Abstract
Purpose
Based on the promising activity and tolerability of flavopiridol administered with a pharmacokinetically-derived dosing schedule in chronic lymphocytic leukemia (CLL), we conducted a phase I study using this schedule in patients with advanced solid tumors.
Experimental Design
Flavopiridol was given IV as a 30-min loading dose followed by a 4-hr infusion weekly for 4 weeks repeated every 6 weeks. Dose-escalation was in cohorts of three patients using the standard 3+3 phase I study design. Blood samples were obtained for pharmacokinetic and pharmacodynamic studies.
Results
Thirty-four eligible patients with advanced solid tumors received a total of 208 doses (median 7, range 1–24). Total doses ranged from 40 – 105 mg/m2. The primary dose limiting toxicity was cytokine release syndrome (CKRS). No antitumor responses were observed. The mean peak plasma concentration across all doses was 1.65 ± 0.86 µM. Area under the concentration-versus-time curve (AUC0–∞) ranged from 4.31 to 32.2 µM·hr with an overall mean of 13.6 ± 7.0 µM·hr. Plasma flavopiridol concentrations and AUC increased proportionally with dose. There was no correlation between cytokine levels and clinical outcomes.
Conclusions
The maximum-tolerated dose of flavopiridol is 20 mg/m2 bolus followed by 20 mg/m2 infusion over 4 hours given weekly for 4 weeks on a 6-week cycle in patients with advanced solid tumors. Flavopiridol PK was notably different, and there was a higher frequency of CKRS, despite prophylactic steroids, seen in this patient group compared to previous studies with CLL using a similar dosing schedule.
Keywords: Flavopiridol, CDK inhibitor, Phase I trial, Solid tumors
Introduction
Flavopiridol is a synthetic flavone pan-cyclin dependent kinase (CDK) inhibitor and has been shown to globally downregulate transcription by potently inhibiting formation of the CDK 9/cyclin T complex, a kinase that activates RNA polymerase II.[1–3] Flavopiridol downregulates the antiapoptotic proteins Mcl-1 and X-linked inactivator of apoptosis (XIAP), and it has also been shown to directly bind DNA which represents another possible mechanism for cytotoxicity. [4–6] In vitro and in vivo studies revealed single agent activity of flavopiridol in a variety of solid tumors.[7–12] Moreover, sequence specific enhancement of chemotherapeutic effect was shown with various cytotoxic agents.[13, 14]
Phase I studies with flavopiridol have used different schedules of 1-, 24-,and 72-hour (hr) intravenous (IV) infusions.[15–17] Two phase I studies using a 72-hr infusion schedule were conducted in refractory solid tumors.[18, 19] Secretory diarrhea was the major dose-limiting toxicity (DLT), and a pro-inflammatory syndrome of fever, hypotension, fatigue and tumor pain was also observed in these studies. One patient with metastatic gastric cancer had a complete response and stayed disease-free for 48 months after stopping therapy.[19] Dose escalation in combination studies with taxanes using the 24-hr schedule of flavopiridol was limited by neutropenia and pulmonary toxicity.[20–22] Neutropenia was the main DLT in studies using the 1-hr infusion schedule.[23, 24]
With the promising pre-clinical activity of flavopiridol and evidence of significant differences in protein binding in humans compared to pre-clinical models, investigators at our institution performed a phase I study using a pharmacokinetically-based schedule of flavopiridol given IV as a 30-minute loading dose followed by a 4-hr infusion in patients with refractory chronic lymphocytic leukemia (CLL). The maximum-tolerated dose (MTD) was 30 mg/m2 bolus followed by 50 mg/m2 infusion. Target concentrations of 1.5 µM were achieved and maintained throughout the infusion using this schedule, and an impressive response rate was observed in this refractory patient population.[25, 26] A subsequent phase II study confirmed the approximately 50% response rate in refractory CLL patients.[27]
Based on the pre-clinical rationale and promising results of the PK-derived schedule in patients with refractory CLL, we performed a dose-escalation study of flavopiridol with a similar schedule in patients with advanced solid tumors with aims of identifying the MTD and studying the toxicity profile, pharmacokinetics and pharmacodynamics in this patient population.
Methods
Patients
Patients with histologically or cytologically confirmed solid tumors for which no curative treatment was available were eligible. Other eligibility criteria included age >18yrs, measurable disease, Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, adequate bone marrow, hepatic, and renal functions, and life expectancy of ≥ six months. Any number of prior therapies was allowed. Key exclusion criteria included uncontrolled brain metastasis and prior therapy with flavopiridol. This study (NCI 7204, OSU 04111) was approved by the Cancer Therapy Evaluation Program (CTEP) of the National Cancer Institute (NCI, Bethesda, MD) and the Institutional Review Board (IRB) of The Ohio State University.
Study design
Flavopiridol was administered through a central venous catheter as an IV loading dose over 30 minutes followed by a 4-hr infusion weekly for four consecutive weeks with a subsequent two week rest period. For the first dose, patients were admitted to the hospital the night before and were given IV hydration and urine alkalization for 10 hr pre- and post-treatment. Patients developing diarrhea during flavopiridol administration were given diarrhea prophylaxis for subsequent treatments. In the absence of cytokine release syndrome (CKRS) of grade 2 or greater severity during the first dose, subsequent doses of flavopiridol were given as an outpatient. Otherwise, doses were given as inpatient until no further symptoms of CKRS were observed.
The standard phase I 3+3 design was followed for dose escalation and 3–6 patients were entered per dose level (Table 1). No intra-patient dose escalation was allowed. After treatment of fourteen patients, amendments were made to the protocol to allow use of dexamethasone (20 mg) either after the 4-hr infusion or as a prophylactic treatment before the bolus (from dose level 2B).
Table 1. Dose limiting toxicities and Cytokine release syndrome.
| Dose level |
Bolus/Infusion mg/m2§ |
No. of Pts |
No. of DLTs |
Grade/Type of DLT (No. of Pts) |
No. of CKRS |
Grade of CKRS (No. of Pts) |
|---|---|---|---|---|---|---|
| 1 | 30/30 | 3 | 0 | 0 | 1 | Grade 1 (1) |
| 2 | 30/45 | 3 | 0 | 0 | 0 | 0 |
| 3 | 30/60 | 9 | 3 | Grade 5 CKRS (1) Grade 3 CKRS (1) Grade 3 LFT (1) |
6 | Grade 1 (1) Grade 2 (3) Grade 3 (1) Grade 5 (1) |
| 4 | 30/75 | 2 | 2 | Grade 3 CKRS (1) Grade 3 LFT (1) |
2 | Grade 2 (1) Grade 3 (1) |
| 2B* | 30/45 | 6 | 3 | Grade 3 CKRS (1) Grade 3 LFT (1) Grade 3 TLS (1) |
1 | Grade 3 (1) |
| 1B* | 30/30 | 3 | 2 | Grade 3 CKRS (1) Grade 3 LFT (1) |
1 | Grade 3 (1) |
| −1B* | 20/20 | 8 | 0 | 0 | 3 | Grade 1 (1) Grade 2 (2) |
Bolus/infusion mg/m2§ : Bolus given over 30 minutes followed by 4 hour infusion, B*: prophylactic dexamethasone 20 mg administered immediately prior to bolus, CKRS: Cytokine release syndrome, DLTs: Dose limiting toxicities, LFT: Liver functions tests (transaminases), TLS: Tumor lysis syndrome
Supportive care guidelines included treatment of diarrhea with loperamide (2 mg po q2 hr up to maximum of 16 mg per 24 hr), cholestyramine or colestipol if loperamide failed. Patients were closely monitored for symptoms of CKRS, including rigors, chills, fever (>101° F), profuse sweating, sinus tachycardia, hypotension, hypoxemia, dyspnea, confusion or disorientation. Patients who developed symptoms of CKRS were immediately treated with repeated doses of dexamethasone 20 mg IV until symptoms resolved or improved.
Objective response was assessed every 12 weeks using the Response Evaluation Criteria in Solid Tumors (RECIST).[28] Adverse events (AEs) were graded according to the National Cancer Institute’s Common Toxicity Criteria (version 3.0).[29] Dose limiting toxicity (DLT) was originally defined as grade 3 or greater non-hematologic AEs (except grade 3 fatigue or diarrhea resolving in 4 days), grade 4 hematological AEs lasting for ≥ 7 days, and recurrent CKRS of grade 2 severity despite prophylactic steroids. After 20 patients were treated, the definition of DLT was amended to exclude grade 3 electrolyte abnormalities, isolated grade 3 elevation of uric acid, grade 3 or 4 liver function abnormalities that persist for ≤ 7 days, and grade 3 tumor lysis syndrome (TLS).
Pharmacokinetic Studies
Venous blood samples drawn into heparinized tubes were obtained during the first dose immediately prior to, and at 0.5, 1, 3, 4, 5, 6, 8, 12, 24, and 48 hrs after start of infusion. Plasma samples were stored until analysis with a validated liquid chromatography/tandem mass spectrometry assay as described previously.[26] WinNonlin version 3.0 (Pharsight, Mountain View, CA) was used to generate non-compartmental PK parameter estimates from individual concentration-time profiles. Flavopiridol protein binding was measured in patient PK samples using an HTD 96 reusable micro-equilibrium dialysis device (HTDialysis, Gales Ferry, CT) as described in the Supplementary Data.
Pharmacodynamic studies
To characterize the immunomodulatory effects of flavopiridol, we obtained 30 mL of peripheral blood in heparinized tubes within 10 days prior to first dose of flavopiridol, immediately prior to the start of the bolus dose, and at 1 and 8 hrs after completion of the 4-hr infusion. Pre-dose and 1-hr post-infusion samples were also obtained on day 15 of cycle 1, days 1 and 15 of cycle 2, and within 4 weeks after the last dose of flavopiridol. Cytokine levels (IL-2, IL-4, IL-6, IL-10, TNF-α and IFNγ) in stored serum samples were determined by cytokine bead array (CBA, BD Biosciences, San Jose, CA) and enzyme linked immunosorbent assay (Pharmingen, San Diego, CA) following manufacturers recommendations.
Results
Between February 2006 and June 2009, a total of 34 patients with advanced solid tumors were enrolled and received treatment on the study at The Ohio State University. The predominant tumor sites were gastrointestinal (N=13), endocrine (N=6) and lung (N=5). Thirty-one patients (91%) had visceral involvement. Seventeen patients (50%) were heavily pretreated with 4 or more prior chemotherapy regimens. Patient demographics are outlined in Table 2.
Table 2. Patient Characteristics.
| Patient Characteristics | N=34 (%) |
|---|---|
| Age | Median 63.5 (44–76) |
| Sex | Males 19 Females 15 |
| ECOG performance status | |
| 0 | 5 (15) |
| 1 | 29 (85) |
| Primary Tumor site | |
| Gastrointestinal Esophagus Colon Small Bowel Rectal Pancreas |
2 (6) 6 (18) 1(3) 3 (9) 1 (3) |
| Endocrine Adrenocortical Neuroendocrine Thyroid |
2 (6) 4 (12) 1 (3) |
| Genitourinary Endometrial Prostate Kidney |
3(9) 1 (3) 1 (3) |
| Lung | 5 (15) |
| Melanoma | 1 (3) |
| Breast | 2 (6) |
| Unknown primary | 1 (3) |
| Metastatic sites | |
| 1–3 | 29 (85) |
| > 3 | 5 (15) |
| Liver | 18 (53) |
| Lung | 20 (59) |
| Non-Visceral sites only | 3 (9) |
| Prior systemic chemotherapy regimens | |
| 0–3 | 17 (50) |
| ≥ 4 | 17(50) |
Treatment administered
Thirty four patients received a total of 208 doses of flavopiridol (median 7, range 1–24) at various dose levels as outlined in Table 1. Patients came off study for the following: 18 patients for progressive disease (PD), 6 patients for grade 3 AEs [CKRS (n=4), AST/ALT (n=2)] and seven withdrew consent (1 patient after 24 doses, 5 after 8 doses, and 1 after 2 doses), two patients died (one died of complications from therapy and another died of PD), and one was removed from study at the discretion of the treating physician because of concerns of toxicity with potentially minimal benefit.
Dose Limiting Toxicity (DLT)
The dose escalation schedule and the DLTs per dose level are outlined in Table 1. The MTD and the recommended phase II dose was established at dose level −1B with 20 mg/m2 bolus flavopiridol followed by 20 mg/m2 infusion for 4 hours, given with prophylactic dexamethasone 20 mg IV immediately prior to the bolus infusion. A total of 8 patients were treated at this dose level with two patients being non-evaluable for DLT as they did not complete the first cycle (one patient withdrew consent and another progressed). The major limiting factor to dose escalation and tolerability of flavopiridol in this patient population was CKRS. Onset of CKRS symptoms occurred approximately 4 hours after completion of flavopiridol infusion, and symptoms usually improved quickly after treatment with dexamethasone and resolved within 6 hours. The most common presenting symptoms of CKRS were sinus tachycardia and profuse sweating. Other symptoms included dizziness, nausea, vomiting and diarrhea, and some patients experienced tumor pain, confusion and syncope (Table 3). Treatment of CKRS included IV dexamethasone 20 mg and other supportive measures such as IV fluids and oxygen. One death on the study was attributed to CKRS. This was a 70 year old man with metastatic gastrinoma and pheochromocytoma as part of the multiple endocrine neoplasia (MEN)-I syndrome. He had co-morbidities of hypertension, hyperlipidemia, diabetes and coronary artery disease. He developed Grade 2 CKRS during the first cycle and so had a dose reduction for cycle 2 and was treated as an inpatient. Approximately 4 hours after completion of the infusion (day 15 cycle 2), he developed diarrhea, profuse sweating, dizziness and dyspnea. He then went into cardiopulmonary arrest with pulseless electrical activity, and resuscitation efforts were unsuccessful. An autopsy showed fibrosis with myocardial disarray in the right and left bundles of the atrio-ventricular node and atherosclerotic deposits in the coronary arteries. Following this an amendment to the protocol was made to exclude patients with significant cardiac history and conduction abnormalities. Administration of prophylactic dexamethasone prior to bolus dose of flavopiridol was mandated, although this did not impact the frequency of CKRS. Other grade 3 or 4 AEs included neutropenia, lymphopenia and elevation of AST and ALT, which were all transient and self-limiting (Table 4).
Table 3. Frequency of symptoms in patients developing CKRS.
| Symptoms | Total no. of CKRS No. of events=23 |
Grade 1 CKRS No. of events=4 |
Grade 2 CKRS No. of events=13 |
Grade 3 CKRS No. of events=6 |
|---|---|---|---|---|
| Fever | 4% | 25% | 0 | 0 |
| Sinus tachycardia | 52% | 25% | 62% | 50% |
| Hypotension | 35% | 0 | 38% | 50% |
| Dizziness | 39% | 75% | 31% | 35% |
| Diaphoresis | 48% | 75% | 23% | 83% |
| Syncope | 13% | 25% | 15% | 0 |
| Tumor pain | 13% | 0 | 15% | 17% |
| Flushing | 4% | 0 | 0 | 17% |
| Confusion/disorientation | 17% | 25% | 0 | 50% |
| Nausea/Vomiting | 35% | 25% | 38% | 33% |
| Rigors/cold extremities | 22% | 25% | 15% | 33% |
| Diarrhea | 13% | 0 | 8% | 33% |
Table 4. Adverse Events.
| Adverse Events | Grade 1–2*: N=34 (%) | Grade 3§: N=34 | Grade 4§: N=34 |
|---|---|---|---|
| Hematologic | |||
| Leucopenia | 8 (24) | 11 | 1 |
| Neutorpenia | 6 (18) | 7 | 11 |
| Thrombocytopenia | 9 (26) | 0 | 0 |
| Anemia | 14 (41) | 9 | |
| Lymphopenia | 6 (18) | 21 | 9 |
| Febrile neutropenia | 1 | ||
| Electrolytes | |||
| Hypophosphatemia | 6 (35) | 7 | |
| Hypokalemia | 6 (35) | 9 | |
| Hypercalcemia | 1 | ||
| Hyperglycemia | 12 | 1 | |
| Hyperuricemia | 4 (12) | 1 | |
| GI/Hepatic | |||
| Nausea and/or vomiting | 20 (59) | ||
| Diarrhea | 19 (56) | 5 | |
| SBO/Ileus | 1 | ||
| Dysphagia | 1 | ||
| Elevated AST | 19 (56) | 3 | |
| Elevated ALT | 14 (41) | 4 | |
| Elevated Alk Phos | 2 | ||
| Hyperbilrubinemia | 4 (12) | ||
| Respiratory | |||
| Dyspnea | 5 | ||
| Hypoxemia | 2 | ||
| Constitutional | |||
| Fever | 4 (12) | ||
| Fatigue | 19 (56) | 8 | |
| Anorexia | 11 (32) | 1 | |
| Diaphories | 8 (24) | ||
| CNS | |||
| Pain | 17 (50) | 3 | |
| Somonolence | 4 (12) | ||
| Dizziness | 6 (35) | ||
| Cytokine release syndrome | 4 (12) | 5 | |
| Tumor lysis syndrome | 1 | ||
| Deep venous Thrombosis | 1 |
Grade 1–2 Adverse Events occuring in > 10% of patients attributable to flavopiridol
Grade 3–4 Adverse events are reported regardless of attribution/frequency.
Responses
Nine patients had stable disease (SD) and seventeen patients had PD after completing two cycles of flavopiridol. Only two of the nine patients with SD after two cycles continued therapy. One patient received a total of 6 cycles (dose level 1) then withdrew because of toxicities (duration of response (DOR)-24 weeks). Another patient received a total of 4 cycles (dose level −1B) then developed progressive disease (DOR-15 weeks). Seven patients who had shown SD as response after two cycles discontinued therapy because of apparent lack of benefit (1 patient showed progression two months after discontinuing flavopiridol (DOR-9 weeks) and received chemotherapy, 5 patients started alternate therapies immediately, 1 patient received no further therapy). The patient who developed tumor lysis syndrome after 1 dose received no further treatment and hence was not evaluable for response.
Pharmacokinetics
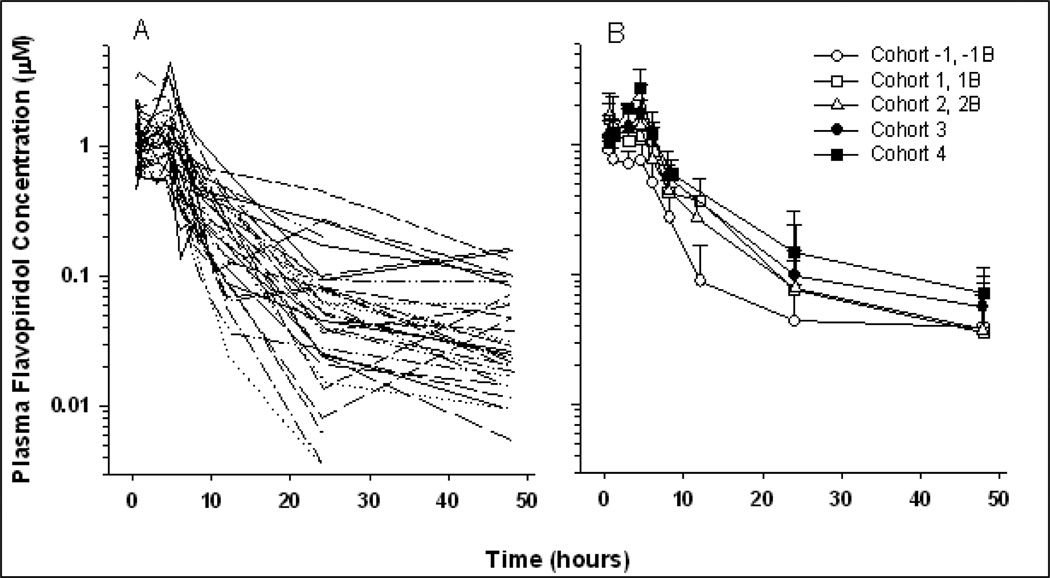
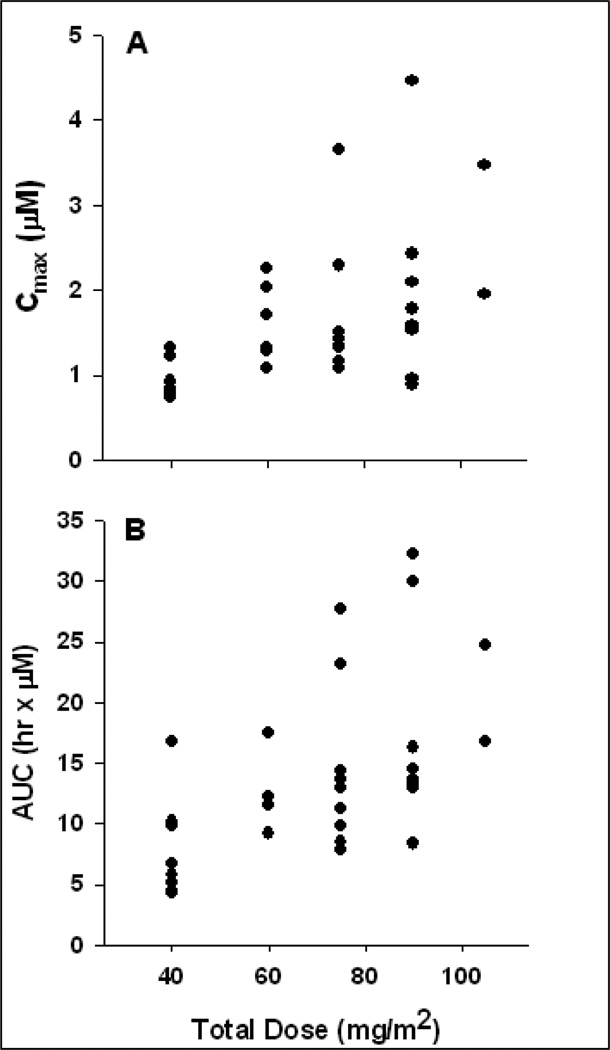
A total of 322 plasma concentrations from 34 patients were available for PK analysis. Figure 1 displays concentration-time profiles for all patients and among cohorts. A summary of non-compartmental parameter estimates is presented in Table 5. Area under the concentration-time curve (AUC0–∞) ranged from 4.31 to 32.2 µM·hr with an overall mean of 13.6 ± 7.0 µM·hr. Dose proportionality was evaluated over the 5 doses ranging from 40 to 105 mg/m2. Analysis of variance indicated a significant increase in maximum observed plasma concentrations with Cmax ranging from 0.94 to 2.71 µM at the lowest (40 mg/m2) and highest (105 mg/m2) doses, respectively (p = .035). This trend is also evident for AUC across the dose ranges (p = .047) with respective mean AUCs of 7.9 and 20.8 hrx·µM. Table 5 lists mean AUC and Cmax values for all dose levels, and Figure 2 displays these relationships graphically. Mean end-of-infusion concentrations (C0.5) for the 20 and 30 mg/m2 doses were 0.91 ± 0.26 and 1.42 ± 0.66 mM, respectively (p = .042).
Figure 1.
Plasma concentration vs. time profiles for individual patients (A) and cohort means ± SD (B).
Table 5. Non-compartmental pharmacokinetic parameter estimates.
Noncompartmental PK parameters were estimated for each individual on study using noncompartmental analysis in WinNonlin. Cohorts with equivalent doses were combined.
| Dose Level (Bolus+Infusion mg/m2) |
No. pts | K 1/hr |
T1/2 hr |
Tmax hr |
Cmax µM |
Conc0.5µM | Conc 4.5 µM |
AUCO-∞ hr x µM |
V2 L/m2 |
CL L/hr/m2 |
|---|---|---|---|---|---|---|---|---|---|---|
| −1, −1B (20+20) | 8 | 0.10 ± 0.08 | 14.8 ± 11.3 | 0.98 ± 1.00 | 0.94 ± 0.22 | 0.91 ± 0.26 | 0.75 ± 0.24 | 7.9 ± 4.2 | 117 ± 99 | 6.1 ± 2.5 |
| 1, 1B (30+30) | 6 | 0.07 ± 0.03 | 11.7 ± 4.6 | 1.09 ± 1.07 | 1.62 ± 0.46 | 1.59 ± 0.49 | 1.17 ± 0.44 | 13.2 ± 3.4 | 198 ± 82 | 11.9 ± 3.0 |
| 2, 2B (30+45) | 9 | 0.06 ± 0.02 | 15.6 ± 11.0 | 1.35 ± 1.50 | 1.79 ± 0.83 | 1.65 ± 0.85 | 1.41 ± 0.94 | 14.3 ± 6.8 | 307 ± 155 | 15.3 ± 5.8 |
| 3 (30+60) | 9 | 0.06 ± 0.02 | 13.8 ± 6.1 | 3.68 ± 1.92 | 1.92 ± 1.07 | 1.15 ± 0.54 | 1.78 ± 1.13 | 16.6 ± 8.6 | 309 ± 168 | 16.5 ± 7.0 |
| 4 (30+75) | 2 | 0.05 ± 0.01 | 14.1 ± 2.4 | 4.53 ± 0.00 | 2.71 ± 1.08 | 1.04 ± 0.05 | 2.71 ± 1.07 | 20.8 ± 5.6 | 260 ± 28 | 13.1 ± 3.6 |
| All | 34 | 0.07 ± 0.05 | 14.2 ± 8.4 | 2.02 ± 1.87 | 1.65 ± 0.86 | 1.30 ± 0.63 | 1.39 ± 0.90 | 13.6 ± 7.0 | 241 ± 149 | 12.7 ± 6.3 |
The table lists mean values ± standard deviations. N, number of patients in each group; k, elimination rate constant; T1/2, elimination half-life; Tmax, time of observed maximum concentration; cmax, maximum observed concentration; Conc0.5, observed concentration at the end of the bolus dose; C4.5, observed concentration at the end of the 4-hour infusion; AUC0-∞, observed area under the curve; Vz, volume of distribution; CL, clearance.
Figure 2.
A and B. Relationships between total dose and flavopiridol PK. Proportional increases in the means of both Cmax (A) and AUC0–∞ (B) were observed in this study.
To evaluate the role of protein binding in flavopiridol PK and outcomes, unbound drug levels were determined in all plasma samples collected in this study. Mean unbound flavopiridol, calculated for each patient by averaging all measurements between 0.5 and 8 hours into treatment, ranged from 0.52% to 4.82% among patients. Baseline albumin levels were generally low in this patient population (mean 2.7, range 1.7 – 3.8 dg/L). No trend was apparent between unbound flavopiridol and baseline albumin, as indicated in supplementary Figure 1. Additionally, protein binding was not correlated with non-compartmental PK parameters or observed maximum or end-of-infusion concentrations.
Pharmacokinetics vs. Outcomes
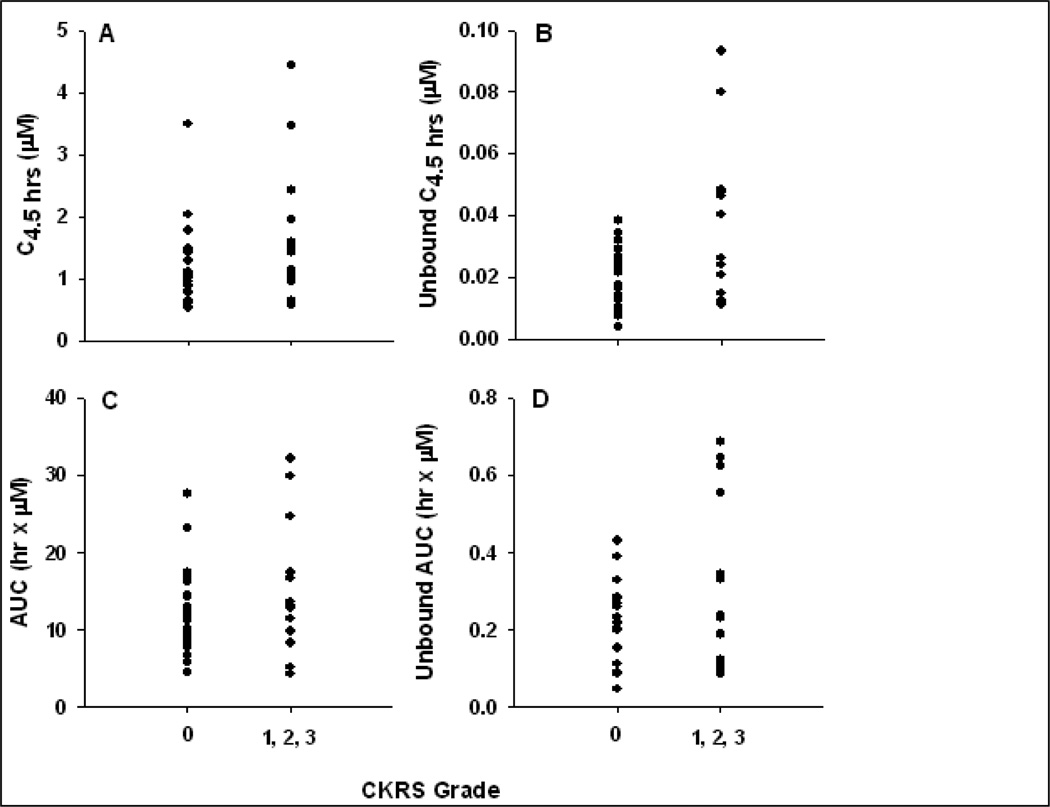
Previous reports have indicated an association between occurrence of CKRS (grade ≥ 1) and plasma flavopiridol AUC.[25, 26, 30] Non-compartmental PK parameter estimates were not significantly associated with CKRS in this study. However, when observed concentrations and AUCs were adjusted for protein binding, the anticipated associations were strengthened. Mean free flavopiridol plasma concentrations at the end of the 4-hour infusion were 20 ± 9 vs. 35 ± 26 nM in patients who did not experience CKRS (N=20) compared to those who experienced grade 1 or higher CKRS during cycle 1 when PK samples were collected (p = .084, two-tail Student’s t-test). Mean free flavopiridol AUC was also higher in patients with CKRS compared to that in patients without observed CKRS during cycle 1 (215 ± 103 vs. 318 ± 220 hr•nM, respectively), although this difference was not statistically significant (p=0.15). Figure 3 displays these relationships.
Figure 3.
Cytokine release vs. total and unbound flavopiridol. The relationship between cytokine release syndrome (CKRS) severity and total (A) or unbound (B) plasma flavopiridol at the end of the 4-hour infusion (C4.5hr) is displayed. Similar plots are displayed for AUC0–∞ calculated from total (C) or unbound (D) flavopiridol concentrations
Pharmacodynamics
Measurement of serum cytokines was performed prior to and after flavopiridol infusion. IL-6 serum levels were found to be associated with flavopiridol infusion. Supplementary figure 2 shows the association of IL-6 serum levels as a function of time following infusion. There were no significant associations observed between IL-6 levels and PK parameters or development of CKRS. (Supplementary Figure 2). The IL-6 levels did decrease in patients who received dexamethasone but this did not impact the incidence of CKRS (data not shown). No other cytokines were associated with flavopiridol infusion or with frequency of CKRS.
Discussion
Previous clinical studies using the 1-, 24-, and 72-hr infusion schedules were largely ineffective with limited activity observed in combination trials.[31–36] A novel PK-directed schedule of single-agent flavopiridol in CLL demonstrated marked activity with a 40% partial response rate, median progression-free survival of 10–12 months among responders, and a DLT of TLS.[25] Furthermore, this dosing schedule was shown to maintain higher plasma drug concentrations which correlated with outcomes.
This is the first report of this alternative schedule in patients with solid tumors. The MTD of this schedule in this patient population (20+20 mg/m2) was lower than that reported in refractory CLL patients (30+50 mg/m2), and the major DLT identified in this patient population was CKRS, which was observed in fourteen (41%) patients.[25] One patient died from grade 5 CKRS at the 30+60 mg/m2 dose level. The previous studies in CLL using this schedule demonstrated that increases in IL-6 levels correlate with CKRS, and that prophylactic administration of dexamethasone prevented both increases in IL-6 levels and severe CKRS. [25, 26, 30] In the current study, dexamethasone was administered to 12 patients prior to the bolus dose of flavopiridol to mitigate CKRS. Although dexamethasone decreased IL-6 levels within 4.5–8 hrs after flavopiridol infusion, this was not associated with decreased occurrence of CKRS. Additionally, CKRS and baseline and/or peak IL-6 levels were not correlated.
Pharmacokinetics for this schedule of flavopiridol was characterized for five dose levels in this study. Increasing doses resulted, on average, in proportional increases in Cmax and AUC, whereas nonlinear PK was reported in previous studies evaluating broad dose ranges with this dosing schedule and with a 72-hr continuous infusion.[37][38] Another notable feature of the PK in the current study is the apparently lower C0.5 compared to other studies utilizing the same 30 mg/m2 bolus dose and schedule in chronic and acute leukemia which reported cohort mean C0.5 concentrations between 1.6 and 2.5 µM. [25, 37, 39] Mean C0.5 for the 26 patients receiving the 30 mg/m2 loading dose in this study was 1.42 µM. We observed that the majority of patients on study had baseline albumin below normal levels with a median of 2.8 g/dL (range 1.7–3.8 g/dL), and we evaluated protein binding as a contributing factor to the apparently different PK of flavopiridol in this patient population compared to those previously reported. Protein binding was variable in these patients with an approximately 10-fold range from 0.5% to 5% mean unbound flavopiridol in plasma. Although no correlations between baseline albumin and PK parameters were apparent, we did note the association between PK and CKRS was strengthened when protein binding was taken into consideration. It is unclear at this point if this inter-subject variability in protein binding contributes to variability in outcomes.
The frequency and range of CKRS-like symptoms observed in this study are reported in Table 4. Diaphoresis was the most consistent symptom noted, although hypotension and sinus tachycardia were other common symptoms. While grade 1–2 hypotension, tumor pain during the infusion, and a proinflammatory syndrome of fever, fatigue and anorexia have been reported in previous studies in solid tumors using both the 1-hr and 72-hr continuous infusions[19, 23], the constellation of symptoms observed in our study may be unique to the bolus/infusion schedule. No correlations were established between cytokine levels and CKRS or other toxicities. Possible reasons for the lack of correlation may be that other type 1 or 2 cytokines were responsible or that the observed cluster of symptoms were related to factors other than cytokine release. Awareness and close surveillance for this side-effect is indeed very critical for future combination studies planned with this agent in this patient population.
The MTD and recommended phase 2 dose of 20 mg/m2 bolus followed by 20 mg/m2 infusion over 4 hrs, along with overall PK, AEs, and treatment tolerability were notably different in this patient population compared to those reported in patients with hematologic malignancies. The rationale for using this PK-derived schedule is based on the need to achieve and maintain active flavopiridol concentrations within the tumor. The observed lower plasma flavopiridol concentrations in this patient population imply drug concentrations that were required for single-agent activity in patients with CLL may not have been achieved within the solid tumors targeted in this study. Therefore, further development of single-agent flavopiridol with this PK-derived schedule in patients with advanced solid tumors will be challenging. No objective responses were observed in this study of heavily pretreated patients, which is consistent with previous reports of single-agent flavopiridol in advanced solid tumors. However, other reports indicate evidence of activity when flavopiridol is combined with chemotherapy, and therefore evaluation of the PK-derived schedule of flavopiridol combined with chemotherapy is warranted.[21, 22, 32, 40, 41]
Supplementary Material
Acknowledgements
This work was supported by Grant No. U01 CA76576 from the National Cancer Institute.
Footnotes
Preliminary results of this study were presented in part at the American Association of Cancer Research meeting, Los Angeles, CA, April 14–18 2007 and at the Annual American Society of Clinical Oncology meeting, Orlando, Fl, May 29-June 1, 2009
References
- 1.Carlson B, Lahusen T, Singh S, et al. Down-regulation of cyclin D1 by transcriptional repression in MCF-7 human breast carcinoma cells induced by flavopiridol. Cancer Research. 1999;59:4634–4641. [PubMed] [Google Scholar]
- 2.Lam LT, Pickeral OK, Peng AC, et al. Genomic-scale measurement of mRNA turnover and the mechanisms of action of the anti-cancer drug flavopiridol. Genome Biology. 2001;2 doi: 10.1186/gb-2001-2-10-research0041. RESEARCH0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Senderowicz AM. Flavopiridol: the first cyclin-dependent kinase inhibitor in human clinical trials. Investigational New Drugs. 1999;17:313–320. doi: 10.1023/a:1006353008903. [DOI] [PubMed] [Google Scholar]
- 4.Gojo I, Zhang B, Fenton RG. The cyclin-dependent kinase inhibitor flavopiridol induces apoptosis in multiple myeloma cells through transcriptional repression and down-regulation of Mcl-1. Clinical Cancer Research. 2002;8:3527–3538. [PubMed] [Google Scholar]
- 5.Kitada S, Zapata JM, Andreeff M, Reed JC. Protein kinase inhibitors flavopiridol and 7-hydroxy-staurosporine down-regulate antiapoptosis proteins in B-cell chronic lymphocytic leukemia. Blood. 2000;96:393–397. [PubMed] [Google Scholar]
- 6.Parker BW, Kaur G, Nieves-Neira W, et al. Early induction of apoptosis in hematopoietic cell lines after exposure to flavopiridol. Blood. 1998;91:458–465. [PubMed] [Google Scholar]
- 7.Bible KC, Kaufmann SH. Flavopiridol: a cytotoxic flavone that induces cell death in noncycling A549 human lung carcinoma cells. Cancer Research. 1996;56:4856–4861. [PubMed] [Google Scholar]
- 8.Drees M, Dengler WA, Roth T, et al. Flavopiridol (L86-8275): selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clinical Cancer Research. 1997;3:273–279. [PubMed] [Google Scholar]
- 9.Patel V, Senderowicz AM, Pinto D, Jr, et al. Flavopiridol, a novel cyclin-dependent kinase inhibitor, suppresses the growth of head and neck squamous cell carcinomas by inducing apoptosis. Journal of Clinical Investigation. 1998;102:1674–1681. doi: 10.1172/JCI3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambol EB, Ambrosini G, Geha RC, et al. Flavopiridol targets c-KIT transcription and induces apoptosis in gastrointestinal stromal tumor cells. Cancer Research. 2006;66:5858–5866. doi: 10.1158/0008-5472.CAN-05-2933. [DOI] [PubMed] [Google Scholar]
- 11.Schwartz GK, Farsi K, Maslak P, et al. Potentiation of apoptosis by flavopiridol in mitomycin-C-treated gastric and breast cancer cells. Clinical Cancer Research. 1997;3:1467–1472. [PubMed] [Google Scholar]
- 12.Wirger A, Perabo FG, Burgemeister S, et al. Flavopiridol, an inhibitor of cyclin-dependent kinases, induces growth inhibition and apoptosis in bladder cancer cells in vitro and in vivo. Anticancer Research. 2005;25:4341–4347. [PubMed] [Google Scholar]
- 13.Bible KC, Kaufmann SH. Cytotoxic synergy between flavopiridol (NSC 649890, L86-8275) and various antineoplastic agents: the importance of sequence of administration. Cancer Research. 1997;57:3375–3380. [PubMed] [Google Scholar]
- 14.Motwani M, Delohery TM, Schwartz GK. Sequential dependent enhancement of caspase activation and apoptosis by flavopiridol on paclitaxel-treated human gastric and breast cancer cells. Clinical Cancer Research. 1999;5:1876–1883. [PubMed] [Google Scholar]
- 15.Byrd JC, Peterson BL, Gabrilove J, et al. Treatment of relapsed chronic lymphocytic leukemia by 72-hour continuous infusion or 1-hour bolus infusion of flavopiridol: results from Cancer and Leukemia Group B study 19805.[see comment] Clinical Cancer Research. 2005;11:4176–4181. doi: 10.1158/1078-0432.CCR-04-2276. [DOI] [PubMed] [Google Scholar]
- 16.Flinn IW, Byrd JC, Bartlett N, et al. Flavopiridol administered as a 24-hour continuous infusion in chronic lymphocytic leukemia lacks clinical activity. Leukemia Research. 2005;29:1253–1257. doi: 10.1016/j.leukres.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Lin TS, Howard OM, Neuberg DS, et al. Seventy-two hour continuous infusion flavopiridol in relapsed and refractory mantle cell lymphoma. Leukemia & Lymphoma. 2002;43:793–797. doi: 10.1080/10428190290016908. [DOI] [PubMed] [Google Scholar]
- 18.Senderowicz AM, Headlee D, Stinson SF, et al. Phase I trial of continuous infusion flavopiridol, a novel cyclin-dependent kinase inhibitor, in patients with refractory neoplasms. Journal of Clinical Oncology. 1998;16:2986–2999. doi: 10.1200/JCO.1998.16.9.2986. [DOI] [PubMed] [Google Scholar]
- 19.Thomas JP, Tutsch KD, Cleary JF, et al. Phase I clinical and pharmacokinetic trial of the cyclin-dependent kinase inhibitor flavopiridol. Cancer Chemotherapy & Pharmacology. 2002;50:465–472. doi: 10.1007/s00280-002-0527-2. [DOI] [PubMed] [Google Scholar]
- 20.El-Rayes BF, Gadgeel S, Parchment R, et al. A phase I study of flavopiridol and docetaxel. Investigational New Drugs. 2006;24:305–310. doi: 10.1007/s10637-005-4343-5. [DOI] [PubMed] [Google Scholar]
- 21.Fornier MN, Rathkopf D, Shah M, et al. Phase I dose-finding study of weekly docetaxel followed by flavopiridol for patients with advanced solid tumors. Clinical Cancer Research. 2007;13:5841–5846. doi: 10.1158/1078-0432.CCR-07-1218. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GK, O'Reilly E, Ilson D, et al. Phase I study of the cyclin-dependent kinase inhibitor flavopiridol in combination with paclitaxel in patients with advanced solid tumors. Journal of Clinical Oncology. 2002;20:2157–2170. doi: 10.1200/JCO.2002.08.080. [DOI] [PubMed] [Google Scholar]
- 23.Tan AR, Headlee D, Messmann R, et al. Phase I clinical and pharmacokinetic study of flavopiridol administered as a daily 1-hour infusion in patients with advanced neoplasms. Journal of Clinical Oncology. 2002;20:4074–4082. doi: 10.1200/JCO.2002.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Whitlock JA, Krailo M, Reid JM, et al. Phase I clinical and pharmacokinetic study of flavopiridol in children with refractory solid tumors: a Children's Oncology Group Study. Journal of Clinical Oncology. 2005;23:9179–9186. doi: 10.1200/JCO.2004.01.0660. [DOI] [PubMed] [Google Scholar]
- 25.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109:399–404. doi: 10.1182/blood-2006-05-020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phelps MA, Lin TS, Johnson AJ, et al. Clinical response and pharmacokinetics from a phase 1 study of an active dosing schedule of flavopiridol in relapsed chronic lymphocytic leukemia. Blood. 2009;113:2637–2645. doi: 10.1182/blood-2008-07-168583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. Journal of Clinical Oncology. 2009;27:6012–6018. doi: 10.1200/JCO.2009.22.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 29.CTEP CTEP. Common Terminology Criteria for Adverse Events, Version 3.0. DCTD, NCI, NIH; DHHS Publish date: May 22,2003. [Google Scholar]
- 30.Messmann RA, Ullmann CD, Lahusen T, et al. Flavopiridol-related proinflammatory syndrome is associated with induction of interleukin-6. Clinical Cancer Research. 2003;9:562–570. [PubMed] [Google Scholar]
- 31.Aklilu M, Kindler HL, Donehower RC, et al. Phase II study of flavopiridol in patients with advanced colorectal cancer. Annals of Oncology. 2003;14:1270–1273. doi: 10.1093/annonc/mdg343. [DOI] [PubMed] [Google Scholar]
- 32.Carvajal RD, Tse A, Shah MA, et al. A phase II study of flavopiridol (Alvocidib) in combination with docetaxel in refractory, metastatic pancreatic cancer. Pancreatology. 2009;9:404–409. doi: 10.1159/000187135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu G, Gandara DR, Lara PN, Jr, et al. A Phase II trial of flavopiridol (NSC #649890) in patients with previously untreated metastatic androgen-independent prostate cancer. Clinical Cancer Research. 2004;10:924–928. doi: 10.1158/1078-0432.ccr-03-0050. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz GK, Ilson D, Saltz L, et al. Phase II study of the cyclin-dependent kinase inhibitor flavopiridol administered to patients with advanced gastric carcinoma. Journal of Clinical Oncology. 2001;19:1985–1992. doi: 10.1200/JCO.2001.19.7.1985. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro GI, Supko JG, Patterson A, et al. A phase II trial of the cyclin-dependent kinase inhibitor flavopiridol in patients with previously untreated stage IV non-small cell lung cancer. Clinical Cancer Research. 2001;7:1590–1599. [PubMed] [Google Scholar]
- 36.Stadler WM, Vogelzang NJ, Amato R, et al. Flavopiridol, a novel cyclin-dependent kinase inhibitor, in metastatic renal cancer: a University of Chicago Phase II Consortium study. Journal of Clinical Oncology. 2000;18:371–375. doi: 10.1200/JCO.2000.18.2.371. [DOI] [PubMed] [Google Scholar]
- 37.Blum WPM, Klisovic RB, Rozewski DM, Ni W, Albanese KA, Rovin B, Kefauver C, Devine SM, Lucas DM, Johnson A, Schaaf LJ, Byrd JC, Marcucci G, Grever MR. Phase I clinical and pharmacokinetic study of a novel schedule of flavopiridol in relapsed or refractory acute leukemias. Haematologica. 2010 Jul;95(7):1098–1105. doi: 10.3324/haematol.2009.017103. Epub 2010 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudek MA, Bauer KS, Jr, Lush RM, 3rd, et al. Clinical pharmacology of flavopiridol following a 72-hour continuous infusion. Annals of Pharmacotherapy. 2003;37:1369–1374. doi: 10.1345/aph.1C404. [DOI] [PubMed] [Google Scholar]
- 39.Lin TS, Blum KA, Fischer DB, et al. Flavopiridol, fludarabine, and rituximab in mantle cell lymphoma and indolent B-cell lymphoproliferative disorders. Journal of Clinical Oncology. 28:418–423. doi: 10.1200/JCO.2009.24.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah MA, Kortmansky J, Motwani M, et al. A phase I clinical trial of the sequential combination of irinotecan followed by flavopiridol. Clinical Cancer Research. 2005;11:3836–3845. doi: 10.1158/1078-0432.CCR-04-2651. [DOI] [PubMed] [Google Scholar]
- 41.Rathkopf D, Dickson MA, Feldman DR, et al. Phase I study of flavopiridol with oxaliplatin and fluorouracil/leucovorin in advanced solid tumors. Clinical Cancer Research. 2009;15:7405–7411. doi: 10.1158/1078-0432.CCR-09-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.