Abstract
A 7-Å cryoelectron microscopy-based reconstruction of Sindbis virus (SINV) was recently generated. Fitting the crystal structure of the SINV capsid protein (Cp) into the density map revealed that the F2-G2 loop of the Cp was shifted away from cytoplasmic domain of E2 (cdE2) in the 7-Å reconstruction relative to its position in the Cp crystal structure. Furthermore, the reconstruction demonstrated that residue E395 in region I of the cytoplasmic domain of the E2 envelope protein (cdE2-RI) and K252 of Cp, part of the Cp F2-G2 loop, formed a putative salt bridge in the virion. We generated amino acid substitutions at residues K250 and K252 of the SINV Cp and explored the resulting phenotypes. In the context of cells infected with wild-type or mutant virus, reversing the charge of these two residues resulted in the appearance of Cp aggregates around cytopathic vacuole type I (CPV-I) structures, the absence of nucleocapsid (NC) formation, and a lack of virus particle release in the infected mammalian cell. However, expressing the same Cp mutants in the cell without the envelope proteins or expressing and purifying the mutants from an Escherichia coli expression system and assembling in vitro yielded NC assembly in all cases. In addition, second-site mutations within cdE2 restored NC assembly but not release of infectious particles. Our data suggest an early temporal and spatial interaction between cdE2-RI and the Cp F2-G2 loop that, when ablated, leads to the absence of NC assembly. This interaction also appears to be important for budding of virus particles.
INTRODUCTION
The alphaviruses are a group of enveloped, positive-sense, single-stranded RNA viruses in the Togaviridae family. Well-studied members include the Eastern, Western, and Venezuelan equine encephalitis viruses, Semliki Forest virus, Ross River virus, Chikungunya virus, and the type member, Sindbis virus (SINV) (42). The emergence of Chikungunya virus as an epidemic threat has reemphasized the medical importance of the alphaviruses (47).
The ∼11.5-kb genome encodes four nonstructural proteins that primarily facilitate genome replication and four structural proteins, capsid, pE2, 6K, and E1, which are initially translated from a subgenomic message as a polyprotein (42). Co- and posttranslational processing by viral and host proteases yields the mature structural proteins. The capsid protein (Cp) is an autoprotease that acts to cotranslationally cleave itself from the growing polypeptide (30) and later associates with viral genomic RNA to form a nucleocapsid (NC) core. The remaining polyprotein inserts into the endoplasmic reticulum (ER) in a signal sequence-mediated manner (2). Signalase processing yields pE2 and E1, the envelope proteins, and 6K, a small membrane-resident protein whose function in the virus life cycle remains unclear (26). pE2 is later cleaved by furin to yield the small protein E3 and the larger protein E2 (52). E1 and E2 are type I integral membrane proteins, with E2 primarily responsible for host receptor engagement (3, 24), while E1 contains pH-dependent fusogenic properties required for virus entry (14, 50). The ectodomains of both E1 and E2 comprise the bulk of each protein, although both E1 and E2 contain some residues, termed cytoplasmic domains, which are found on the inner face of the envelope and, at least in the case of E2, interact with the NC (15, 17, 28).
The icosahedrally arranged virus particle, released by budding from the host plasma membrane (PM), is comprised of two protein shells (4). In the outer shell, 240 copies each of E1 and E2, arranged as 80 trimers of E1-E2 heterodimers, are embedded in a plasma membrane-derived lipid bilayer (33). The membrane layer also contains substoichiometric amounts of 6K and surrounds the NC core. The NC is comprised of 240 copies of Cp and one copy of genomic RNA and also demonstrates icosahedral symmetry (4). The icosahedral symmetry of the NC may derive from the 1:1 protein-protein interaction between each molecule of Cp in the NC and a molecule of E2 in the envelope, as the icosahedral arrangement of prebudded, cytoplasmic NCs remains loosely defined (4, 12, 32).
The alphaviruses have long proven a model system for structural studies of enveloped mammalian viruses owing, in part, to the T=4 icosahedral arrangement of both the envelope and the NC core of budded virus particles. Genetic and structural evidence suggests that a hydrophobic pocket on the face of the Cp interacts with the cytoplasmic domain of E2 (cdE2) in the context of a budded virion (36, 38, 44). Specifically, a conserved YxL motif in cdE2 is involved in side-chain interactions with residues resident in the Cp hydrophobic pocket, and other evidence suggests interactions of C-terminal cdE2 residues, relative to the YxL domain, with Cp (23, 25, 31, 36, 51). Thus, Cp and E2 interact during budding, and this interaction is required for budding to occur (43, 53). However, details regarding the arrangements of, and the interactions between, the Cp and the E1 and E2 envelope proteins prior to budding events remain unclear.
It is thought that the trimer of E1-E2 heterodimers, often referred to as spikes, forms a two-dimensional (2D) crystalline array at the plasma membrane and probably already arrives at the plasma membrane in a spike-like conformation (20, 41, 49). However, the site of the initial E1-E2 heterodimerization, as well as the subcellular compartment in which trimerization occurs, is not clear, although these processes likely occur soon after translation (34). Likewise, a detailed understanding of the NC assembly pathway is not available. While Cp homodimers are thought to be key assembly intermediates, and charge neutralization between genomic RNA and the basic Cp likely provides the source for the initial assembly nucleation, no NC assembly intermediates have been identified by either biophysical or microscopy-based techniques (27, 46). Additionally, the site of NC assembly is unclear, although NC may assemble at or near the site of viral genome replication (11, 40). Interestingly, Cp mutants have been described in which NC formation in the cytoplasm is disrupted but in which wild-type (wt)-like virus particles are nevertheless budded from the cell (9).
While it has long been established that NCs and envelope protein spikes interact during budding at the plasma membrane, emerging data suggest that the interaction of NC and envelope proteins may occur before either arrives at the plasma membrane, in the form of membranous virus-induced type II cytopathic vacuoles (CPV-II) (16). These CPV-II molecules have been implicated in the traffic of envelope proteins to the plasma membrane and appear to be decorated with NCs (19, 41). The precise origin of CPV-II vacuoles and the temporal and spatial aspects of the initial interaction between NC and envelope proteins on these CPV-II structures have yet to be detailed. However, the cotrafficking of NC and E2 to the site of budding hints at interactions between Cp and the envelope proteins well before virus budding at the plasma membrane.
A 7-Å cryoelectron microscopy (cryo-EM)-based reconstruction of SINV virus demonstrated several novel molecular links between the E2 envelope protein and the NC in the context of a virus particle (44). Among these data, it was shown that a flexible loop on the edge of the hydrophobic pocket of Cp, the F2-G2 loop composed of amino acids 248-NSKGK-252, was shifted ∼3.5 Å away from cdE2 relative to the loop position in the SINV Cp crystal structure. Furthermore, a salt bridge between cdE2 E395 and Cp K252, part of the F2-G2 loop, was proposed. On the basis of those results and the observation that Cp residue K250 was implicated in cross-capsomere contacts (45), we performed a mutational analysis of Cp residues K250 and K252.
Simultaneous mutation of SINV Cp residues K250 and K252 led to production of Cp aggregates around cytopathic type I replication vacuoles (CPV-I) and the complete abrogation of NC formation in the cell concomitant with a lack of virus particle release. Surprisingly, though, when the mutant proteins were expressed and assembled in vitro (45), expressed in the absence of the envelope proteins, or expressed via a replicon or by a mammalian expression plasmid, formation of core-like particles (CLPs) was detected in all cases. Double SINV mutants were generated in which both cdE2-RI (cdE2 391-KARRE-395) and the Cp residues K250 and K252 were simultaneously mutated. These double mutants restored NC formation for the Cp K250D/K252D and K250E/K252E mutant backgrounds but did not restore the release of virions.
Taken together, our data suggest (i) that an early temporal and spatial interaction occurs between Cp and E2 in the infected cell, likely at or near the CPV-I structure, (ii) validation of the modeling performed in the SINV 7-Å reconstruction with regard to a functional interaction between cdE2-RI and the Cp F2-G2 loop, and (iii) a new set of cdE2:Cp amino acid interactions, involving the N-terminal region of cdE2, that are important for alphavirus assembly and budding.
MATERIALS AND METHODS
Cell culture.
BHK-15 cells obtained from the American Type Culture Collection were maintained in minimal essential medium (MEM) (Life Technologies) supplemented with 10% fetal bovine serum (FBS), except for enzyme-linked immunosorbent assay (ELISA) studies in which the FBS was omitted.
Generation of mutants in virus cDNA.
All mutants were generated in the pToto64 SINV cDNA plasmid using standard overlap PCR mutagenesis with Platinum Pfx DNA polymerase (Invitrogen). Infectious full-length RNA was produced by linearizing the plasmids with SacI followed by SP6-mediated in vitro transcription (Ambion). The Cp-expressing replicon was created in the pToto64 background by deleting the nucleotides corresponding to E3, E2, 6K, and E1 via overlap PCR mutagenesis. The first nine nucleotides of E3 were retained in addition to the stop codon utilized by E1. Plaque diameter analysis was performed by incubating BHK cells seeded in 6-well culture dishes with 1.5 ml of 0.2 mg/ml DEAE dextran–50 mM Tris (pH 7.4) at 37°C for 1 h, followed by incubation with in vitro-transcribed RNA at 25°C for 30 min and subsequent overlay with 1% agarose–MEM.
NC accumulation assays.
BHK cells were electroporated (1.5 kV, 25 μF, 200 Ω, 0.2-cm-gap cuvette) with 10 μg of in vitro-transcribed RNAs derived from the Toto64 cDNA clones. Infection was allowed to proceed for 8 h, at which time media were removed and cells washed 3 times with phosphate-buffered saline (PBS) and then scraped off the cell culture dish using 1 ml PBS. Cells were subjected to pellet formation for 10 min at 750 × g and resuspended in hypotonic buffer (10 mM NaCl, 10 mM Tris [pH 7.4], 20 mM EDTA) to swell for 20 min on ice. Triton X-100 (20%) was added to achieve a final concentration of 4% (vol/vol). Lysed cell debris and nuclei were subjected to pellet formation at 2,000 × g for 10 min. The solution was then loaded onto a continuous iodixanol gradient (0% to 30%) in TNE-Triton X-100 (50 mM Tris [pH 7.4], 100 mM NaCl, 1 mM EDTA, 0.1% Triton X-100) and centrifuged at 32,000 rpm in a Beckman SW-41 rotor for 2.5 h. All steps were carried out at 4°C. The gradient was fractionated, and each fraction was analyzed by SDS-PAGE on a 12% polyacrylamide gel. Separated proteins were electroeluted and adsorbed to 0.4-μm-pore-size nitrocellulose membranes before being probed with a rabbit polyclonal anti-Cp primary antibody and an IRDye 680 goat anti-rabbit secondary antibody conjugate (Li-COR). Stained membranes were scanned with a Li-COR Odyssey infrared imager. Cp intensities were measured using the Odyssey software and relative intensity values plotted as a function of fraction number.
In vitro NC assembly.
The wild-type or mutant Cp genes were subcloned into pET-11a prokaryotic expression vector (Novagen). Protein expression and purification and in vitro assembly of NCs were performed as described previously (45), using an arbitrary single-stranded DNA (ssDNA) 109-mer (5′-GATCCGAAAGCGCGCCGTGAGTGCCTGACGCCATACGCCCTGGCCCCAAACGCCGTAATCCCAGCGGCCGCTGCCGCCGCGTGCTGCGTTAGGTCGGCCAATGCTTAGC-3′).
Transient transfection and expression of SINV Cp or Cp plus envelope proteins.
The SINV sequences encoding the structural proteins (Cp, pE2, 6K, E1), or the sequences encoding just the Cp, were cloned into pcDNA4/TO/myc/his/A mammalian expression vector (Invitrogen). BHK cells were seeded into 60-mm-diameter culture dishes at ∼70% confluence and then transiently transfected with 10 μg of plasmid with Lipofectamine (Invitrogen) 18 h prior to lysis and gradient analysis as described above.
Multistep growth curve analysis.
BHK cells were infected with stock virus at a multiplicity of infection (MOI) of 0.5. Virus was allowed to adsorb to cells at 25°C for 1 h followed by incubation at 37°C. Media were removed and replaced with fresh media at 30-min intervals for the first 2 h and then at 1-h intervals thereafter. The amount of infectious virus release was quantified by titration on BHK cells.
Plaque assay.
Serial dilutions (10-fold) of culture media or virus were made in PBS containing 1% FBS plus 10 mM CaCl2 and 10 mM MgCl2. BHK cells at ∼90% confluence were inoculated with 250 μl of the dilutions for 1 h at room temperature and then overlaid with MEM containing 5% FBS and 1% agarose. Cells were incubated at 37°C for 48 h and stained with neutral red.
ELISA.
Posttransfection media (2 ml) were concentrated to 100 μl using Amicon 100-kDa centrifugal filters and applied to a 96-well Polysorp ELISA plate (Sigma) overnight at 4°C. The samples were blocked with 5% BSA–PBS for 2 h followed by three washes with PBS-T (PBS, 0.1% Tween 20). A 1:500 dilution of a monoclonal anti-E2 antibody (202) was applied to the sample for 2 h, followed by three washes with PBS-T. A 1:1,500 dilution of a horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibody was applied to the sample for 1 h, followed by three PBS-T washes. A TMB peroxidase detection system (KPL) was used to develop the ELISA according to the manufacturer's instructions. The optical density (OD) was determined at 450 nm.
Electron microscopy analyses.
BHK cells were electroporated with in vitro-transcribed RNAs or transfected (Lipofectamine 2000) with plasmids expressing the viral Cp or structural polyprotein. Cells were fixed and processed at 8 h postelectroporation of RNA or 18 h posttransfection of plasmid. Samples were fixed in 2% glutaraldehyde–cacodylate buffer (0.1 M sodium cacodylate, 2 mM MgCl2, 2 mM CaCl2, 0.5% NaCl, pH 7.4) and then washed in cacodylate buffer followed by water. Samples were then postfixed with 2% OsO4 and 1.5% K4Fe(CN)6 and then rinsed with water. Processing of cells for immunostaining omitted the osmium tetroxide and potassium ferrocyanide. Cells were subjected to pellet formation at 4,000 × g in 1% low-melt agarose. The pellet was dehydrated in sequential concentrations of ethanol from 10% to 100%. Pellets were then embedded in Durcupan resin and hardened at 60°C for 72 h. Sections (50 to 70 nm thick) were collected on carbon-coated copper mesh grids (carbon-coated gold mesh grids for immunostaining) using a diamond knife. Sections received a postembedding stain of 2% uranyl acetate and Sato's lead. Images were collected on a Philips CM-10 Biotwin transmission electron microscope (TEM) with film at 80 kV, a FEI CM200F cryoelectron microscope with a 1-k charge-coupled-device (CCD) camera at 200 kV (FEI Company, Hillsboro, OR), or an FEI Titan Krios TEM at 300 kV with a 4-k CCD camera. For immunostaining, sections were etched with 200 mM NaOH in ethanol and rinsed in ethanol and water followed by incubation in 0.005% Triton X–PBS for 10 min. Grids were then blocked with 2% bovine serum albumin–1% fish gelatin–PBS for 30 m, followed by exposure to anti-Cp antibody (1:150 in 10% blocking solution–PBS) for 1.5 h. Samples were then rinsed in PBS and blocked with 3% normal goat serum in PBS for 10 min followed by exposure of anti-rabbit antibody conjugated to 6-nm-diameter gold (EMS) (1:40 in PBS) for 1.5 h. Samples were rinsed in PBS and cross-linked with 2.5% glutaraldehyde for 5 min and then poststained with 2% uranyl acetate and Sato's lead.
RESULTS
Phenotype of the SINV Cp F2-G2 loop mutants.
It has previously been suggested that the SINV Cp F2-G2 loop is involved in a putative interaction with cdE2 (44). Furthermore, Cp K250 was proposed to mediate cross-capsomere contacts within the NC (46) and Cp K252 was postulated to participate in salt bridge contacts with cdE2. However, analysis of virus assembly phenotypes after mutation of these residues has not yet been described. Thus, we generated SINV mutants in which K250 and K252 were simultaneously mutated to alanine (K250/2A), aspartic acid (K250/2D), or glutamic acid (K250/2E) and a mutant in which the sequence encoding the tripeptide sequence 250-KGK-252 was deleted (ΔKGK).
After BHK cells were electroporated with in vitro-transcribed viral RNA, infectious virus was recovered from only the wild type and the K250/2A mutant; no infectious virus was recovered from the K250/2D, K250/2E, or ΔKGK mutant. Growth curve analysis of the wild type and the K250/2A mutant indicated that the K250/2A mutant released ∼1.5-log-fewer infectious units than the wild type throughout the 12-h time course (Fig. 1A).
Fig 1.

Analysis of infectious particle release. (A) Growth curve of the wild type and the K250/2A Sindbis capsid mutant. Infectious stocks of the wild type or the K250/2A mutant were used to inoculate a monolayer of BHK cells at a multiplicity of infection of 0.5. Media was collected at various time intervals postinoculation, and the amount of infectious particles released from the cells was determined via titration on BHK cells. (B) ELISA to detect total Sindbis particles released after electroporation of in vitro-transcribed viral RNA. BHK cells were electorporated with RNA, and the media were collected at 8 h posttransfection. In parallel, the titer was determined by plaque assay on BHK cells and the total amount of virions determined by ELISA. The mock sample was derived from BHK cells electroporated without RNA and processed as described above. n.p., no plaques; n/a, not applicable.
To ascertain if noninfectious particles were being released in the K250/2D, K250/2E, or ΔKGK backgrounds, we collected media 8 h after electroporation of in vitro-transcribed viral RNAs and assayed the amount of infectious particles in the media and the amount of total virus particles in parallel by plaque assay and ELISA, respectively (Fig. 1B). The K250/2A mutant showed a decrease in signal in the ELISA relative to the growth curve, indicating no modulation in specific infectivity in that mutant. The K250/2D, K250/2E, and ΔKGK mutants, however, failed to release any virus particles as gauged by the ELISA, indicating that, in these backgrounds, virus particle release is completely abrogated. Electroporating BHK cells with in vitro-transcribed RNA generated from wild-type or mutant cDNAs and growing the electroporated cells at 30°C, rather than 37°C, did not rescue infectious virus particle release for the K250/2D, K250/2E, and ΔKGK mutants (data not shown). Thus, when the lysines present at Cp positions 250 and 252 were replaced with a small, neutral, and nonpolar residue, release of infectious virus was attenuated. However, reversing the charge of these two lysines, as in the K250/2D and K250/2E mutants, or deleting the residues led to a complete disruption of virus particle release from cells.
Reversing the charge of Cp residues K250 and K252 leads to incomplete NC assembly in the mammalian cell.
Tang et al. (44) suggested that the Cp F2-G2 loop, which contains the K250 and K252 residues, might shift position when cdE2 inserts into the Cp hydrophobic pocket; thus, these Cp residues may be important for budding. Tellinghuisen and Kuhn suggested that Cp K250 may be involved in cross-capsomere contacts in the context of an assembled NC core (46). Thus, disruption of the lysine residues in the Cp F2-G2 loop could be expected to affect NC assembly or budding or both.
To determine if NC assembly was affected in the F2-G2 loop mutants, we performed a NC accumulation assay in BHK cells after electroporation of in vitro-transcribed viral RNAs in a fashion similar to that described previously (29). Briefly, 8 h postelectroporation, lysates of BHK cells were applied to a continuous rate-zonal gradient and centrifuged and the migration of Cp-containing complexes was determined by fractionation and densitometry after immunoblot analysis. With the exception of the ΔKGK mutant, equivalent amounts of total Cp were detected in the cell (data not shown). The migration of viral genomic RNA-containing complexes was also determined by fractionation and quantitative reverse transcriptase PCR (qRT-PCR).
As shown in Fig. 2, free Cp (45) and free in vitro-transcribed viral genomic RNA (assayed independently of each other) showed only slight sedimentation into the gradient. Lysates from cells transfected with the wild type and the K250/2A mutant demonstrated cosedimentation of Cp and viral genomic RNA to fraction 7, the position typically occupied by NC (data not shown). The K250/2D and K250/2E mutants, however, demonstrated sedimentation of the bulk of Cp- and genomic RNA-containing complexes to fractions 4 and 5, unlike the wild type, suggesting that, in these mutants, Cp-genomic RNA complexes are formed but are conformationally different from that of authentic NC. In addition to mock-electroporated cells, the ΔKGK mutant failed to generate full-length Cp in the cell (data not shown) and was not considered further. We unsuccessfully attempted to visualize the putative aggregates isolated from the K2502/2D or K250/2E gradient fractions by negative-stain electron microscopy.
Fig 2.
Rate-zonal gradient analysis of infected cell lysates. BHK cells were electroporated with in vitro-transcribed full-length RNA and hypotonically lysed at 8 h posttransfection. Clarified cell lysates were loaded onto an iodoxinol gradient and fractionated from the top of the gradient after 2.5 h. Fraction 1 refers to the top of the gradient, whereas fraction 11 was collected from the bottom. The amount of capsid protein and genomic RNA in each fraction was determined by immunoblotting and densitometry and by qRT-PCR, respectively. The y axis represents the normalized intensity in each fraction relative to the fraction containing the most abundant signal.
To validate the lack of NC formation in the K250/2D and K250/2E mutant backgrounds, we examined transfected BHK cells by electron microscopy. In thin-section EM, we were unable to identify NC structures in the cells transfected with the K250/2D and K250/2E mutants (data not shown), whereas we were able to identify such structures in the cells transfected with the wild type (Fig. 3A). Using immuno-EM with an anti-Cp antibody, however, we identified what appeared to be aggregates of Cp surrounding CPV-I replication vacuoles (Fig. 3B). These aggregates did not resemble the canonical NC structure. Taken together, the gradient and EM analyses suggested that, whereas the K250/2A mutant was restricted at a point after NC assembly, but before completion of the budding process, the K250/2D and K250/2E mutants were deficient in NC assembly.
Fig 3.
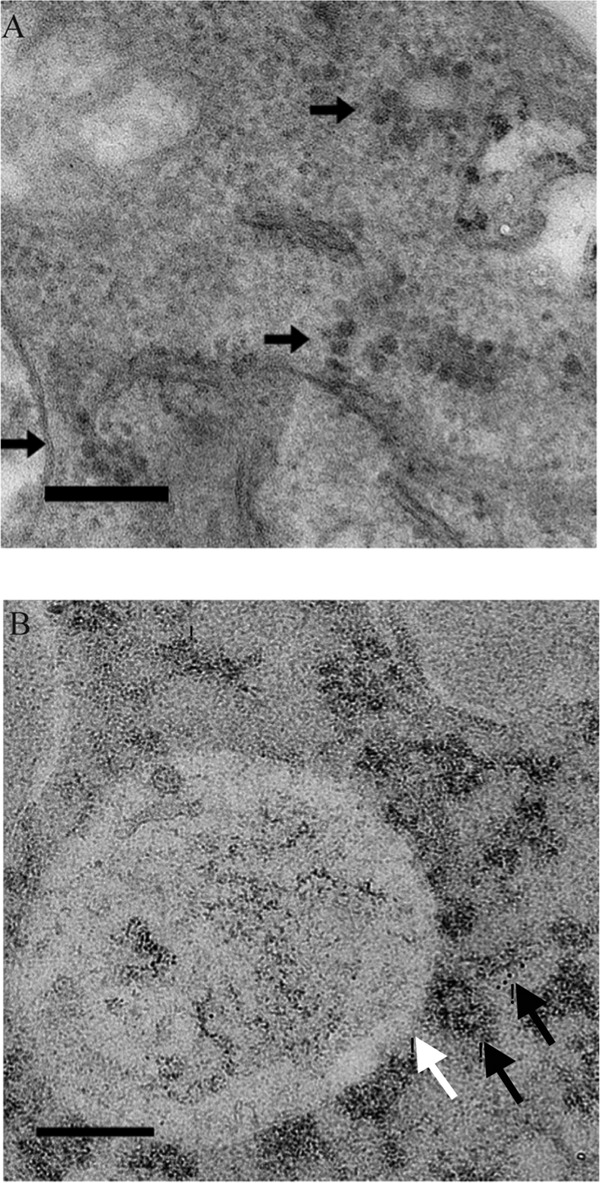
Electron microscopic analysis of infected BHK cells. BHK cells were electroporated with in vitro-transcribed infectious RNA and processed for EM at 8 h posttransfection, followed by staining with uranyl acetate. (A) Thin-section EM analysis of a cell infected with the wild type. Nucleocapsid cores in close proximity to CPV-II replication structures are readily apparent (black arrows). Cores were not detected via thin-section EM of cells infected with the K250/2D or K250/2E mutant (data not shown). Bar, 200 nm. (B) Immunogold EM analysis of a cell infected with the K250/2E mutant. Cells were stained with an anti-capsid primary antibody and a gold-conjugated secondary antibody in addition to uranyl acetate. Aggregates of protein assuming no regular shape were stained with the gold label (black arrows), and they lie on or near the surface of CPV-I replication vacuoles (white arrow). Bar, 200 nm.
The SINV Cp F2-G2 loop mutants assemble into core-like particles in vitro.
Given the lack of knowledge of the NC assembly process, we sought to interrogate NC assembly in vitro using the K250/2D and K250/2E mutants, as the gradient analysis suggested that the Cp aggregates found after expression of mutant Cp might represent a NC assembly intermediate. The wild-type and mutant Cp was expressed in Escherichia coli and purified using chromatography as previously described (45).
Like the wild type, the K250/2A mutant readily assembled into core-like particles (CLPs) in vitro using a single-stranded DNA template but, surprisingly, the K250/2D and K250/2E mutants also assembled into CLPs under identical standard assembly reaction conditions as gauged by native agarose gel electrophoresis (Fig. 4A) and negative-stain electron microscopy of assembly reactions (data not shown). Interestingly, though, when subjected to a temperature gradient, the K250/2D (data not shown) and K250/2E NC mutants appeared less thermostable than the wild type and the K250/2A mutants (Fig. 4B), suggesting that these charged residues may indeed be involved in intercapsomere contacts in the context of an assembled NC as previously suggested (46). Nevertheless, the mutant Cp is capable of assembling into NC-like structures in vitro and, thus, some other factor is responsible for the lack of NC formation in vivo for the K250/2D and K250/2E Cp mutants.
Fig 4.

Agarose gel electrophoretic analysis of in vitro assembly reactions. Capsid protein was expressed and purified in vitro. (A) Capsid and mutant proteins all assemble into core-like particles in a nucleic acid-dependent fashion. Assembly reactions were carried out as described in the text. Aliquots of each reaction were electrophoresed on 0.8% agarose and stained with 0.05% Coomassie blue. The small shift in mobility of the assembled CLPs was due to the difference in the pI of the proteins. The top of the image represents the cathode and the bottom the anode. The basic unassembled capsid protein can be seen migrating toward the cathode when nucleic acid is absent. (B) Assembled CLPs demonstrate a difference in levels of thermostability. Assembled CLPs were subjected to an increasing temperature gradient at the temperatures indicated for 15 min. Treated CLPs were analyzed by agarose gel electrophoresis as described above.
When expressed ectopically, the SINV Cp F2-G2 loop mutants assemble into NCs in the cell.
The disparate results with respect to NC assembly for the K250/2D and K250/2E mutants between infected cells and in vitro results led us to assay the ability of the mutant Cp to assemble into NC in the cell when expressed alone using a mammalian expression vector. BHK cells were transfected with a plasmid expressing wild-type or mutant Cp and were lysed 18 h after transfection. The cell lysate was subjected to the same ultracentrifugation and densitometric analyses as before. As seen in Fig. 5, all mutants produced Cp-containing structures that migrated in a manner similar to that seen with the wild type. Previously published work has shown that expression of the alphavirus structural proteins ectopically leads to the formation and release of virus-like particles, and alphavirus NC assembly in cells that lack nucleic acid has not been described; thus, NCs presumably assemble here with the aid of cellular RNAs (1). Therefore, the complexes found in these cell lysates likely represent NCs and, as such, the K250/2D and K250/2E mutant Cp are also capable of NC assembly in cells. Taken further, there is apparently some factor or environment in the infected cell that prevents authentic NC assembly using K250/2D and K250/2E mutant Cp.
Fig 5.
Rate-zonal gradient analysis of cells transfected with a mammalian expression vector producing capsid protein. BHK cells were transfected with an expression plasmid producing wild-type or mutant capsid proteins and hypotonically lysed at 16 h posttransfection. Clarified cell lysates were loaded onto an iodoxinol gradient and fractionated after 2.5 h. The amount of capsid protein in each fraction was determined by immunoblotting and densitometry. The y axis represents the normalized intensity in each fraction relative to the fraction containing the most abundant signal.
When expressed via a SINV replicon, the SINV Cp F2-G2 loop mutants assemble into NCs in the cell.
In the infected cell, NCs are found in close proximity to CPV-I structures which comprise both the site of viral RNA synthesis and the site of NC assembly (11). NCs also interact with the envelope proteins (23, 25, 31, 36, 51) frequently during the viral life cycle. Thus, in an effort to determine whether it is the viral replicase, the viral envelope proteins, or the environment in an infected cell that is responsible for the conflicting results seen with infected cells versus the other experimental conditions with regard to NC assembly for the K250/2D and K250/2E mutants, we generated a SINV replicon expressing only the wild-type or mutant Cp. This construct, which lacked the sequence encoding the viral glycoproteins, was expected to better mimic the environment of an infected cell, given the expression of the viral enzymatic proteins and the concurrent establishment of viral replicases on CPV-I replication vacuoles. As before, BHK cells were electroporated and the cell lysates were subjected to the same ultracentrifugation and densitometric analysis. Again (Fig. 6A), the mutants all produced Cp-containing structures that migrated in a manner similar to that seen with the wild type and apparently represented NCs. Indeed, electron microscopy studies validated the presence of NC structures for both the wild type and the mutants (Fig. 6B). To exclude the possibility that the shorter length of the replicon nucleic acid, relative to that of the full-length viral genomic RNA molecule, played a role in rescuing NC assembly, we created a separate construct in which we replaced the fourth and fifth codons of E3 with stop codons. In this system, only Cp is produced via the subgenomic message, as in the replicon, but the replicated nucleic acid is the same length as in the full-length genome. Both the wt and the K250/2D Cp mutant were tested in this background, and we detected NC assembly in cell lysates after sedimentation analysis in the ultracentrifuge (Fig. 7). Therefore, it appeared that it was the envelope proteins that were responsible for the lack of NC assembly in infected cells expressing the K250/2D and K250/2E mutant Cp.
Fig 6.
Analysis of cells transfected with Sindbis replicon expressing the capsid. BHK cells were electroporated with in vitro-transcribed replicon RNA. (A) Cells were hypotonically lysed at 8 h posttransfection. Clarified cell lysates were loaded onto an iodoxinol gradient and fractionated after 2.5 h. The amount of capsid protein in each fraction was determined by immunoblotting and densitometry. The y axis represents the normalized intensity in each fraction relative to the fraction containing the most abundant signal. (B) Cells transfected with a SINV replicon expressing wt capsid protein were processed for negative-stain electron microscopy at 8 h posttransfection. Clusters of cores are indicated by black arrows. Bar, 200 nm. (C) Cells transfected with a SINV replicon expressing the K250E/K252E capsid protein were processed for negative stain and EM at 8 h posttransfection. Clusters of cores are indicated by black arrows. Bar, 200 nm.
Fig 7.

Rate-zonal gradient analysis of cells transfected with viral RNA containing premature stop codons in E3. BHK cells were transfected with in vitro-transcribed viral RNA producing either wild-type or K250/2D capsid protein in the absence of viral envelope protein expression. Cells were hypotonically lysed at 8 h posttransfection. Clarified cell lysates were loaded onto an iodoxinol gradient and fractionated after 2.5 h. The amount of capsid protein in each fraction was determined by immunoblotting and densitometry. The y axis represents the normalized intensity in each fraction relative to the fraction containing the most abundant signal.
Double mutants containing mutations in both the Cp F2-G2 loop and cdE2 region I rescue NC assembly but not virus particle release.
Given the 7-Å SINV reconstruction, as well as previously published biophysical analyses of residues involved in Cp:E2 interactions (22, 44), we speculated that region I of the cytoplasmic domain of E2 (cdE2-RI), when present, may be responsible for the lack of NC assembly in the K250/2D and K250/2E mutant Cp backgrounds. We therefore created a set of double mutants in which cdE2-RI (cdE2 391-KARRE-395) and Cp residues K250 and K252 were simultaneously changed. Interestingly, gradient analysis of the double mutants demonstrated restoration of NC assembly in the K250/2D and K250/2E Cp backgrounds (Table 1). Lysates were scored as containing NCs (+) if the most intense Cp signal was found in fraction 6 or 7 or scored as lacking NCs if the most intense Cp signal was found in fraction 1, 2, 3, 4, or 5. Negative-stain electron microscopy analyses of a subset of the double mutants validated the gradient findings (Fig. 8). Specifically, we identified electron-dense NC structures adjacent to CPV-I structures for both the wt and K250/2E Cp mutant backgrounds concurrently with a lack of aggregates of Cp around these CPV-I structures using immuno-EM (data not shown). However, recovery of infectious particles as determined by plaque titration of posttransfected BHK cell media, or immediate agarose overlay after transfection of infectious RNA, was not restored for the K250/2D or K250/2E Cp background despite the formation of pinpoint-sized syncytium-derived plaques (Table 2). Furthermore, modulation of the sequence of cdE2 R-I residues 393-RRE-395 also impaired the ability of virus with a wild-type or K250/2A Cp background to release infectious particles. Taken together, these data suggest that cdE2-RI is important for efficient budding but also that cdE2-RI may interact with Cp at a prebudding stage, perhaps before or during NC assembly.
Table 1.
Rate zonal gradient analysis of cell infected with Sindbis virus containing mutations in both capsid and cdE2a
| Sequence | Presence or absence of NC |
|||
|---|---|---|---|---|
| 250KGK252 (wt) | 250AGA252 | 250DGD252 | 250EGE252 | |
| 391KARRE395 (wt) | + | + | − | − |
| 391KAERE395 | + | + | + | + |
| 391KARRK395 | + | + | + | + |
| 391KARAA395 | + | + | + | + |
| 391KAARE395 | + | + | + | + |
| 391KAAAA395 | + | + | + | + |
Column headings represent the capsid protein primary sequences, and rows represent the cdE2 primary sequences. Lysates were scored as containing NCs (+) if the most intense Cp signal was found in fraction 6 or 7 or scored as lacking NCs if the most intense Cp signal was found in fraction ≤5. Boldface represents the amino acids mutated in this study.
Fig 8.

EM analysis of cells infected with double cdE2/capsid mutants. (A) Cells transfected with a SINV full-length infectious RNA expressing wt capsid protein in the cdE2 R393E background were processed for negative-stain electron microscopy at 8 h posttransfection. Clusters of cores are indicated by black arrows. Bar, 200 nm. (B) Cells transfected with a SINV full-length infectious RNA expressing the K250E/K252E capsid protein in the cdE2 R393E background were processed for negative-stain electron microscopy at 8 h posttransfection. Clusters of cores are indicated by black arrows. Bar, 200 nm.
Table 2.
Plaque diameter analysis of cells infected with Sindbis virus containing mutations in both capsid and cdE2a
| Sequence | Plaque diam (mm) |
|||
|---|---|---|---|---|
| 250KGK252 (wt) | 250AGA252 | 250DGD252 | 250EGE252 | |
| 391KARRE395 (wt) | 3 | 2 | <1 | <1 |
| 391KAERE395 | <1 | <1 | <1 | <1 |
| 391KARRK395 | 2 | 1.5 | <1 | <1 |
| 391KARAA395 | <1 | 1.5 | <1 | <1 |
| 391KAARE395 | <1 | <1 | <1 | <1 |
| 391KAAAA395 | <1 | <1 | <1 | <1 |
Column headings represent the capsid protein primary sequences, and rows represent the cdE2 primary sequences.
Ectopic expression of the entire structural polyprotein with Cp F2-G2 loop mutants yields NC assembly in the mammalian cell.
Next, we generated a set of mammalian expression plasmids in which only the structural polyprotein, containing the wild-type envelope proteins, with either the wild-type Cp or the F2-G2 loop mutant Cp, was produced from a single message. Given the presence of the wild-type cdE2 R-I sequence, and its implication in NC assembly defects as described above, we expected to find a lack of NC structures in cells transfected with plasmids expressing the wild-type envelope proteins but the K250/2D and K250/2E mutant Cp. Cell lysates were subjected to the same gradient analysis described above, and, surprisingly, we detected molecules that sedimented like wild-type NC structures in all cases (Fig. 9). Given the absence of a viral replicase in this study, and the transfected cells devoid of CPV-I replication vacuoles, the data suggest that NC assembly may occur in a noncanonical fashion when the structural proteins are expressed ectopically or perhaps due to a lack of viral RNA synthesis. Therefore, the proposed cdE2:Cp interactions may occur only on virus-induced structures, which were absent in the transfected cells in the experiment described here. Indeed, although cdE2 R-I has been shown to interact with NC in vitro (22), we were unable to inhibit in vitro NC assembly of the wild type or the F2-G2 loop Cp mutants when GFP:cdE2 protein (22) was spiked into assembly reaction mixtures at up to a 10-mole excess (data not shown).
Fig 9.
Rate-zonal gradient analysis of cells transfected with a mammalian expression vector producing the Sindbis structural polyprotein. BHK cells were transfected with an expression plasmid producing wild-type or mutant capsid protein, in addition to the wild-type envelope proteins, and hypotonically lysed at 16 h posttransfection. Clarified cell lysates were loaded onto an iodoxinol gradient and fractionated after 2.5 h. The amount of capsid protein in each fraction was determined by immunoblotting and densitometry. The y axis represents the normalized intensity in each fraction relative to the fraction containing the most abundant signal.
DISCUSSION
Genetic and other evidence has convincingly implicated an interaction of Cp with a conserved YxL motif on cdE2 as necessary for budding to occur (36, 43, 53). However, whether there are additional Cp:cdE2 interactions, as well as the pathway by which the assembled NCs as well as the envelope proteins traffic to the site of budding, remains unclear.
Recent evidence has suggested that NCs and envelope proteins cotraffic from cytoplasmic domains to the plasma membrane on virus-induced membrane structures termed cytopathic vacuoles type II (CPV-II), and NC and the envelope proteins likely interact on these CPV-II structures (19, 41). Furthermore, a morphologically distinct virus-induced membrane structure, termed cytopathic vacuole type I (CPV-I), likely contains the viral replicase and has been implicated as the site of initial NC assembly (11). Thus, NCs likely assemble on or near CPV-I structures prior to their association with envelope proteins on CPV-II structures. It is unknown, however, whether any interactions exist between Cp/NCs and the envelope proteins prior to their association on CPV-II vacuoles.
Here, we provide evidence that Cp and the E2 envelope protein may interact earlier than previously thought in the virion assembly pathway, perhaps in an event that precedes cotrafficking of NC and envelope proteins to the PM via CPV-II structures.
Modeling of cdE2 into and out of the hydrophobic pocket of Cp in a 7-Å cryo-EM reconstruction of SINV virus provided the opportunity for structure-guided mutagenesis (44). Specifically, Tang and colleagues suggested that a flexible loop on the Cp, the F2-G2 loop, was shifted ∼3.5 Å from its position observed in the crystal structure of SINV Cp (5, 48). One residue in this loop, lysine 250, had been previously implicated in intercapsomere contacts within the NC (46), and Tang et al. suggested that the F2-G2 loop might move when the NC encounters the cdE2 domain of E2 (44). Furthermore, a second lysine in the F2-G2 loop, K252, was implicated as being a part of a salt bridge pair with D395 of cdE2 (44). Given these data, we performed structure-guided mutagenesis, simultaneously mutating both K250 and K252 in the Cp F2-G2 loop to alanine, aspartic acid, or glutamic acid, and expressed these proteins in a variety of ways.
Disruption of the lysines present in the F2-G2 loop led to a lack of NC assembly in the infected cell, with Cp found in aggregates around CPV-I vacuoles. In vitro assembly of the same mutant Cp, however, led to formation of core-like particles (CLPs). Interestingly, though, the NCs assembled from K250/2D and K250/2E Cp were not as thermostable as the wild type and the K250/2A mutant, in line with the proposal that at least one residue, K250, in the F2-G2 loop might be involved in cross-capsomere contacts in the context of an assembled NC (46). A crystal structure of the SINV Cp revealed that K252 is involved in a salt bridge with Cp E111 (25). Residue E111 is part of the N-terminal arm of Cp shown to interact with the hydrophobic pocket of an adjacent Cp molecule in the crystal lattice and, thus, perhaps in the context of a NC prior to binding of cdE2 into the hydrophobic pocket. Taken together, these observations could explain the difference in thermostability. Despite these differences in thermostability, CLPs assembled with both wild-type and mutant Cp remained intact during gradient analysis of in vitro assembly reactions (data not shown), and thus the differences in thermostability are unlikely to be related to the phenotype observed in the cell. Therefore, importantly, and unexpectedly, the in vitro assembly data demonstrated that the mutant Cp contains the information necessary to assemble into NC-like structures.
Expression of Cp in the cell by a SINV replicon or by a mammalian expression plasmid with or without the viral envelope proteins also readily led to NC assembly in the cell, as gauged by gradient and negative-stain electron microscopy analyses. In the former system, not only were the viral nonstructural and the viral envelope proteins not produced but the reorganization of internal cellular membranes that are a hallmark of alphavirus infection (37) was also absent. Previously, overexpression of the alphavirus structural proteins in a similar fashion was shown to yield the formation and release of virus-like particles (1). In the latter system, the replicon that we created contained a SINV RNA in which the envelope protein gene segment was missing. In this replicon, Cp is still produced via translation of a subgenomic message; the viral replicase, the genomic RNA packaging signal, and enzymatic proteins are present and CPV-I vacuoles are observed in the transfected cell (data not shown), but the envelope proteins are not produced and CPV-II vacuoles are not observed (data not shown). Additionally, in a separate experiment, we excluded the length of the capsid-expressing nucleic acid as a factor in rescuing NC assembly. Taken together, the data suggested that the envelope proteins were responsible for the NC assembly defect seen in the infected cell.
Previously published genetic and structural data strongly suggested that cdE2 interacts closely with a hydrophobic pocket on the surface of Cp (36, 38, 44). Jose and colleagues recently demonstrated that region I of cdE2 (cdE2-RI), comprised of E2 391-KARRE-395, specifically interacts with both NCs isolated from cells infected with SINV, as well as in vitro-assembled CLPs (22). cdE2 E395 has been specifically implicated in virus assembly (13), and Tang et al. proposed that cdE2 residue E395, part of region I, formed a salt bridge with Cp K252, a residue in the F2-G2 loop under investigation here (44). Thus, we speculated that cdE2-RI, when present, was responsible for the lack of NC assembly in the K250/2D and K250/2E mutant Cp backgrounds.
Double mutants in which the primary sequences of both the Cp F2-G2 loop and cdE2-RI were mutated showed that multiple different alterations of the cdE2 sequence 393-RRE-395 were capable of restoring the ability of the K250/2D and K250/2E mutant Cp to incorporate into NCs in the cell. The fact that multiple different substitutions in this region of cdE2 rescued the NC assembly defect suggests that it is not necessarily one particular Cp:cdE2 side-chain interaction that is responsible for the K250/2D and K250/2E mutant phenotypes but perhaps more likely the shape or conformation of cdE2. Indeed, it has been suggested that, given the conservation of the positions of prolines and cysteines in cdE2 across the alphavirus genus, cdE2 might adopt a conserved conformation important for Cp binding (42).
While mutation of cdE2-RI restored the capacity for NC formation in the K250/2D and K250/2E backgrounds, it did not restore the release of infectious particles. Except for the E2 E395K mutant, all other cdE2-RI mutants prevented release of infectious particles by virus containing wild-type Cp. That cdE2 region I might be important in virus assembly was also recently proposed by Jose et al. (22). Thus, cdE2-RI may have a multifaceted role in the virus assembly process through interaction with the Cp. Interestingly, as previously noted, some degree of covariance exists between cdE2-RI and the F2-G2 loop of Cp (22). Specifically, in most alphaviruses sequenced to date, when the Cp residue corresponding to SINV K250 is basic, the cdE2 residue corresponding to E395 is acidic, and when the Cp residue corresponding to K250 is acidic, the cdE2 residue is basic. Note that it is Cp residue K252, not K250, that is reported to form a salt bridge with cdE2 residue E395 (44); thus, modeling based on static images of virus particles and isolated NC may underscore the true fluidity of the cdE2 and Cp domains that apparently interact with one another.
When we assayed the ability of the K250/2D and K250/2E Cp mutants to assemble when they were ectopically expressed as part of the complete structural polyprotein, we expected that the K250/2D and K250/2E Cp mutants would not assemble into NCs. However, gradient analysis demonstrated the presence of Cp-containing structures that sedimented just like the wild type. These data suggest that, while cdE2 does play a role in the NC assembly defect phenotypes, the ultrastructure of the infected cell does as well. Indeed, in this system, CPV-I vacuoles are absent, and the polyprotein is likely translated in a canonical fashion rather than proximal to CPV-I structures. The nonstructural proteins, likely compartmentalized at CPV-I vacuoles in a regular infection, have been shown to interact with numerous host proteins (6, 18, 35). In short, the NC assembly defects described here may not be able to be recapitulated via ectopic expression of the structural polyprotein because of the inherent differences in the organization or arrangement of viral proteins in the uninfected versus infected cell. Indeed, when GFP:cdE2 protein was added at up to a 10-mole excess into in vitro assembly reactions using wild-type or mutant F2-G2 loop Cp, assembly of CLPs was unaffected, again demonstrating that the presence of cdE2 alone is not sufficient to reconstitute the Cp:cdE2 interactions that occur in the infected cell.
The results described here provide additional insights into the temporal and spatial interactions between SINV Cp and cdE2. We demonstrated that Cp containing mutations in the F2-G2 loop still retained the information necessary to assemble into NCs. CPV-I vacuoles are proposed to be the site of normal NC assembly (11), but in cells transfected with in vitro transcripts containing the Cp mutation K250/2D or K250/2E, an accumulation of aggregates of Cp and viral genomic RNA complex at CPV-I vacuoles was detected. Given that this phenotype can be rescued by modulation of the primary sequence of cdE2-RI, a region already implicated in Cp:E2 interactions (44), and that, in the absence of these second-site mutations, K250/2D and K250/2E Cp form aggregates on CPV-I vacuoles, our data suggest that Cp and E2 may interact on or near CPV-I vacuoles, the site of NC assembly (11), soon after Cp synthesis.
It is tempting to speculate that cdE2 might act as a chaperone, aiding the assembly of NCs. Other viruses employ such a mechanism (7); however, additional data are required to interrogate this hypothesis. Another mechanistic possibility, and perhaps one that is more likely, is that changing the local charge of the Cp F2-G2 loop in the K250/2D and K2502/E mutants increases the affinity of Cp for cdE2. This could lead to an altered rate of nucleation between Cp and cdE2 and the formation of aggregates. In the infected cell, the K250/2D Cp and the K2502/E Cp may become kinetically trapped by this interaction, unable to continue down the NC assembly pathway. Computational studies with other RNA viruses have demonstrated, counterintuitively, that increasing the affinity of a viral Cp for its cognate RNA leads to an aberrant and incorrect NC assembly pathway by altering the rate of nucleation (8, 21).
Nevertheless, the data presented here have provided additional knowledge concerning the interactions of Cp and E2 in the infected cell and, coupled with recent additions to the literature, have provided a new level of insight into the interactions and trafficking of the alphavirus NC and envelope proteins and allow us to propose a more detailed model regarding the NC assembly pathway. Wilkinson et al. proposed that Cp and RNA may initially associate on the cytoplasmic faces of intracellular membranes (51). Here, we show that Cp and cdE2 may interact on CPV-I vacuoles, although direct evidence of the presence of E2 in CPV-I structures has yet to be published. However, the analysis of alphavirus chimeras with respect to genome replication kinetics as well as work with an anti-idiotypic antibody in which the original antigen was cdE2 have led to speculation that molecules of E2 might indeed be present in the replicase complex (42, 43). Given that CPV-I vacuoles are derived from the plasma membrane (10), the ultimate destination for E2, the presence of the envelope proteins in or near the viral replicase may not actually be that surprising.
Soonsawad and colleagues showed that CPV-II vacuoles contain envelope proteins spikes, are decorated with NCs, and traffic from sites internal to the cell to the plasma membrane (41). Unpublished electron tomography data (T. J. Edwards and R. J. Kuhn) have demonstrated the movement of assembled NCs from CPV-I vacuoles to CPV-II vacuoles, and recent studies using microinjection of viral RNA into envelope protein-expressing cells demonstrated a preference for newly synthesized envelope proteins during envelopment of NCs (39).
Taking these data together, we can propose a more detailed model regarding the fate of Cp and envelope proteins in the cell. Specifically, newly translated Cp may associate with CPV-I membranes, interact with viral genomic RNA, and assemble into NC structures. Molecules of cdE2 resident in the CPV-I structures may temporarily interact with Cp before or during NC assembly. Assembled NCs may then traffic from CPV-I vacuoles to CPV-II vacuoles, where they interact with additional molecules of cdE2. Presumably, NC and cdE2 interact strongly here, perhaps via the conserved cdE2 YxL motif and the hydrophobic pocket on Cp. The C-terminal domain of cdE2 (cdE2-CTD) is reported to initially transverse the membrane, flipping back and exposing cdE2-CTD to the cytosol well after translation in a phosphorylation-dependent manner (28). Thus, cdE2 may assume different conformations within the cell, providing scaffolds of different levels of affinity for Cp when embedded in CPV-I versus CPV-II membranous structures. A strong interaction between NC and cdE2 at CPV-II may tether NCs to CPV-II, and NCs and envelope proteins could cotraffic to the plasma membrane (41). The structure, or perhaps composition, of CPV-II is such that premature budding is inefficient (41). Only when CPV-II reaches and fuses with the PM do the envelope proteins form a 2D lattice that is planar enough to envelop the NC, providing the free energy to bud a virus particle through the PM. This model, while attractive, is almost certainly overly simplistic and requires further studies be performed to completely elucidate the alphavirus virion assembly pathway.
The work presented here correlates with previously published genetic and structural analyses and expands our knowledge of alphavirus assembly. Taken together, our data (i) suggest that an early temporal and spatial interaction occurs between Cp and E2 at or near the CPV-I replication structures and (ii) provide a description of a new set of cdE2:Cp amino acid interactions, involving the N-terminal region of cdE2, that are important for alphavirus NC assembly as well as budding.
ACKNOWLEDGMENTS
We thank Anita Robinson for clerical assistance as well as the Life Science Microscopy Facility and Biological Electron Microscopy Facility at Purdue University.
We acknowledge support from the NIH through NIGMS award GM56279 to R.J.K. and NIH Biophysics Training Grants 5T32GM008296-21 and 5T32GM008296-22 to J.E.S.
Footnotes
Published ahead of print 5 September 2012
REFERENCES
- 1. Akahata W, et al. 2010. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 16:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bonatti S, Migliaccio G, Blobel G, Walter P. 1984. Role of signal recognition particle in the membrane assembly of Sindbis viral glycoproteins. Eur. J. Biochem. 140:499–502 [DOI] [PubMed] [Google Scholar]
- 3. Byrnes AP, Griffin DE. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72:7349–7356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng RH, et al. 1995. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell 80:621–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi HK, et al. 1991. Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature 354:37–43 [DOI] [PubMed] [Google Scholar]
- 6. Cristea IM, et al. 2010. Host factors associated with the Sindbis virus RNA-dependent RNA polymerase: role for G3BP1 and G3BP2 in virus replication. J. Virol. 84:6720–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dokland T. 1999. Scaffolding proteins and their role in viral assembly. Cell. Mol. Life Sci. 56:580–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elrad OM, Hagan MF. 2010. Encapsulation of a polymer by an icosahedral virus. Phys Biol. 7:045003 doi:10.1088/1478-3975/7/4/045003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Forsell K, Griffiths G, Garoff H. 1996. Preformed cytoplasmic nucleocapsids are not necessary for alphavirus budding. EMBO J. 15:6495–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frolova EI, Gorchakov R, Pereboeva L, Atasheva S, Frolov I. 2010. Functional Sindbis virus replicative complexes are formed at the plasma membrane. J. Virol. 84:11679–11695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Froshauer S, Kartenbeck J, Helenius A. 1988. Alphavirus RNA replicase is located on the cytoplasmic surface of endosomes and lysosomes. J. Cell Biol. 107:2075–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fuller SD, Berriman JA, Butcher SJ, Gowen BE. 1995. Low pH induces swiveling of the glycoprotein heterodimers in the Semliki Forest virus spike complex. Cell 81:715–725 [DOI] [PubMed] [Google Scholar]
- 13. Gaedigk-Nitschko K, Schlesinger MJ. 1991. Site-directed mutations in Sindbis virus E2 glycoprotein's cytoplasmic domain and the 6K protein lead to similar defects in virus assembly and budding. Virology 183:206–214 [DOI] [PubMed] [Google Scholar]
- 14. Garoff H, Frischauf AM, Simons K, Lehrach H, Delius H. 1980. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature 288:236–241 [DOI] [PubMed] [Google Scholar]
- 15. Garoff H, Simons K. 1974. Location of the spike glycoproteins in the Semliki Forest virus membrane. Proc. Natl. Acad. Sci. U. S. A. 71:3988–3992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garoff H, Sjoberg M, Cheng RH. 2004. Budding of alphaviruses. Virus Res. 106:103–116 [DOI] [PubMed] [Google Scholar]
- 17. Garoff H, Soderlund H. 1978. The amphiphilic membrane glycoproteins of Semliki Forest virus are attached to the lipid bilayer by their COOH-terminal ends. J. Mol. Biol. 124:535–549 [DOI] [PubMed] [Google Scholar]
- 18. Gorchakov R, Garmashova N, Frolova E, Frolov I. 2008. Different types of nsP3-containing protein complexes in Sindbis virus-infected cells. J. Virol. 82:10088–10101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Griffiths G, et al. 1989. The dynamic nature of the Golgi complex. J. Cell Biol. 108:277–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gualtieri EJ, et al. 2011. Detection of membrane protein two-dimensional crystals in living cells. Biophys. J. 100:207–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hagan MF, Elrad OM. 2010. Understanding the concentration dependence of viral capsid assembly kinetics—the origin of the lag time and identifying the critical nucleus size. Biophys. J. 98:1065–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jose J, et al. 2012. Interactions of the cytoplasmic domain of Sindbis virus E2 with nucleocapsid cores promote alphavirus budding. J. Virol. 86:2585–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kail M, et al. 1991. The cytoplasmic domain of alphavirus E2 glycoprotein contains a short linear recognition signal required for viral budding. EMBO J. 10:2343–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klimstra WB, Ryman KD, Johnston RE. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357–7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee S, et al. 1996. Identification of a protein binding site on the surface of the alphavirus nucleocapsid and its implication in virus assembly. Structure 4:531–541 [DOI] [PubMed] [Google Scholar]
- 26. Liljeström P, Garoff H. 1991. Internally located cleavable signal sequences direct the formation of Semliki Forest virus membrane proteins from a polyprotein precursor. J. Virol. 65:147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Linger BR, Kunovska L, Kuhn RJ, Golden BL. 2004. Sindbis virus nucleocapsid assembly: RNA folding promotes capsid protein dimerization. RNA 10:128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu N, Brown DT. 1993. Transient translocation of the cytoplasmic (endo) domain of a type I membrane glycoprotein into cellular membranes. J. Cell Biol. 120:877–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lopez S, Yao JS, Kuhn RJ, Strauss EG, Strauss JH. 1994. Nucleocapsid-glycoprotein interactions required for assembly of alphaviruses. J. Virol. 68:1316–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Melancon P, Garoff H. 1987. Processing of the Semliki Forest virus structural polyprotein: role of the capsid protease. J. Virol. 61:1301–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Metsikkö K, Garoff H. 1990. Oligomers of the cytoplasmic domain of the p62/E2 membrane protein of Semliki Forest virus bind to the nucleocapsid in vitro. J. Virol. 64:4678–4683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mukhopadhyay S, Chipman PR, Hong EM, Kuhn RJ, Rossmann MG. 2002. In vitro-assembled alphavirus core-like particles maintain a structure similar to that of nucleocapsid cores in mature virus. J. Virol. 76:11128–11132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mukhopadhyay S, et al. 2006. Mapping the structure and function of the E1 and E2 glycoproteins in alphaviruses. Structure 14:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mulvey M, Brown DT. 1996. Assembly of the Sindbis virus spike protein complex. Virology 219:125–132 [DOI] [PubMed] [Google Scholar]
- 35. Neuvonen M, et al. 2011. SH3 domain-mediated recruitment of host cell amphiphysins by alphavirus nsP3 promotes viral RNA replication. PLoS Pathog. 7:e1002383 doi:10.1371/journal.ppat.1002383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Owen KE, Kuhn RJ. 1997. Alphavirus budding is dependent on the interaction between the nucleocapsid and hydrophobic amino acids on the cytoplasmic domain of the E2 envelope glycoprotein. Virology 230:187–196 [DOI] [PubMed] [Google Scholar]
- 37. Schwartz M, Chen J, Lee WM, Janda M, Ahlquist P. 2004. Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication. Proc. Natl. Acad. Sci. U. S. A. 101:11263–11268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Skoging U, Vihinen M, Nilsson L, Liljestrom P. 1996. Aromatic interactions define the binding of the alphavirus spike to its nucleocapsid. Structure 4:519–529 [DOI] [PubMed] [Google Scholar]
- 39. Snyder JE, et al. 2011. Rescue of infectious particles from preassembled alphavirus nucleocapsid cores. J. Virol. 85:5773–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Söderlund H. 1973. Kinetics of formation of the Semliki Forest virus nucleocapsid. Intervirology 1:354–361 [DOI] [PubMed] [Google Scholar]
- 41. Soonsawad P, et al. 2010. Structural evidence of glycoprotein assembly in cellular membrane compartments prior to alphavirus budding. J. Virol. 84:11145–11151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Strauss JH, Strauss EG. 1994. The alphaviruses: gene expression, replication, and evolution. Microbiol. Rev. 58:491–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suomalainen M, Liljestrom P, Garoff H. 1992. Spike protein-nucleocapsid interactions drive the budding of alphaviruses. J. Virol. 66:4737–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tang J, et al. 2011. Molecular links between the E2 envelope glycoprotein and nucleocapsid core in Sindbis virus. J. Mol. Biol. 414:442–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tellinghuisen TL, Hamburger AE, Fisher BR, Ostendorp R, Kuhn RJ. 1999. In vitro assembly of alphavirus cores by using nucleocapsid protein expressed in Escherichia coli. J. Virol. 73:5309–5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tellinghuisen TL, Kuhn RJ. 2000. Nucleic acid-dependent cross-linking of the nucleocapsid protein of Sindbis virus. J. Virol. 74:4302–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thiboutot MM, et al. 2010. Chikungunya: a potentially emerging epidemic? PLoS Negl. Trop. Dis. 4:e623 doi:10.1371/journal.pntd.0000623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tong L, Choi HK, Minor W, Rossmann MG. 1992. The structure determination of Sindbis virus core protein using isomorphous replacement and molecular replacement averaging between two crystal forms. Acta Crystallogr. A 48(Pt 4):430–442 [DOI] [PubMed] [Google Scholar]
- 49. von Bonsdorff CH, Harrison SC. 1978. Hexagonal glycoprotein arrays from Sindbis virus membranes. J. Virol. 28:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wahlberg JM, Bron R, Wilschut J, Garoff H. 1992. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J. Virol. 66:7309–7318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wilkinson TA, Tellinghuisen TL, Kuhn RJ, Post CB. 2005. Association of Sindbis virus capsid protein with phospholipid membranes and the E2 glycoprotein: implications for alphavirus assembly. Biochemistry 44:2800–2810 [DOI] [PubMed] [Google Scholar]
- 52. Zhang X, Fugere M, Day R, Kielian M. 2003. Furin processing and proteolytic activation of Semliki Forest virus. J. Virol. 77:2981–2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao H, Lindqvist B, Garoff H, von Bonsdorff CH, Liljestrom P. 1994. A tyrosine-based motif in the cytoplasmic domain of the alphavirus envelope protein is essential for budding. EMBO J. 13:4204–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]






