Abstract
Adenovirus (Ad) cell attachment is initiated by the attachment of the fiber protein to a primary receptor (usually CAR or CD46). This event is followed by the engagement of the penton base protein with a secondary receptor (integrin) via its loop region, which contains an Arg-Gly-Asp (RGD) motif, to trigger virus internalization. To understand the well-orchestrated adenovirus cell attachment process that involves the fiber and the penton base, we reconstructed the structure of an Ad5F35 capsid, comprising an adenovirus type 5 (Ad5) capsid pseudotyped with an Ad35 fiber, at a resolution of approximately 4.2 Å. The fiber-penton base interaction in the cryo-electron microscopic (cryo-EM) structure of Ad5F35 is similar to that in the cryo-EM structure of Ad5, indicating that the fiber-penton base interaction of adenovirus is conserved. Our structure also confirms that the C-terminal segment of the fiber tail domain constitutes the bottom trunk of the fiber shaft. Based on the conserved fiber-penton base interaction, we have proposed a model for the interaction of Ad5F35 with its primary and secondary receptors. This model could provide insight for designing adenovirus gene delivery vectors.
INTRODUCTION
Human adenoviruses (Ads) are nonenveloped, double-stranded DNA viruses and are responsible for respiratory, gastrointestinal, and ocular infections. In addition, Ads have demonstrated great potential as vaccine vectors (7, 13, 22). Ad has a large, complex, icosahedral capsid with a diameter of ∼920 Å, not including the elongated homotrimeric fibers at each of its 5-fold vertices. The Ad capsid is composed of three major proteins: 240 hexon trimers that form the 20 facets of the icosahedral capsid, 12 penton bases, and 12 fiber trimers at the 12 icosahedral vertices (8, 36). A structure of Ad5F35, comprising an adenovirus type 5 (Ad5) capsid pseudotyped with an Ad35 fiber (25, 35), has been determined at 6-Å resolution (33). Combined with secondary structure prediction for minor proteins, the 6-Å structure has enabled the precise locations of minor proteins IIIa, VI, VIII, and IX to be determined (33). The locations of these minor proteins have been further confirmed by two nearly atomic resolution Ad structural analyses: the Ad5 structure analyzed by cryo-electron microscopy (cryo-EM) and the Ad5F35 structure determined using X-ray crystallography (17, 30). The minor capsid proteins, including IIIa, VIII, and IX, are thought to form protein-protein networks to enhance the stability of the Ad capsid (17).
The Ad fiber, which is formed by three copies of fiber proteins, plays a role in the initial attachment of the Ad capsid to the host cell surface through its interaction with the cellular receptor. Although Ad fibers are of various lengths depending on the serotype, the fiber proteins of all serotypes share an overall architecture: an N-terminal tail domain that interacts with the pentameric penton base, a C-terminal globular knob domain that functions as the attachment site for the host cell receptors, and a central shaft domain that connects the tail and the knob domains. The shaft domain consists of a number of repeating β-strands, and the number of β-strands determines the fiber length for the different serotypes (23, 37, 41). The tail domain of the trimeric fiber attaches to the pentameric penton base at the 5-fold axis, resulting in a symmetry mismatch interaction. This interaction is of particular interest because the fiber and penton base play very important roles in the onset of Ad infection. Ad entry into a cell is initiated by a two-step receptor-binding process. The first step of cell entry is the attachment of the fiber to a cell receptor, tethering the viral capsid to the cell surface (3, 11, 12, 21). This attachment is followed by the interaction of an Arg-Gly-Asp (RGD) loop in the penton base protein with the host cell integrins to trigger virus internalization into clathrin-coated vesicles or macropinosomes, thereby promoting membrane penetration (4, 14, 16, 39, 40). Due to the symmetry mismatch between the trimeric fiber and penton base, it is difficult to resolve the structure of the interaction by X-ray crystallography and cryo-EM. Using multiresolution filtering and computational simulation of mismatched symmetries, a model of the fiber-penton base interaction has been proposed recently based on the in situ structure of fiber in Ad5 virion (19). According to this model, the bottom of the fiber shaft interacts with the hydrophobic ring of the penton base, and the three fiber tails of each fiber act as stay-cables, residing inside three of the five grooves between the neighboring penton base monomers. This model of fiber tail-penton base interaction is consistent with earlier observations of the crystal structure of the Ad2 penton base bound to the N-terminal fiber peptide (45) and the cryo-EM structure of a dodecahedral penton particle (10). However, this model of fiber-penton base interaction is different from that proposed by Reddy et al. (30). In the crystal structure of Ad5F35, the densities that were proposed to represent a fiber structure were located in the central pore of the penton base.
Previous studies have shown that the C-terminal knob domain, together with the length and flexibility of the Ad fiber shaft domain, determine viral tropism and infectivity (34, 41). Indeed, Ad5 and Ad5F35 have fibers that differ strikingly in their lengths and utilize different receptors for cell entry. It remains unclear whether these differences between Ad5 and Ad5F35 cause a difference in the type of fiber-penton base interaction. Similarly, it is currently uncertain how Ads with short fibers (i.e., Ad35 fiber) interact with the receptors. Aiming to address these uncertainties in the field, we reconstructed the structure of an Ad5F35 capsid with Ad35 tropism at nearly atomic resolution using cryo-EM. The Ad5F35 capsid studied here consisted of an Ad5 capsid and a chimeric fiber composed of an Ad35 fiber shaft, an Ad35 C-terminal knob, and an Ad5 N-terminal fiber tail (residues 1 to 43) (24), while the Ad5F35 capsid analyzed by X-ray crystallography (30) consists of an Ad5 capsid and an entire Ad35 fiber. Our Ad5F35 structure reveals that the fiber-penton base interaction in the cryo-EM structure of Ad5F35 is similar to that in the cryo-EM structure of Ad5 (17); however, it is different from the interaction proposed based on the crystal structure of Ad5F35 (30). The fiber-penton base interaction observed in the crystal structure of Ad5F35 could possibly reflect an intermediate stage. By comparing the fiber structure from the Ad5F35 density map with a pseudo homology model of the Ad35 fiber, comprising the C-terminal knob and shaft domains, we confirmed that the C-terminal segment (residues 22 to 43) of the fiber tail domain is located at the bottom trunk of the fiber shaft and contributes to the length of the shaft. Moreover, a model of the interaction of the Ad5F35 capsid with CD46 and integrin receptors based on our Ad5F35 structure suggests that Ads with short fibers may be capable of binding integrin and CD46 at the same time.
MATERIALS AND METHODS
Sample preparation, cryo-EM imaging, and three-dimensional (3-D) reconstruction.
The Ad5F35 sample was prepared as previously described (24). An aliquot of 3.5 μl purified Ad5F35 sample was applied to a 200-mesh quantifoil grid and blotted for 4 s in a chamber at 100% humidity using an FEI Vitrobot Mark IV. Viruses on the grid, embedded in thin ice, were imaged with an FEI 300-kV Titan Krios electron microscope equipped with a Gatan UltraScan4000 (model 895) 16-megapixel charge-coupled-device (CCD) camera. The microscope was carefully aligned before image collection such that a maximum visible contrast transfer function ring of a dry portion of the carbon film image (20 to 25 electrons/Å2 dose) at 1/4 to 1/3 Å−1 spatial frequency was observed. The defocus values of all micrographs were set to approximately 1.5 to 2.5 μm. The contrast transfer function correction for each micrograph was determined manually using the CTFIT program from the EMAN package (20) based on incoherently averaged Fourier transforms of each image. The orientations and centers of all virus particles were determined using the IMIRS package (15) based on the common-line strategy (9). Approximately 21,000 selected particles (68% of all particles) from 4,500 micrographs were combined and reconstructed using a reconstruction program based on icosahedral symmetry-adapted functions (18).
Structure analysis and model building of the Ad35 fiber and integrin.
Protein subunit densities were segmented from the Ad5F35 map and visualized using UCSF Chimera (28). The segmented densities of the hexon protein monomer were aligned and averaged using UCSF Chimera (28) and the AVG3D program from the EMAN package (20) to improve the resolution. The fiber tail density map was segmented out from the cryo-EM density map of Ad5F35. We used the fiber-tail model from the cryo-EM model of Ad5 (PDB identifier [ID] 3IZO) to fit to our density map. We then used Coot (6) to improve the geometry and the fit of the tail atomic model to our density map. By sequence alignment of the fiber shaft, we built a pseudoatomic model of the shaft with 5 β-repeats as described previously (19). This model was then merged with the atomic model of the crystal structure of the Ad35 fiber knob (26). The density model of the fiber shaft and the knob was generated from its pseudoatomic model using the pdb2mrc program in EMAN (20). The model of integrin that adopts an extended conformation was built as a composite of the αvβ3 integrin crystal structures with PDB IDs 1L5G and 1U8C (43, 44) as described previously (16).
Electron microscopy density map accession numbers.
The 7-Å- and 4.2-Å-resolution electron density maps have been deposited in EMDataBank with accession numbers EMD-5454 and EMD-5467, respectively.
RESULTS
Structure determination.
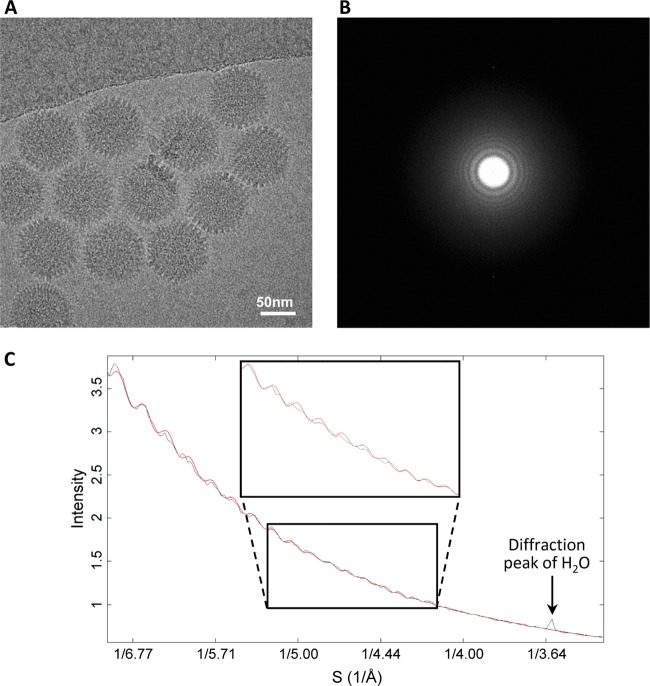
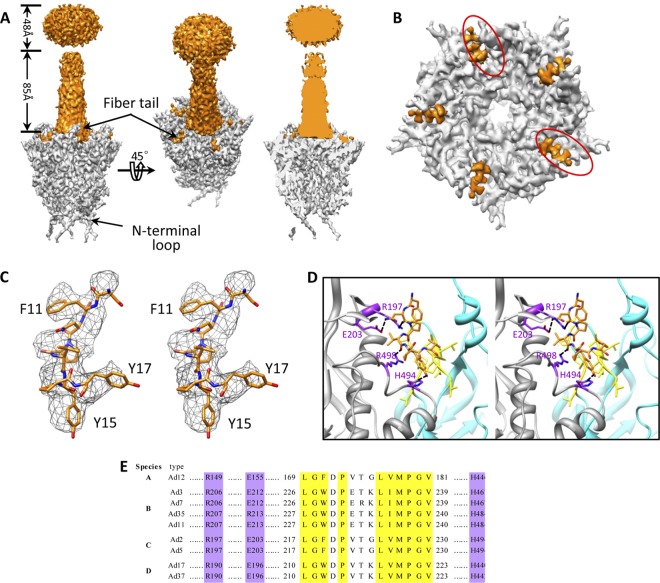
We obtained a 3-D structure of the Ad5F35 virion by cryo-EM and single-particle reconstruction (Fig. 1A). The final 3-D reconstruction was generated from approximately 21,000 particle images from 4,500 micrographs collected by CCD camera with an FEI Titan Krios microscope (Fig. 2). There are 12 copies of the hexon protein monomer in each icosahedral asymmetrical unit. By averaging the segmented density maps of the 12 hexon protein monomers, the density map of the hexon protein was further improved (Fig. 1B and C). We evaluated the Ad5F35 structure by fitting the atomic model of Ad5 capsid proteins (17) to our Ad5F35 density map. Except for the fiber structure and some hypervariable regions in the hexon protein, the major proteins and minor proteins in our Ad5F35 structure are almost identical to their counterparts in the atomic model of Ad5 capsid proteins (Fig. 1B, C, and D). An N-terminal segment of the penton base protein is resolved in our structure but not in the Ad5F35 crystal structure (Fig. 1D). Based on the clarity of the resolved features, which includes the separated β-strands, α-helix turns, and certain clearly visible side chains (Fig. 1B, C, and D), we estimated that the Ad5F35 structure has a resolution of approximately 4.2 Å. In contrast to the partially resolved fiber structure of Ad5 (19), the fiber in our Ad5F35 structure is fully resolved (Fig. 1A).
Fig 1.
Cryo-EM structure of Ad5F35. (A) Overall structures (left) of the Ad5F35 virion filtered at a 10-Å resolution along a 2-fold axis and zoomed-in view of a fiber (right). (B) Transparent view of the density map of a hexon protein monomer with its atomic model (only the backbone is shown) superimposed (left). Transparent view of a segmented β-hairpin density map with its atomic model superimposed (middle). Zoomed-in view of a β-strand density map (mesh) superimposed on its atomic model (right). (C) Cross-eye stereo views of density maps (mesh) of a β-sheet and an α-helix segmented from a hexon protein superimposed on their atomic models. (D) Transparent view of a penton-base protein with its atomic model superimposed (only the backbone is shown) and a zoomed-in view of its N-terminal segment map (mesh) superimposed on the atomic model.
Fig 2.
Resolution assessment of a cryo-EM image of Ad5F35. (A) Cryo-EM image of Ad5F35 recorded using a CCD camera. (B) Fourier transform of the cryo-EM image. (C) Rotationally averaged power spectrum (black curve) of the Fourier transform in panel B, showing the structure-factor profile modulated by the contrast transfer (CTF). The red curve is a simulated CTF curve.
Sequence analysis and homology modeling of the Ad35 fiber.
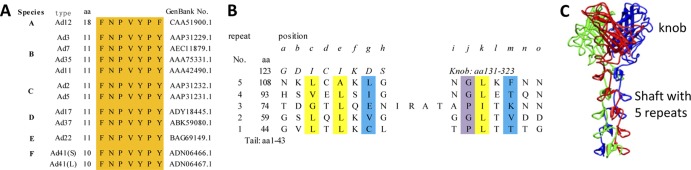
Sequence alignment indicates that amino acid residues 11 to 17 in the Ad35 fiber tail domain are conserved among the six human Ad subgroups, A to F (Fig. 3A), implying that they have conserved interactions with the penton base proteins. X-ray crystallography analysis of the recombinant Ad2 fiber protein containing the four distal repeats of the shaft and knob domains shows that the shaft domain is formed by a number of β-repeats (37). Although the amino acid sequence of the Ad35 fiber shaft domain is much shorter than that of the Ad2 fiber shaft domain, we were able to align 5 β-repeats in the Ad35 fiber (Fig. 3B) in the same way that the 22 β-repeats were aligned in the Ad2 shaft (37). The Ad35 fiber contains an insert of residues (IRAT) in the third β-repeat of the shaft from the N-terminal tail end (Fig. 3B) and is similar to the amino acid sequence of the Ad2 fiber (37). The analogous periodic sequence organization of the Ad35 fiber shaft domain, namely, the hydrophobic core residues, the residues forming the peripheral hydrophobic patches, and the conserved glycines or prolines in the turns, suggests that the shaft domains of the Ad35 fiber and the Ad2 fiber are structurally conserved. We also built a pseudo model (Fig. 3C) for the Ad35 fiber knob and shaft domains in accordance with the crystal structures of the Ad2 fiber shaft (37) and the Ad35 knob (26, 38).
Fig 3.
Sequence analysis and the pseudo model of the Ad35 fiber. (A) Sequence alignment of the conserved tail segments of fiber from different human Ad serotypes in six species (A to F). The GenBank accession numbers of the fiber sequences are provided. (B) Sequence alignment of the Ad35 fiber-shaft domain. The conserved glycines or prolines in the repeats, the residues involved in the hydrophobic patches, and the residues possibly forming the peripheral hydrophobic patches are colored in purple, yellow, and blue, respectively. (C) Pseudo model of a trimeric Ad35 fiber knob and shaft.
Fiber structure in Ad5F35 and its interaction with the penton base.
The knob, shaft, and tail domains of the Ad35 fiber are well resolved in our Ad5F35 structure (Fig. 1A), but the density map for the fiber shaft is weaker at progressively greater distances from the penton base, suggesting that the Ad35 fiber is flexible. The fiber is located at the 5-fold axis on a central pore in the penton base. The size of the central pore is approximately 25 Å in diameter in our Ad5F35 structure. No density was observed inside the central pore (Fig. 4A and B). Five fiber tails that bind at the 5 grooves between neighboring penton base monomers are clearly visible in our Ad5F35 structure (Fig. 4A and B), resembling those reported for Ad5 (19) and Ad2 (45). It is reasoned that five copies of tail fibers are observed due to the imposed icosahedral 5-fold symmetry, although only three tails bind the penton base. Considering that residues 11 to 17 in the fiber tail domain are conserved among the human Ads (Fig. 3A), we fitted these residues into the tail density map (Fig. 4C). The interactions between the fiber tail and the penton base include hydrophobic interactions and probable hydrogen bonds (Fig. 4D), which are similar to the interaction reported for Ad5. These interaction sites in the penton base protein are fully conserved among the six human Ad subgroups, A to F (Fig. 4E).
Fig 4.
Fiber-penton base interaction. (A) Density maps of a penton base (gray) and a fiber (orange) segmented from our 4.2-Å-resolution structure of the Ad5F35 virion (left and middle). Cut-away view of the density map of a penton base interacting with a fiber shows the absence of densities inside the central pore (right). (B) Penton base (gray) and fiber tail (orange). The fiber has been computationally removed for clarity. Five fiber tail densities are visible due to the imposed 5-fold symmetry; two positions (circled) should be left unoccupied. (C) Cross-eye stereo view of the atomic model of the fiber tail (amino acids 11 to 17) superimposed on its density map (mesh). (D) Cross-eye stereo view of the interactions (ribbon) between the fiber tail (orange stick view) and the two copies of adjacent penton base proteins (gray and cyan ribbons) show a hydrophobic groove formed by a number of hydrophobic residues (yellow) in one subunit of the penton base protein (cyan) and a few putative hydrogen bonds (purple) involving residues Asn12 (fiber) and Glu203, Asn12 (fiber) and Arg197, Val14 (fiber) and Arg496, and Tyr15 (fiber) and His494 between the fiber tail and another subunit of the penton base protein (gray). (E) Amino acid sequence alignment of penton base protein from different human Ad serotypes in six species (A to F) shows that the residues involved in hydrophobic interactions (yellow) and hydrogen interactions (purple) with fiber tail are fully conserved.
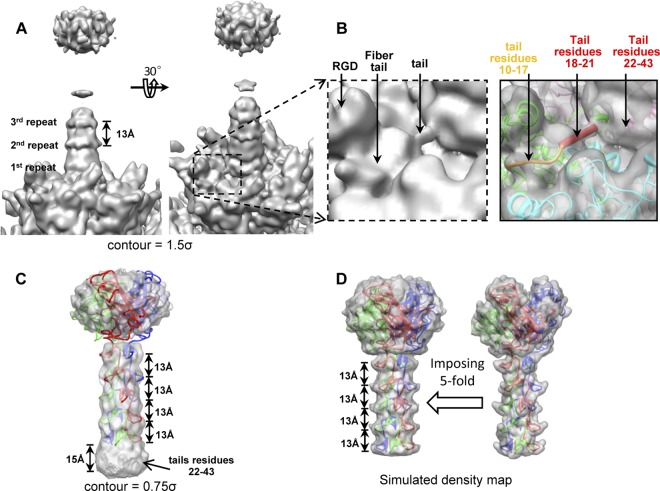
On filtering the structure of the fiber and the penton base to a 7-Å resolution and setting a display contour level of 1.5σ (σ is the standard deviation of the density map) (Fig. 5A and B), a connection between the shaft bottom and the penton base is observed, which is apparently missing in the 4.2-Å-resolution density map (Fig. 4A and B). The length of this connection is approximately 14 Å, which accounts for 4 residues (residues 18 to 21) in the fiber tail (assuming a length of 3.6 Å per residue). Alignment of the β-repeats in the shaft indicates that the shaft domain starts with residue 44, leaving residues 22 to 43 unaccounted for. We segmented out the fiber shaft and knob domains (Fig. 5C) from the 7-Å-resolution structure of Ad5F35 and compared this with a simulated fiber density map containing the knob and the shaft (Fig. 5D) generated from the pseudo model (Fig. 3C) of the Ad35 fiber. Because the 5-fold symmetry is imposed on the Ad35 trimeric fiber during 3-D reconstruction, we simulated the effect of imposing a 5-fold symmetry on our pseudo model of the Ad35 trimeric fiber for comparison. When segmented from the 7-Å-resolution Ad5F35 density map and with the display contour set to 0.75σ, an intact fiber structure is visible, showing 5 layered repeats at approximately 13-Å distances (Fig. 5C). Compared with the simulated fiber density map (Fig. 5D), these repeats correspond to the β-repeats in the shaft. However, the fiber appears longer than the pseudo model, and a 15-Å-long extra region at the bottom of the fiber is observed that cannot be attributed to the β-repeats. Considering that the pseudo fiber model only contains the knob and shaft domains (residues 44 to 323) and that residues 11 to 21 in the tail domain are assigned to the peptide that binds to the penton base and to the connection between the shaft bottom and the penton base (Fig. 5B), the extra length of 15 Å in the density map can be assigned to the residues previously unaccounted for, that is, residues 22 to 43 in the tail domain. This assignment is in agreement with the ideal suggested by a previous structural analysis of Ad5F35 (32). A clearer structure of the β-repeats is observed when the display contour is set to a higher level of 1.5σ although only β-repeats 1 to 3 are visible (Fig. 5A). When the display contour is set to an even lower level to show the weakest density, the upper part of the shaft only enlarges slightly, indicating that the fiber bend is limited to a very narrow angle and that the fiber shaft is relatively rigid (Fig. 6).
Fig 5.
Structure of the fiber. (A) The structure of the fiber and part of the penton base filtered at a resolution of 7 Å shows that the fiber tail connects with the penton base. The display contour level was set to 1.5σ. (B) Zoomed-in view of a fiber tail in panel A (left) and transparent view of the tail and the penton base fitted with the atomic model (right). The connection (shown as a red rod) between the tail and penton base is approximately 14 Å in length. (C) Structure of the Ad35 fiber segmented from the structure of the Ad5F35 virion at 7 Å with the display contour level set to 0.75σ. (D) Simulated density map of an Ad35 fiber using the pseudo model (right), and simulated density map of an Ad35 fiber with imposed 5-fold symmetry (left).
Fig 6.

Fiber structure. (A) Structure of the fiber and part of the penton base is displayed at a very low contour level to show the weakest density. (B) Schematic illustrations of the fiber and the penton base, showing that the fiber bend is limited to a very narrow angle. Only one of the three fiber tails and one of the five RGD loops (black dashed line) are illustrated here.
Model of Ad5F35 interaction with receptors.
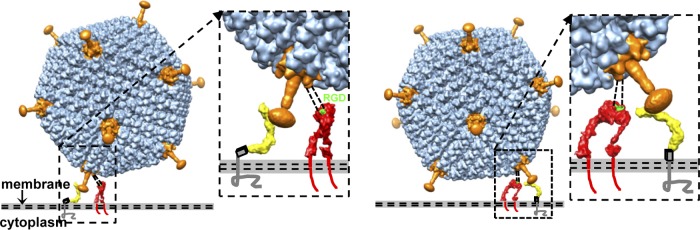
The attachment of the Ad35 fiber knob to its primary receptor, CD46 (11), is followed by the interaction of an RGD motif in the penton base with integrin, a secondary receptor, to promote subsequent steps for cell entry (16, 39, 40). The two-step receptor binding process involves concerted efforts from the fiber and the penton base. Previous studies indicated that the flexibility and length of the fiber could affect the fiber-receptor interaction or prevent the penton base from interacting with the integrin due to steric collision between the host cell membrane and the virus capsid (5, 41). Based on the model proposed in reference 5, for Ads with short and rigid fibers, only the top of the knob but not the side must be available for binding to the receptor. A recent structural analysis of the CD46 extracellular domain complexed with the Ad11 knob shows that CD46 interacts with the side of the Ad11 knob (27). Thus, how does the Ad5F35, with short and rigid fibers, interact with its receptor, CD46? Given that Ad35 and Ad11 belong to the same human Ad subgroup (subgroup B) and that their fiber knobs share a high sequence identity of 50%, it is reasonable to postulate that they have similar interactions with CD46. We docked the crystal structure of the Ad11 knob complexed with CD46 into our Ad5F35 structure in an attempt to reveal the interaction between Ad5F35 and CD46 on a host cell membrane. There are two extreme conformational possibilities of CD46 on the cell surface (27). Although it is difficult to ascertain the positioning of CD46 on the cell surface, using both of the extreme conformations of CD46 in our analysis, we concluded that Ad35 fibers can correctly interact with CD46 without introducing any steric collision between the host cell membrane and the virus capsid (Fig. 7).
Fig 7.
Model of the interaction between Ad5F35 and its receptors. The penton bases and the fibers are in orange. The left view and right view show two extreme possibilities for the conformation of CD46 (yellow) on the cell surface. The penton base can always bind integrin (red) due to the flexible RGD loop (shown as black dashed lines). The RGD motif that binds integrin is in green. The gray-and-black box stands for the STP region (rich in serine, threonine, and proline), comprising approximately 30 amino acids that are not included in the CD46 structure (see reference 27).
The penton base protein in our Ad5F35 structure is missing a loop of 78 residues (residues 297 to 374) containing the RGD motif, perhaps due to its flexible nature. The RGD motif in the loop serves to bind integrin, a secondary receptor, on the cell surface (40, 44). The RGD motif comprises residues 340 to 342 in the middle of the loop. Based on the alignment of the Ad5 and Ad35 penton base proteins, the Ad35 penton base protein has a similar RGD loop of 59 residues (residues 307 to 365) and the RGD motif comprises residues 341 to 343 in the loop. The flexible loop can therefore project the RGD motif ∼115 Å (for Ad5) or ∼84 Å (for Ad35) away from the surface of the penton base (assuming a length of 3.6 Å per residue) to bind to the secondary receptor, integrin. We chose to use a model of integrin that adopts an extended conformation described previously (16). The sufficient mobility of the RGD loop allows the RGD motif to easily bind to the integrin irrespective of whether the integrin adopts an extended or a bent conformation (Fig. 6B and 7).
DISCUSSION
Ad5 is the most commonly used vector in gene therapy applications. However, the widespread distribution of Ad5 receptors in the host allows the Ad5 vector to infect a wide range of cells in the host, resulting in unsuccessful attempts to restrict it to specific tissues for safety and efficiency. Moreover, a number of tissues which represent important targets for gene therapy are refractory to Ad5 infection due to the lack of these receptors (2). One possible approach to alter the Ad5 tropism is genetic modification of the Ad5 fiber. Understanding the fiber-penton base interaction is thus of significance to design Ad vectors with different tropisms. The Ad5F35 capsid with Ad35 tropism (24) was resolved at near-atomic resolution. We observe a different fiber-penton base interaction from that observed for the crystal structure of Ad5F35. By comparing our structure with the cryo-EM structure of Ad5 (19) and the crystal structure of an Ad2 penton base bound with an N-terminal fiber peptide (45), we demonstrated that these fiber-penton base interactions are structurally conserved and independent of fiber length. Indeed, our Ad5F35 fiber is a chimeric fiber composed of the Ad35 fiber shaft, the Ad35 C-terminal knob, and the Ad5 N-terminal fiber tail domains, while the Ad5F35 capsid analyzed by X-ray crystallography has an entire Ad35 fiber. However, the amino acids in the fiber tails of Ad5 and Ad35 are highly conserved and the residues in the Ad5 fiber tail that are involved in binding the penton base are fully conserved with those in the Ad35 fibers (Fig. 3A). We suggest that the fiber-penton base interaction observed in the crystal structure of Ad5F35 may reflect an intermediate stage during the virus's cell entry or assembly. It is worth noting that the crystallization by Reddy et al. took many weeks at room temperature, and it is also possible that this resulted in alterations to the capsid structure (29).
Based on our Ad5F35 structure, together with the model of CD46 complexed with the Ad11 knob and the model of integrin, we propose a new model of Ad-receptor interaction that reveals the interaction of Ads with short and rigid fibers with their primary and secondary receptors. The model of interaction between Ads and their receptors indicates that Ad5F35, with its short and rigid fibers, can bind the primary receptor CD46 and the secondary receptor integrin simultaneously. Our model negates a previous model in which only the top region of the knobs of Ads with short and rigid fibers is available for receptor binding, due to steric hindrance between the viral capsid and the host cell membrane (5). The previous model did not consider the importance of the conformation of the primary receptor (CD46), which could allow the relative positions of the Ad capsid and the cell membrane be situated to avoid steric hindrance between the viral capsid and the host cell membrane. This was most likely because at that time, CD46 was not known to be an Ad receptor (42); the crystal structure of CD46 complexed with Ad11 reveals such an Ad-CD46 interaction (27) that was not previously known. It is noteworthy that Ads with long fibers (i.e., human Ads of subgroups A, C, E, and F) use CAR as their primary receptors, while Ads with short and rigid fibers (i.e., human Ads of subgroup B) use CD46 or other non-CAR receptors as their primary receptors (1). The Ads with moderate fiber lengths (i.e., human Ads of subgroup D) use both CAR and CD46 as their primary receptors (1). Note that Ad41 in subgroup F has both short and long fibers, but only the long fiber binds to CAR on the host (31). These results are consistent with our model in that Ads with short and rigid fibers can attach to receptors CD46 and integrin simultaneously and with the model proposed by Wu et al. in that the attachment of Ads to the primary receptor CAR requires long and flexible fibers (41). The two models for long-fiber Ad–receptor and short-fiber Ad–receptor interactions can be taken into consideration for designing Ads with altered tropism by genetically modifying the Ad fibers.
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (grants 31070663, 31170706, and 31000333), the Scientific Foundation of the Ministry of Education and the Education Department of Hunan Province, China (grants 212119 and 11A124), the Program for Changjiang Scholars and Innovative Research Team at the University of China (IRT0964), and the National Basic Research Program of China (grant 2010CB912403).
Footnotes
Published ahead of print 5 September 2012
REFERENCES
- 1. Arnberg N. 2009. Adenovirus receptors: implications for tropism, treatment and targeting. Rev. Med. Virol. 19:165–178 [DOI] [PubMed] [Google Scholar]
- 2. Barnett BG, Crews CJ, Douglas JT. 2002. Targeted adenoviral vectors. Biochim. Biophys. Acta 1575:1–14 [DOI] [PubMed] [Google Scholar]
- 3. Bergelson JM, et al. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320–1323 [DOI] [PubMed] [Google Scholar]
- 4. Chiu CY, Mathias P, Nemerow GR, Stewart PL. 1999. Structure of adenovirus complexed with its internalization receptor, alpha(v)beta 5 integrin. J. Virol. 73:6759–6768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chiu CY, et al. 2001. Structural analysis of a fiber-pseudotyped adenovirus with ocular tropism suggests differential modes of cell receptor interactions. J. Virol. 75:5375–5380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60:2126–2132 [DOI] [PubMed] [Google Scholar]
- 7. Everts M, Curiel DT. 2004. Transductional targeting of adenoviral cancer gene therapy. Curr. Gene Ther. 4:337–346 [DOI] [PubMed] [Google Scholar]
- 8. Fabry CM, et al. 2005. A quasi-atomic model of human adenovirus type 5 capsid. EMBO J. 24:1645–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuller SD, Butcher SJ, Cheng RH, Baker TS. 1996. Three-dimensional reconstruction of icosahedral particles: the uncommon line. J. Struct. Biol. 116:48–55 [DOI] [PubMed] [Google Scholar]
- 10. Fuschiotti P, et al. 2006. Structure of the dodecahedral penton particle from human adenovirus type 3. J. Mol. Biol. 356:510–520 [DOI] [PubMed] [Google Scholar]
- 11. Gaggar A, Shayakhmetov DM, Lieber A. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408–1412 [DOI] [PubMed] [Google Scholar]
- 12. Greber UF, Willetts M, Webster P, Helenius A. 1993. Stepwise dismantling of adenovirus-2 during entry into cells. Cell 75:477–486 [DOI] [PubMed] [Google Scholar]
- 13. Henaff D, Salinas S, Kremer EJ. 2011. An adenovirus traffic update: from receptor engagement to the nuclear pore. Future Microbiol. 6:179–192 [DOI] [PubMed] [Google Scholar]
- 14. Kalin S, et al. 2010. Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35. J. Virol. 84:5336–5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liang Y, Ke EY, Zhou ZH. 2002. IMIRS: a high-resolution 3D reconstruction package integrated with a relational image database. J. Struct. Biol. 137:292–304 [DOI] [PubMed] [Google Scholar]
- 16. Lindert S, Silvestry M, Mullen TM, Nemerow GR, Stewart PL. 2009. Cryo-electron microscopy structure of an adenovirus-integrin complex indicates conformational changes in both penton base and integrin. J. Virol. 83:11491–11501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H, et al. 2010. Atomic structure of human adenovirus by cryo-EM reveals interactions among protein networks. Science 329:1038–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu H, et al. 2008. Symmetry-adapted spherical harmonics method for high-resolution 3D single-particle reconstructions. J. Struct. Biol. 161:64–73 [DOI] [PubMed] [Google Scholar]
- 19. Liu H, Wu L, Zhou ZH. 2011. Model of the trimeric fiber and its interactions with the pentameric penton base of human adenovirus by cryo-electron microscopy. J. Mol. Biol. 406:764–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ludtke SJ, Baldwin PR, Chiu W. 1999. EMAN: semiautomated software for high-resolution single-particle reconstructions. J. Struct. Biol. 128:82–97 [DOI] [PubMed] [Google Scholar]
- 21. Nakano MY, Boucke K, Suomalainen M, Stidwill RP, Greber UF. 2000. The first step of adenovirus type 2 disassembly occurs at the cell surface, independently of endocytosis and escape to the cytosol. J. Virol. 74:7085–7095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nemerow GR, Pache L, Reddy V, Stewart PL. 2009. Insights into adenovirus host cell interactions from structural studies. Virology 384:380–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicklin SA, Wu E, Nemerow GR, Baker AH. 2005. The influence of adenovirus fiber structure and function on vector development for gene therapy. Mol. Ther. 12:384–393 [DOI] [PubMed] [Google Scholar]
- 24. Nilsson M, et al. 2004. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J. Gene Med. 6:631–641 [DOI] [PubMed] [Google Scholar]
- 25. Ophorst OJAE, et al. 2004. An adenoviral type 5 vector carrying a type 35 fiber as a vaccine vehicle: DC targeting, cross neutralization, and immunogenicity. Vaccine 22:3035–3044 [DOI] [PubMed] [Google Scholar]
- 26. Pache L, Venkataraman S, Nemerow GR, Reddy VS. 2008. Conservation of fiber structure and CD46 usage by subgroup B2 adenoviruses. Virology 375:573–579 [DOI] [PubMed] [Google Scholar]
- 27. Persson BD, et al. 2010. Structure of the extracellular portion of CD46 provides insights into its interactions with complement proteins and pathogens. PLoS Pathog. 6:e1001122 doi:10.1371/journal.ppat.1001122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pettersen EF, et al. 2004. UCSF chimera: a visualization system for exploratory research and analysis. J. Comput. Chem. 25:1605–1612 [DOI] [PubMed] [Google Scholar]
- 29. Reddy VS, et al. 2010. Crystallization and preliminary X-ray diffraction analysis of human adenovirus. Virology 402:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reddy VS, Natchiar SK, Stewart PL, Nemerow GR. 2010. Crystal structure of human adenovirus at 3.5 angstrom resolution. Science 329:1071–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Roelvink PW, et al. 1998. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 72:7909–7915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saban SD, Nepomuceno RR, Gritton LD, Nemerow GR, Stewart PL. 2005. CryoEM structure at 9A resolution of an adenovirus vector targeted to hematopoietic cells. J. Mol. Biol. 349:526–537 [DOI] [PubMed] [Google Scholar]
- 33. Saban SD, Silvestry M, Nemerow GR, Stewart PL. 2006. Visualization of alpha-helices in a 6-angstrom resolution cryoelectron microscopy structure of adenovirus allows refinement of capsid protein assignments. J. Virol. 80:12049–12059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shayakhmetov DM, Lieber A. 2000. Dependence of adenovirus infectivity on length of the fiber shaft domain. J. Virol. 74:10274–10286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith TA, et al. 2003. Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum. Gene Ther. 14:777–787 [DOI] [PubMed] [Google Scholar]
- 36. Stewart PL, Burnett RM, Cyrklaff M, Fuller SD. 1991. Image reconstruction reveals the complex molecular organization of adenovirus. Cell 67:145–154 [DOI] [PubMed] [Google Scholar]
- 37. van Raaij MJ, Mitraki A, Lavigne G, Cusack S. 1999. A triple beta-spiral in the adenovirus fibre shaft reveals a new structural motif for a fibrous protein. Nature 401:935–938 [DOI] [PubMed] [Google Scholar]
- 38. Wang H, et al. 2007. Identification of CD46 binding sites within the adenovirus serotype 35 fiber knob. J. Virol. 81:12785–12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wickham TJ, Filardo EJ, Cheresh DA, Nemerow GR. 1994. Integrin alpha v beta 5 selectively promotes adenovirus mediated cell membrane permeabilization. J. Cell Biol. 127:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. 1993. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 73:309–319 [DOI] [PubMed] [Google Scholar]
- 41. Wu E, et al. 2003. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 77:7225–7235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wu E, et al. 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78:3897–3905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiong JP, Stehle T, Goodman SL, Arnaout MA. 2004. A novel adaptation of the integrin PSI domain revealed from its crystal structure. J. Biol. Chem. 279:40252–40254 [DOI] [PubMed] [Google Scholar]
- 44. Xiong JP, et al. 2002. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 296:151–155 [DOI] [PubMed] [Google Scholar]
- 45. Zubieta C, Schoehn G, Chroboczek J, Cusack S. 2005. The structure of the human adenovirus 2 penton. Mol. Cell 17:121–135 [DOI] [PubMed] [Google Scholar]