyB cry1 triple mutant was identified by crossing the phyBcry1 double mutant with the phyAphyB double mutant, allowing the F1 plants to self-fertilize, and isolating long plants from an F2 population grown for 3 d in far-red light followed by 2 d in blue light.
PCR-Based Identification of Mutant Alleles
The genotypes of each of the mutant combinations were confirmed by the following PCR reactions. The phyB-5 mutation creates a dCAPS marker (Neff et al., 1998). PCR products using the primers 5′-CCATTTGATTTCTTTCGCAGTGTGAGATCGGAA-3′ and 5′-GTGATATGCTTCTGCGTG-3′ were resolved on a 2.5% MetaPhor agarose gel (FMC BioProducts, Rockland, ME) after digestion with the restriction endonuclease NlaIV. The phyA-201 mutation was identified by using the primers 5′-GAAGTGTTGACTGCTTCCACGAGT-3′ and 5′-TAGCAAGATGCACAGAACGCC-3′, followed by digestion with HinfI. The cry1 (hy4-2.23n) mutation, a result of a chromosomal rearrangement, was identified by amplifying genomic DNA with the primers 5′-GAAATACTGAACTGGAGA-3′ and 5′-TTGAAACTTACTGAAAAT-3′, followed by resolution of approximately 130- and 180-bp fragments for the wild type and approximately 180-bp fragments for the cry1 mutant. The 180-bp product may have been the result of amplification of CRY-like sequences. The intensity of this band is affected by the annealing temperature.
Seedling Growth and Light Conditions
All experiments were performed at least three times. Seedlings of each genotype were grown on the same 100-mm Petri dish for each repeat. Seeds were sterilized by soaking for 5 min in 70% ethanol with 0.05% Triton X-100, followed by 5 min in 95% ethanol. The seeds were allowed to imbibe for 2 d in sterile, distilled water at 4°C before being plated on one-half-strength Murashige-Skoog medium with 1.5% Suc (Valvekens et al., 1988) and stored for an additional 2 d at 4°C in the dark. Plates were placed into white light for 24 h before being transferred to the appropriate experimental light conditions. All seedlings were grown at 21°C in an incubator (model E-30B, Percival, Boone, IA). White light was supplied by six cool-white, 35-W fluorescent bulbs (F24T12/CS/HO, Sylvania) and two 25-W, clear, incandescent bulbs (25T10, General Electric). Red light was supplied by three 20-W fluorescent bulbs (F20T12/GRO Gro-Lux, Sylvania) filtered through red acrylic (Gavrieli no. 2423, Ridout Plastics, San Diego, CA). Blue light was supplied by three 20-W fluorescent bulbs (Sylvania) filtered through blue glass (no. 5–57 Kopp Glass, Pittsburgh, PA). The far-red-light source was described previously (Nagatani et al., 1993). A dose-response curve in white light for each of the genotypes determined that 50 μmol m−2 s−1 was not saturating for the hypocotyl-growth response (data not shown). Time-course experiments from 1 to 10 d at 50 μmol m−2 s−1 in white light demonstrated that growth was still linear at the 5-d time point (data not shown). Based on the dose-response curves and time-course analysis, 5-d-old seedlings grown in 50 μmol m−2 s−1 of continuous light were used for each of the experiments described.
Analysis of Seedling Morphology
Cotyledon unhooking and unfolding were measured by two different methods, depending on the overall morphology of the plant. Seedlings with small, folded cotyledons were placed on transparent tape without altering the hook morphology. The seedlings were sandwiched between the tape and a sheet of acetate, digitized using a 35-mm slide scanner at a resolution of 2700 points per inch, and the images were saved as TIFF files for further analysis. Seedlings with expanded, and open cotyledons were placed on a microscope slide and digitized as TIFF files using a gel-documentation system (Speedlight, Lighttools Research, Encinitas, CA). The two acquisition systems were necessary for these measurements because of the wide range of phenotypes examined. Cotyledon unfolding was calculated by drawing a line between the apical meristem and the tip of each cotyledon and measuring the angle between these two lines. Cotyledon-hook opening was calculated by measuring the angle between a line drawn from the apical meristem to the center of the hypocotyl and a second line drawn from the apical meristem bisecting the cotyledon-unfolding angle described above.
The cotyledon area was measured by placing cotyledons flat on transparent tape and sandwiching the cotyledons between the tape and an acetate sheet. The sandwiched cotyledons were digitized using a 35-mm slide scanner at a resolution of 2700 points per inch. Hypocotyl length was measured by scanning seedlings that were sandwiched between two sheets of acetate in a flatbed scanner at a resolution of 300 points per inch. This resolution was sufficient for identification of the transition between hypocotyl and root. All digitized images were analyzed with imaging software from the National Institutes of Health.
Analysis of Chlorophyll Content
Total chlorophyll was extracted in 450 μL of N,N-dimethylformamide by shaking in the dark at 4°C. For each experiment, at least two groups of four seedlings from each treatment were collected and frozen at −20°C. Relative chlorophyll levels were determined by fluorescence using a luminescence spectrometer (model LS-50B, Perkin-Elmer). The samples were diluted so that all readings were in the linear range of accuracy for this assay as determined by a standard curve of fluorescence versus concentration of chlorophyll. The excitation wavelength was 620 nm and the emission wavelength was 673 nm. The absolute amount of chlorophyll was calculated based on comparisons with a wild-type sample of known concentration using the extinction coefficients calculated by Porra et al. (1989). Each experiment was performed at least three times.
Analysis of Anthocyanin Content
Relative anthocyanin levels were determined by collecting at least two groups of eight seedlings from each of the light treatments and incubating them overnight in 150 μL of methanol acidified with 1% HCl. After the addition of 100 μL of distilled water, anthocyanins were separated from chlorophylls with 250 μL of chloroform. Total anthocyanins were determined by measuring the A530 and A657 of the aqueous phase using a spectrophotometer (DU-530, Beckman). By subtracting the A657 from the A530, the relative amount of anthocyanin per seedling was calculated.
Determination of Flowering Time
Seedlings were grown in continuous white light for 5 d, and then transferred to individual pots of soil and grown in a 16-h light/8-h dark regime. Plants were grown 30 cm away from a bank of three cool-white bulbs and one fluorescent bulb (40 W each). Flowering time was determined by counting both the rosette and cauline leaves at the time of bolting. More than 50 plants were used for each measurement.
RESULTS
To examine genetic interactions between the three photoreceptors phyA, phyB, and cry1, we grew each of the single, double, and triple null mutants in a variety of light conditions and compared aspects of their seedling and adult morphologies with those of the wild type. We also compared dark-grown wild-type and mutant plants. In all of the growth responses measured, there was no significant difference between the dark phenotypes for each of the genotypes measured. Therefore, we have included only the wild-type dark measurements for comparison of seedling phenotypes.
phyA, phyB, and cry1 All Contribute to Seedling Development in White Light
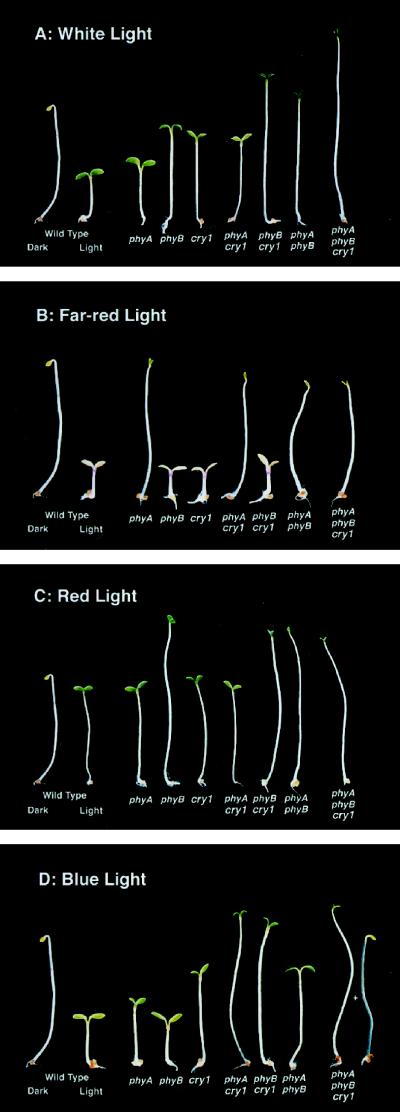
Lines carrying null mutations in PHYA, PHYB, and CRY1 or combinations of these were grown in continuous white light for 5 d to examine the roles of these photoreceptors during de-etiolation (Fig. 1A). Cotyledon unhooking (Table I, column 2) was controlled in a completely redundant manner by all three photoreceptors, since only the phyAphyBcry1 triple mutant showed an inability to fully unhook in white light. The partial unhooking of the triple mutant was similar to the dark phenotype exhibited by the wild type and each of the mutant combinations. phyB and cry1 were the primary photoreceptors involved in cotyledon unfolding in white light (Table I, column 3). These photoreceptors acted in a redundant manner, since only the phyBcry1 double mutant had an aberrant unfolding phenotype (57% of wild type). The triple mutant had the most severe cotyledon-unfolding phenotype in white light (18% of wild type), indicating that phyA contributed to this growth response in the absence of phyB and cry1 under these growth conditions.
Figure 1.
Five-day-old seedlings in various light conditions.
Table I.
White-light-grown seedlings
| Genotype | Angle
|
Cotyledon Area | Hypocotyl Length | Chlorophyll Content | Anthocyanin Content | |
|---|---|---|---|---|---|---|
| Hook | Cotyledon | |||||
| degrees | mm2 | mm | μg seedling−1 | (A530−A657) × 1000 seedling−1 | ||
| La-er | 172 (1.6) | 206 (3.2) | 2.2 (0.08) | 3.1 (0.2) | 0.76 (0.03) | 0.75 (0.15) |
| phyA | 174 (1.1) | 195 (5.2) | 2.1 (0.06) | 3.7 (0.2) | 0.86 (0.05) | 0.97 (0.1) |
| phyB | 172 (1.4) | 177 (5.4) | 0.8 (0.04) | 7.7 (0.4) | 0.56 (0.04) | 0.86 (0.3) |
| cry1 | 174 (1.1) | 166 (5.5) | 1.1 (0.06) | 7.4 (0.2) | 0.58 (0.04) | 0.53 (0.23) |
| phyAcry1 | 173 (1.1) | 203 (3.3) | 1.7 (0.05) | 6.0 (0.2) | 0.66 (0.04) | N.D.a |
| phyBcry1 | 168 (1.7) | 118 (8.8) | 0.3 (0.01) | 13.4 (0.3) | 0.26 (0.02) | N.D. |
| phyAphyB | 174 (1.0) | 152 (8.4) | 0.5 (0.02) | 11.1 (0.6) | 0.29 (0.02) | N.D. |
| phyAphyBcry1 | 132 (5.8) | 38 (7.6) | 0.2 (0.007) | 13.9 (0.6) | 0.19 (0.01) | N.D. |
| La-er (dark) | 132 (4.4) | 15 (1.8) | 0.2 (0.007) | 9.7 (0.6) | N.D. | N.D. |
Values in parentheses are se.
N.D., Not detected.
Cotyledon expansion involved all three photoreceptors in white light (Table I, column 4). phyB and cry1 contributed in an additive manner to this growth response. phyB contributed more than cry1 to this growth response, since the removal of cry1 (i.e. the cry1 mutant compared with the wild type) caused a 50% reduction in cotyledon area, whereas the removal of phyB caused a 64% reduction. Cotyledons in the triple mutant were as small as dark-grown cotyledons, demonstrating that these three photoreceptors were sufficient to account for all of the white-light-mediated cotyledon expansion. By comparing the phyAphyBcry1 mutant with the phyBcry1 mutant, we observed that phyA had little effect on white-light-mediated cotyledon expansion in the absence of phyB and cry1. In the presence of either phyB or cry1, phyA had the opposite effect. The phyA mutation enhanced the phyB phenotype, since the double mutant was 38% smaller than phyB. In contrast, the phyA mutation partially suppressed cry1 defects, since the removal of phyA caused a 1.5-fold increase in cotyledon area in a cry1 mutant background.
The inhibition of hypocotyl growth in white light was driven primarily by phyB and cry1, with both single mutants being approximately 2.4-fold longer than the wild type (Table I, column 5). The phyA single mutant had only a 1.2-fold longer hypocotyl than the wild type. Therefore, as noted previously (Reed et al., 1994), the role of phyA in the hypocotyl-growth response in white light was uncovered only in the double and triple mutant combinations. As with the cotyledon-growth response, the phyA mutation enhanced phyB (phyAphyB 1.4-fold longer than phyB) and partially suppressed cry1 (phyAcry1 19% shorter than cry1).
The same trends were observed for chlorophyll accumulation in white light (Table I, column 6). The phyB and cry1 mutants both accumulated approximately 75% of wild-type chlorophyll levels, whereas the phyA mutant had normal levels. As was seen with the growth responses of both the hypocotyl and the cotyledon, the phyA mutation partially suppressed cry1, since the phyAcry1 double mutant accumulated 87% of wild-type chlorophyll levels. This relationship between phyA and cry1 was dependent on phyB, since the phyAphyBcry1 triple mutant was more severe than any of the other mutant combinations (25% of wild type). Anthocyanin accumulation in white light required at least two functional photoreceptors (Table I, column 7). Although there was no significant difference between the wild type and the single-photoreceptor mutants, the double and triple mutants accumulated no detectable anthocyanins, indicating a partially redundant role between these three photoreceptors.
phyA Is Epistatic to phyB and cry1 in Far-Red Light
Because our white light contained red, far-red, and blue light, we chose to examine the growth responses of each of the mutant combinations in monochromatic light. Our results confirm and reinforce that all of the growth responses in far-red light were governed primarily by phyA (Fig. 1B; Table II). For most growth responses measured, every mutant combination lacking phyA showed the same growth response as the phyA single mutant. One exception to this generalization was cotyledon unhooking (Table II, column 2). Although the phyB mutant had no aberrant unhooking response in far-red light, phyB clearly had some activity in a phyA mutant background, since the phyAphyB double mutant and the phyAphyBcry1 triple mutant each conferred 18% to 27% less unhooking than the phyA single mutant.
Table II.
Far-red-light-grown seedlings
| Genotype | Angle
|
Cotyledon Area | Hypocotyl Length | Anthocyanin Content | |
|---|---|---|---|---|---|
| Hook | Cotyledon | ||||
| degrees | mm2 | mm | (A530 − A657) × 1000 seedling−1 | ||
| La-er | 171 (1.2) | 131 (5.2) | 0.4 (0.2) | 2.3 (0.1) | 11.2 (1.3) |
| phyA | 153 (2.2) | 27 (4.7) | 0.2 (0.005) | 8.8 (0.3) | N.D.a |
| phyB | 170 (1.4) | 141 (7.0) | 0.5 (0.02) | 2.3 (0.1) | 12.1 (1.0) |
| cry1 | 168 (1.8) | 140 (3.6) | 0.5 (0.02) | 2.4 (0.07) | 12.2 (1.8) |
| phyAcry1 | 151 (4.1) | 22 (3.8) | 0.2 (0.004) | 9.5 (0.2) | N.D. |
| phyBcry1 | 166 (1.7) | 129 (6.4) | 0.5 (0.02) | 2.5 (0.08) | 7.4 (0.5) |
| phyAphyB | 125 (7.8) | 14 (4.2) | 0.1 (0.005) | 9.9 (0.2) | N.D. |
| phyAphyBcry1 | 111 (10.4) | 17 (2.9) | 0.1 (0.006) | 10.1 (0.2) | N.D. |
| La-er (dark) | 132 (4.4) | 15 (1.8) | 0.2 (0.007) | 9.7 (0.6) | N.D. |
Values in parentheses are se.
N.D., Not detected.
phyA was the major photoreceptor for the induction of anthocyanin accumulation in far-red light, since all mutant combinations lacking phyA had no detectable anthocyanin under these conditions. phyB and cry1 mutants accumulated anthocyanin to normal levels, yet the phyBcry1 double mutant accumulated 66% of the wild-type levels of anthocyanin, indicating that phyB and cry1 influence anthocyanin accumulation in far-red light in a redundant manner. Chlorophyll accumulation was very low in far-red light and just above the noise level for all genotypes in our experiments. Therefore, we did not include these measurements in Table II.
phyB Is the Major Photoreceptor Involved in De-etiolation in Red Light
De-etiolation in red light was driven primarily by phyB, although some responses also involved the other two photoreceptors (Fig. 1C; Table III). Cotyledon unhooking was driven by phyA and phyB in a fully redundant manner, since all single mutants behaved in the same manner as the wild type and only the phyAphyB double mutant unhooked 42% less than the wild type (Table III, column 2). In contrast, cotyledon unfolding was controlled by phyB, with a 61% reduction in the phyB mutant (Table III, column 3). However, there was clearly some activity of phyA and cry1 in the unfolding response, since removal of either phyA or cry1 in a phyB mutant background conferred a more dramatic mutant phenotype (14% and 22% of the wild type, respectively). Cotyledon expansion was driven almost entirely by phyB, since all mutant combinations lacking phyB had a similar phenotype to the phyB single mutant, with 20% of the wild-type cotyledon-growth response (Table III, column 4). Hypocotyl growth inhibition was also driven primarily by phyB, since all mutant combinations lacking phyB had hypocotyls approximately 1.5-fold longer than the wild type (Table III, column 5).
Table III.
Red-light-grown seedlings
| Genotype | Angle
|
Cotyledon Area | Hypocotyl Length | Chlorophyll Content | |
|---|---|---|---|---|---|
| Hook | Cotyledon | ||||
| degrees | mm2 | mm | μg seedling−1 | ||
| La-er | 172 (0.8) | 196 (7.0) | 1.5 (0.04) | 8.7 (0.2) | 0.54 (0.04) |
| phyA | 169 (1.4) | 186 (6.9) | 1.4 (0.04) | 8.5 (0.3) | 0.5 (0.04) |
| phyB | 157 (4.1) | 76 (5.6) | 0.3 (0.01) | 14.2 (0.5) | 0.39 (0.03) |
| cry1 | 168 (1.6) | 201 (5.6) | 1.4 (0.05) | 9.5 (0.2) | 0.49 (0.03) |
| phyAcry1 | 169 (1.6) | 230 (4.1) | 1.3 (0.04) | 7.0 (0.2) | 0.49 (0.03) |
| phyBcry1 | 152 (2.9) | 44 (4.6) | 0.3 (0.008) | 13.2 (0.3) | 0.25 (0.03) |
| phyAphyB | 99 (5.7) | 28 (2.7) | 0.2 (0.006) | 15.6 (0.3) | 0.14 (0.02) |
| phyAphyBcry1 | 123 (5.8) | 37 (4.5) | 0.2 (0.004) | 13.3 (0.4) | 0.17 (0.02) |
| La-er (dark) | 132 (4.4) | 15 (1.8) | 0.2 (0.007) | 9.7 (0.6) | N.D.a |
Values in parentheses are se.
N.D., Not detected.
Chlorophyll measurements demonstrated a complex relationship between phyB and the other two photoreceptors (Table III, column 6). The phyB mutant was approximately 25% less green than the wild type or either of the other single mutants. However, the removal of either cry1 or phyA from a phyB mutant background reduced chlorophyll levels by 36% or 64%, respectively. phyA had a strong effect on phyB-mediated greening in red light, since removal of phyA from a phyB mutant (64% reduction) or a phyBcry1 mutant (32% reduction) had a dramatic effect on chlorophyll levels. In comparison, removal of cry1 from a phyB mutant reduced chlorophyll levels by 36%, yet removal of cry1 from a phyAphyB double-mutant background had no effect. Anthocyanin accumulation was very low in red light and for all genotypes in our experiments, so these measurements were not included in Table III.
phyA, phyB, and cry1 Are All Involved in the De-etiolation Response in Blue Light
phyA, phyB, and cry1 acted in a fully redundant manner with respect to cotyledon unhooking in blue light, since only the phyAphyBcry1 triple mutant was similar to the wild-type phenotype in the dark, having approximately 74% of the wild-type unhooking response in the light (Fig. 1D; Table IV, column 2). In contrast to unhooking, cotyledon unfolding was controlled mainly by cry1, since only the cry1 single mutant had a 24% reduced unfolding response compared with the wild type (Table IV, column 3). phyA and phyB influenced the blue-light-induced cotyledon-unfolding growth response in a redundant manner, since the phyAphyBcry1 triple mutant had a phenotype similar to dark-grown plants (8.5% of wild type) and was not seen in either the cry1 single mutant or the phyAcry1 and phyBcry1 double mutants.
Table IV.
Blue-light-grown seedlings
| Genotype | Angle
|
Cotyledon Area | Hypocotyl Length | Chlorophyll Content | Anthocyanin Content | |
|---|---|---|---|---|---|---|
| Hook | Cotyledon | |||||
| degrees | mm2 | mm | μg seedling−1 | (A530 − A657) × 1000 seedling−1 | ||
| La-er | 169 (1.7) | 176 (4.7) | 1.9 (0.08) | 3.1 (0.09) | 0.54 (0.03) | 3.2 (0.2) |
| phyA | 168 (1.6) | 201 (6.6) | 1.2 (0.05) | 5.5 (0.1) | 0.45 (0.04) | N.D.a |
| phyB | 172 (1.8) | 248 (5.0) | 1.2 (0.05) | 3.9 (0.09) | 0.57 (0.04) | 3.2 (0.2) |
| cry1 | 168 (1.6) | 134 (5.1) | 0.6 (0.03) | 8.8 (0.1) | 0.37 (0.03) | N.D. |
| phyAcry1 | 164 (2.4) | 128 (6.6) | 0.3 (0.01) | 11.7 (0.2) | 0.19 (0.03) | N.D. |
| phyBcry1 | 169 (1.6) | 119 (6.0) | 0.5 (0.04) | 11.6 (0.2) | 0.25 (0.02) | N.D. |
| phyAphyB | 172 (1.4) | 197 (8.2) | 0.7 (0.04) | 8.0 (0.1) | 0.29 (0.02) | N.D. |
| phyAphyBcry1 | 125 (4.8) | 15 (2.6) | 0.2 (0.004) | 13.9 (0.5) | 0.06 (0.01) | N.D. |
| La-er (dark) | 132 (4.4) | 15 (1.8) | 0.2 (0.007) | 9.7 (0.6) | N.D. | N.D. |
Values in parentheses are se.
N.D., Not detected.
Cotyledon expansion in blue light was influenced by phyA, phyB, and cry1 (Table IV, column 4). The independent activity of each photoreceptor was demonstrated by the observation that each of the single mutants had smaller cotyledons than the wild type (phyA, 63% of wild type; phyB, 63%; hy4, 32%). Furthermore, the triple mutant had smaller cotyledons (10% of wild type) than each of the double mutant combinations, demonstrating activity of each photoreceptor in the absence of the other two. phyB had a small effect in the absence of cry1, since the phyBcry1 and phyAphyBcry1 mutants had only slightly smaller cotyledons compared with the cry1 or phyAcry1 mutants, respectively. The same relationship was not seen between phyA and cry1.
phyA and cry1 were the major photoreceptors involved in the hypocotyl-growth response in blue light, since the phyA and cry1 mutant hypocotyls were 1.8- and 2.8-fold longer than the wild type, respectively (Table IV, column 5). This increased length of the phyA mutant in blue light has been reported previously (Whitelam et al., 1993). Regardless of the state of cry1 or phyA, the removal of phyB caused a 1.2- to 1.4-fold increase in hypocotyl length. cry1 had activity in the absence of phyA and phyB, since the triple mutant had a 1.7-fold longer hypocotyl than the phyAphyB double mutant.
cry1 was the major photoreceptor involved in chlorophyll accumulation in blue light, since the only single mutant to have significantly less chlorophyll (68% of wild type) was cry1 (Table IV, column 6). The phyAcry1 double mutant had a more dramatic phenotype (35% of wild type), demonstrating that the phyA mutation can enhance this cry1 mutant growth response. The phyBcry1 double mutant had 46% of the wild-type chlorophyll levels, demonstrating that the phyB mutation can also enhance this cry1 defect. Interaction between phyA and phyB was also observed, since the phyAphyB double mutant (and not the single mutants) had 46% less chlorophyll than the wild type. cry1 had activity in the phyAphyB double mutant background, since the triple mutant had only 11% of the wild-type chlorophyll content. Anthocyanin measurements demonstrated that phyA and cry1 were both necessary for anthocyanin production, since the loss of either of these photoreceptors prevented the accumulation of this pigment (Table IV, column 7).
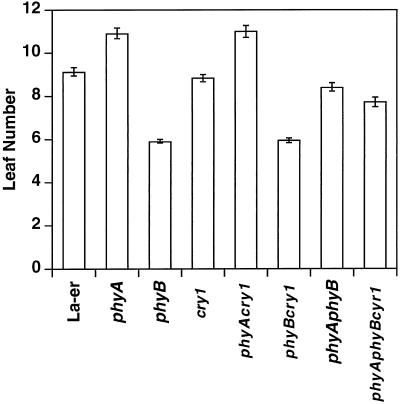
phyA and phyB Affect Flowering in Long-Day Conditions
Analysis of seedling morphology in both broad-spectrum white light and monochromatic lights demonstrated complex and varying interactions between the three photoreceptors phyA, phyB, and cry1. To test the possibility that these photoreceptors interact during the later stages of plant development, we chose to examine the flowering response of these mutants under long-day conditions (Fig. 2). phyA accelerated flowering, since the phyA mutant flowered with two more leaves than the wild type. In contrast, phyB delayed flowering, since the phyB mutant flowered with three fewer leaves than the wild type. The phyAphyB double mutant was intermediate between the single mutants, demonstrating additivity or antagonism between these photoreceptors. The same relationship between phyA and phyB was shown in a cry1 mutant background; cry1 had little effect on flowering in long-day conditions, since the presence or absence of this photoreceptor had no significant effect on the roles of phyA and phyB in this growth response.
Figure 2.
Flowering time of the wild type and mutant combinations in a 16-h light/8-h dark regime. Leaf number refers to both cauline and rosette leaves on the primary inflorescence stem. Error bars indicate ± se.
DISCUSSION
phyA, phyB, and cry1 Drive Cotyledon Unhooking and Expansion in White Light
The white-light mediated deetiolation responses of the wild type and single, double, and triple mutant combinations of phyA, phyB, and cry1 demonstrate the complex interplay that these three photoreceptors have during seedling development (Table I). Although these growth responses suggest interaction between photoreceptors and/or shared partners in the signaling pathways, it is difficult to interpret these results, since broad-spectrum white light delivers a group of signals capable of activating multiple photoreceptors.
Comparing the growth of the phyAphyBcry1 triple mutant in white light with that of dark- and light-grown wild-type seedlings can determine whether a growth response is driven entirely by these three photoreceptors or if other photoreceptors are involved. Cotyledon unhooking (Table I, column 2) and expansion in the triple mutant (Table I, column 4) is the same as that in wild-type dark-grown plants, demonstrating that this growth response is completely accounted for by the activity of these three photoreceptors. Hypocotyls of white-light-grown phyBcry1 and phyAphyBcry1 are significantly longer than those of dark-grown seedlings, indicating that the hypocotyl is physiologically capable of longer autotrophic growth than the heterotrophic growth seen in the dark (Table I, column 5). Indeed, phyBcry1 and phyAphyBcry1 are the only mutant combinations to continue to elongate after 10 d of growth (data not shown). That the hypocotyls of these mutants grow longer in the light than those of dark-grown plants can be explained by two distinct developmental pathways for hypocotyl growth. These two pathways are genetically separated by the Arabidopsis prc1 mutant (Desnos et al., 1996), and have also been described as a phytochrome-mediated response in wild-type plants (Gendreau et al., 1997, 1998).
Interactions between Photoreceptors in Monochromatic Light
To address the interactions of phyA, phyB, and cry1 signaling pathways, the deetiolation responses were measured after exposure to monochromatic red, far-red, or blue light. In some cases a single photoreceptor is implicated in a growth response (e.g. phyA and cotyledon expansion in far-red light), indicating a simple signal transduction pathway. In other cases, multiple photoreceptors contribute to a growth response in an additive manner (e.g. hypocotyl-growth inhibition in blue light), suggesting convergence of these pathways downstream of the signal. However, additive interactions do not rule out the possibility of parallel, completely independent pathways affecting the growth response through different mechanisms.
Certain mutant combinations and responses suggest direct genetic interactions between multiple photoreceptor pathways. The strongest correlations are presented in Tables V and VI and are enumerated for each light condition below.
Table V.
Redundant interactions between phyA, phyB, and cry1 signal transduction pathways
| Photoreceptor | Light | Response |
|---|---|---|
| phyA (phyB)a | Far-red | Cotyledon unhooking |
| phyA, phyB | Red | Cotyledon unhooking |
| phyA, phyB, cry1 | Blue | Cotyledon unhooking |
Partial redundancy.
Table VI.
Effector/modulator interactions between phyA, phyB, and cry1 signal transduction pathways
| Effector | Modulator | Light | Response |
|---|---|---|---|
| phyA | phyB and cry1 redundantly | Far-red | Anthocyanin accumulation |
| phyB (phyA)a | cry1 | Red | Chlorophyll accumulation |
| cry1 | phyB | Blue | Cotyledon expansion |
Partial redundancy.
In far-red light, two growth responses may involve the co-action of other photoreceptors with phyA (Table II). phyB is partially redundant with phyA for cotyledon unhooking. In contrast, phyB and cry1 act as redundant enhancers of phyA-mediated anthocyanin accumulation, since the phyBcry1 double mutant accumulated significantly less anthocyanin than the wild type in far-red light. Therefore, we placed phyA as the effector proper, with phyB and cry1 as redundant modulators in the model of interaction between photoreceptor systems put forth by Mohr (1994) (Table VI). It is surprising that phyB and cry1 can act as redundant modulators of a far-red-light-driven, phyA-mediated growth response. Although there may be a small pool of active phyB (Pfr) in far-red light, cry1, which is not activated by far-red light, was also capable of eliciting the response in the absence of phyB.
In red light two responses suggest an interaction between multiple photoreceptor signal transduction pathways (Table III). phyA and phyB act in a completely redundant manner in cotyledon unhooking. In a more complex relationship, cry1 enhances both phyB's strong effect and phyA's partially redundant effect on chlorophyll accumulation in red light. Because no cry1 activity is detected in a phyAphyB double mutant, we considered cry1 to be the modulator of this response, affected primarily by phyB and secondarily by phyA (Table VI).
By comparing the blue-light-grown triple mutant with dark-grown plants, it was shown that these three photoreceptors can account for all of the cotyledon unhooking, unfolding, and expansion in our experimental conditions. Three growth responses demonstrate interactions between the photoreceptor systems in blue light (Table IV). In cotyledon unhooking, phyA, phyB, and cry1 play completely redundant roles. In cotyledon unfolding and chlorophyll accumulation, there was no phyA or phyB mutant phenotype, although their activity could be seen in a background lacking cry1, suggesting an interplay between phyA and phyB in this growth response. In cotyledon expansion all three photoreceptor single mutants have smaller cotyledons than the wild type. Comparison of double mutants with single mutants or the triple mutant shows cry1 and phyA activity in the absence of the other photoreceptors. However, there is little to no activity of phyB in the absence of cry1, demonstrating an interaction between these two photoreceptors. Therefore, we consider cry1 to be the effector and phyB the modulator of cotyledon expansion in blue light (Table VI). In contrast to cotyledon growth, there is clear additivity of hypocotyl inhibition by blue light between the three null mutations, demonstrating activity for each of these photoreceptors in the absence of the other two.
Phytochrome as a Blue-Light Receptor
Ahmad and Cashmore (1997) put forth a model stating that cry1 activity requires functional phyA or phyB in blue light. The reasoning behind this model is that the phyAphyB double mutant has a blue-light mutant phenotype similar to a mutant lacking cry1 (cry1), whereas the phyA and phyB single mutants behave the same as the wild type. This model predicts that there would be no cry1 activity in a phyAphyB double mutant. Our cotyledon and hypocotyl measurements in blue light demonstrate that cry1 has significant activity in a phyAphyB double-mutant background. This activity of cry1 is also seen in cotyledon unhooking, unfolding, expansion, and chlorophyll accumulation. cry1 activity in the phyAphyB double mutant has also been demonstrated (J.J. Casal and A. Mazzella, personal communication).
One explanation for the blue-light phenotype seen in the phyAphyB double-mutant background is that phyA and phyB act directly as blue-light photoreceptors. Although Ahmad and Cashmore (1997) observed a wild-type blue-light phenotype in these phytochrome mutants, we have clearly shown that phyA, and to a lesser extent phyB, have longer hypocotyls in blue light, and that phyA and phyB cotyledons are smaller in blue light. The phyB cotyledon phenotype in blue light has been described previously (Neff and Van Volkenburgh, 1994). phyA mutants have been shown to have a blue-light mutant defect with respect to hypocotyl-growth inhibition (Whitelam et al., 1993) and CAB gene induction (Hamazato et al., 1997). In agreement with the aberrant CAB gene induction in phyA, we observed less chlorophyll accumulation in this mutant grown in blue light (Table IV, column 6). Further support for blue light driving phytochrome responses is shown in action spectra examining the roles of phyA and phyB in seed germination in Arabidopsis (Shinomura et al., 1996).
Interaction between Cryptochrome and Phytochrome Pathways
Based on the observations leading to their model, Ahmad and Cashmore (1993) suggested that cry1 shares a common reaction intermediate with phytochromes. Although our data do not support their model for hypocotyl inhibition, they do not rule out the possibility of shared intermediates between the signal transduction pathways mediated by these photoreceptors. Furthermore, we have demonstrated that phyB's effect on cotyledon expansion in blue light requires functional cry1, strongly suggesting an interaction between these two photoreceptor systems during this growth response.
Many growth responses are driven by multiple photoreceptors in an additive manner. In other cases, the contribution of some photoreceptors could only be identified in the absence of others. These relationships could be the result of parallel pathways or interactions between pathways. Because the experiments presented in this paper do not distinguish between these two possibilities, we have not included them in Tables V and VI. By examining these mutants in dichromatic light, such as blue added to a background of red, one may be able to distinguish between interacting and parallel signal transduction pathways.
Without knowing the early intermediates in the photoreceptor signaling pathways, it is difficult to speculate on the mechanism of interaction between these photoreceptor systems. The results of two studies may shed light on possible interactions between these systems. Arabidopsis phyB and phyA contain weak nuclear-localization sequences, with phyB showing light-dependent nuclear localization (Sakamoto and Nagatani, 1996). Therefore, it is possible that phyB and phyA induce light-regulated gene expression by interacting with transcription factors after this nuclear localization. This possibility is further supported by the presence of two periodic-arnt-sim domains, which are thought to be involved in protein-protein interactions with a class of basic helix-loop-helix transcription factors, in the C-terminal half of these phytochromes (Lagarias et al., 1995). cry1 could facilitate partitioning of phytochrome or one of its interacting partners. In addition, recent analysis of a cyanobacterial phytochrome showed it to be a light-dependent protein kinase (Yeh et al., 1997), suggesting that phosphorylation of early signaling components, some of which may be common to multiple phytochromes, is involved.
Given the complexity of the interactions between phyA, phyB, and cry1, it is difficult to describe the interplay between these pathways with a simple linear model. The interplay may be more accurately described as a web of interactions. The janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways in mammals and Drosophila illustrate a method of integrating multiple signals and pathways into a coordinated activation of specific genes. The JAK/STAT family of signaling molecules is involved in specific responses to a broad family of receptors, the cytokine receptors. Cytokine receptors have an extracellular, ligand-binding domain and cytoplasmic JAK/STAT-interacting domains. Upon ligand binding, cytokine receptors dimerize, allowing the activation of cytokine-specific JAKs, which then phosphorylate Tyr residues in the receptor. Specific STATs are then phosphorylated by both the cytokine receptor and the JAK, leading to STAT dimerization, nuclear localization, and transcriptional activation of target genes. The STATs are capable of either hetero- or homodimerization, allowing coordination of multiple cytokine-mediated signaling pathways into a single growth response (for reviews, see Ihle, 1996; Winston and Hunter, 1996).
It is possible that phytochromes and cryptochromes have a similar method of coordinating their signal transduction pathways. Early components in each of these pathways may be photoreceptor specific (similar to cytokine receptor/JAK interactions). Later steps in signal transduction may be specific or capable of interacting with other pathways by forming heterodimers or transcriptional activating complexes in a manner similar to the STATs. JAK/STAT pathways have not been identified in plants; however, their ability to coordinate multiple signals demonstrates a possible mechanism for interactions between different photoreceptor systems. The cloning of specific or shared phytochrome and cryptochrome signal transduction components will allow us to test their similarity with pathways that have been well described in other organisms.
ACKNOWLEDGMENTS
We thank Jorge Casal and Agustine Mazzella for sharing and discussing data before publication. We thank Christian Fankhauser for critical reading of the manuscript and the members of the Chory lab for helpful discussions.
Abbreviations:
- CAB
chlorophyll a/b-binding protein
- cry1
cryptochrome 1
- phyA
phytochrome A
- phyB
phytochrome B
Footnotes
This work was supported by a grant from the National Institutes of Health (no. RO1GM52413 to J.C.). J.C. is an Associate Investigator of the Howard Hughes Medical Institute. M.M.N. was supported by National Research Service Award postdoctoral fellowship no. GM17577.
LITERATURE CITED
- Ahmad M, Cashmore AR. HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. Plant J. 1997;11:421–427. doi: 10.1046/j.1365-313x.1997.11030421.x. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Boccalandro H. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta. 1995;197:213–218. doi: 10.1007/BF00202639. [DOI] [PubMed] [Google Scholar]
- Desnos T, Orbovic V, Bellini C, Kronenberger J, Caboche M, Traas J, Höfte H. Procuste1 mutants identify two distinct genetic pathways controlling hypocotyl cell elongation, respectively in dark- and light-grown Arabidopsis seedlings. Development. 1996;122:683–693. doi: 10.1242/dev.122.2.683. [DOI] [PubMed] [Google Scholar]
- Drumm H, Mohr H. The mode of interaction between blue (UV) light photoreceptor and phytochrome in anthocyanin formation of Sorghum seedling. Photochem Photobiol. 1978;27:241–248. [Google Scholar]
- Drumm-Herrel H, Mohr H. A novel effect of UV-B in a higher plant. Photochem Photobiol. 1981;33:391–398. [Google Scholar]
- Fankhauser C, Chory J (1997) Light control of plant development. In JA Spudich, J Gerhart, SL McKnight, R Schekman, eds, Annual Review of Cell and Developmental Biology, Vol 13. Annual Reviews, Palo Alto, CA, pp 203–229 [DOI] [PubMed]
- Gendreau E, Höfte H, Grandjean O, Brown S, Traas J. Phytochrome controls the number of endoreduplication cycles in the Arabidopsis thaliana hypocotyl. Plant J. 1998;13:221–230. doi: 10.1046/j.1365-313x.1998.00030.x. [DOI] [PubMed] [Google Scholar]
- Gendreau E, Traas J, Desnos T, Grandjean O, Caboche M, Höfte H. Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 1997;114:295–305. doi: 10.1104/pp.114.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazato F, Shinomura T, Hanzawa H, Chory J, Furuya M. Fluence and wavelength requirements for Arabidopsis CAB gene induction by different phytochromes. Plant Physiol. 1997;115:1533–1540. doi: 10.1104/pp.115.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle JN. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Rolff E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Lagarias DM, Wu S-H, Lagarias JC. Atypical phytochrome gene structure in the green alga Mesotaenium caldariorum. Plant Mol Biol. 1995;29:1127–1142. doi: 10.1007/BF00020457. [DOI] [PubMed] [Google Scholar]
- Mohr H. Coaction between pigment systems. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 353–373. [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Neff JD, Chory J, Pepper AE (1998) dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J (in press) [DOI] [PubMed]
- Neff MM, Van Volkenburgh E. Light-stimulated cotyledon expansion in Arabidopsis seedlings. The role of phytochrome B. Plant Physiol. 1994;104:1027–1032. doi: 10.1104/pp.104.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmüller R, Mohr H. Responsivity amplification by light in phytochrome-mediated induction of chloroplast glyceraldehyde-3-phosphate dehydrogenase (NADP-dependent, EC 1.2.1.1.13) in the shoot of milo (Sorghum vulgare Pers) Plant Cell Environ. 1984;7:29–37. [Google Scholar]
- Oelmüller R, Mohr H. Mode of coaction between blue/UV light and light absorbed by phytochrome in light-mediated anthocyanin formation in milo (Sorghum vulgare Pers) Proc Natl Acad Sci USA. 1985a;82:6124–6128. doi: 10.1073/pnas.82.18.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmüller R, Mohr H. Specific action of blue light on phytochrome-mediated enzyme synthesis in the shoot of milo (Sorghum vulgare Pers) Plant Cell Environ. 1985b;8:27–31. [Google Scholar]
- Parks BM, Quail PH. hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. Phytochromes: photosensory perception and signal transduction. Science. 1995;268:675–680. doi: 10.1126/science.7732376. [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Nagatani A. Nuclear localization activity of phytochrome B. Plant J. 1996;10:859–868. doi: 10.1046/j.1365-313x.1996.10050859.x. [DOI] [PubMed] [Google Scholar]
- Shinomura T, Nagatani A, Hanzawa H, Kubota M, Watanabe M, Furuya M. USA. 1996;93:8129–8133. doi: 10.1073/pnas.93.15.8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire W, Mohr H. Gradient formation of anthocyanin in seedlings of Fagopyrum and Sinapis unilaterally exposed to red and far-red light. Photochem Photobiol. 1970;12:145–149. doi: 10.1111/j.1751-1097.1970.tb06046.x. [DOI] [PubMed] [Google Scholar]
- Valvekens D, Van Montagu M, Van Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston LA, Hunter T. Intracellular signaling: putting JAKS on the kinase MAP. Curr Biol. 1996;6:668–671. doi: 10.1016/s0960-9822(09)00445-x. [DOI] [PubMed] [Google Scholar]
- Woitzik F, Mohr H. Control of hypocotyl phototropism by phytochrome in a dicotyledonous seedling (Sesamum indicum L.) Plant Cell Environ. 1988;11:653–661. [Google Scholar]
- Yeh K-C, Wu S-H, Murphy JT, Lagarias JC. A cyanobacterial two-component light sensory system. Science. 1997;227:1505–1508. doi: 10.1126/science.277.5331.1505. [DOI] [PubMed] [Google Scholar]