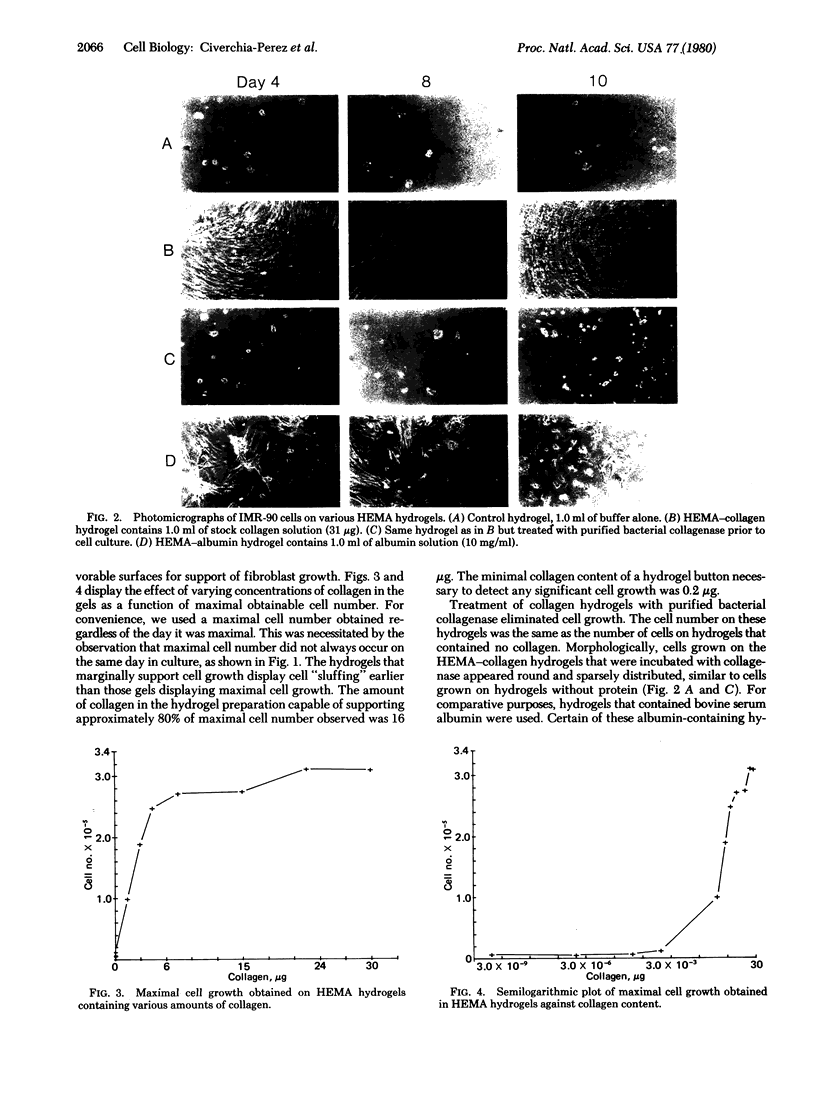
Abstract
Collagen-hydroxyethylmethacrylate hydrogels were prepared by polymerizing monomeric hydroxyethylmethacrylate in the presence of various concentrations of soluble native collagen. The resulting transparent hydrogels were evaluated as substrata for growth of IMR-90 human embryonic lung fibroblasts. Without collagen no significant growth occurred, whereas a dose-response curve expressing maximal cell growth against collagen concentration could be constructed quite readily by the use of appropriate hydrogels. The method allows for quantification of the collagen contribution to cell growth and, in a more general sense, provides the foundation for a relatively easy procedure to probe mechanisms of cell adhesion and cell differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruck S. D. Aspects of three types of hydrogels for biomedical applications. J Biomed Mater Res. 1973 Sep;7(5):387–404. doi: 10.1002/jbm.820070503. [DOI] [PubMed] [Google Scholar]
- Chung E., Miller E. J. Collagen polymorphism: characterization of molecules with the chain composition (alpha 1 (3)03 in human tissues. Science. 1974 Mar;183(130):1200–1201. doi: 10.1126/science.183.4130.1200. [DOI] [PubMed] [Google Scholar]
- Chvapil M., Holusa R., Kliment K., Stoll M. Some chemical and biological characteristics of a new collagen-polymer compound material. J Biomed Mater Res. 1969 Jun;3(2):315–332. doi: 10.1002/jbm.820030211. [DOI] [PubMed] [Google Scholar]
- Dillingham E. O., Webb N., Lawrence W. H., Autian J. Biological evaluation of polymers. I. Poly(methyl methacylate). J Biomed Mater Res. 1975 Nov;9(6):569–596. doi: 10.1002/jbm.820090605. [DOI] [PubMed] [Google Scholar]
- Dunn M. W., Nishihara T., Stenzel K. H., Branwood A. W., Rubin A. L. Collagen-derived membrane: corneal implantation. Science. 1967 Sep 15;157(3794):1329–1330. doi: 10.1126/science.157.3794.1329. [DOI] [PubMed] [Google Scholar]
- Dunn M. W., Stenzel K. H., Rubin A. L., Miyata T. Collagen implants in the vitreous. Arch Ophthalmol. 1969 Dec;82(6):840–844. doi: 10.1001/archopht.1969.00990020832021. [DOI] [PubMed] [Google Scholar]
- Dunn M., Shafer D., Stenzel K. H., Rubin A. L., Miyata T., Hopkins L., Powers M. J. Collagen as a vitreous heterograft. Trans Am Soc Artif Intern Organs. 1971;17:421–423. [PubMed] [Google Scholar]
- Faris B., Snider R., Levine A., Moscaritolo R., Salcedo L., Franzblau C. Effect of ascorbate on collagen synthesis by lung embryonic fibroblasts. In Vitro. 1978 Dec;14(12):1022–1027. doi: 10.1007/BF02616217. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Galin M. A., Chowchuvech E., Galin A. Tissue culture methods for testing the toxicity of ocular plastic materials. Am J Ophthalmol. 1975 Apr;79(4):665–669. doi: 10.1016/0002-9394(75)90808-9. [DOI] [PubMed] [Google Scholar]
- Galin M. A., Chowchuvech E., Galin A. Tissue culture studies of contact lenses. Ann Ophthalmol. 1976 Jun;8(6):669–672. [PubMed] [Google Scholar]
- Hay E. D. Interaction between the cell surface and extracellular matrix in corneal development. Soc Gen Physiol Ser. 1977;32:115–137. [PubMed] [Google Scholar]
- Hisada T. [The adhesion of culture cells to some polymers (in vitro)]. Shika Rikogaku Zasshi. 1976 May;17(38):91–101. [PubMed] [Google Scholar]
- Holly F. J., Refojo M. F. Wettability of hydrogels. I. Poly (2-hydroxyethyl methacrylate). J Biomed Mater Res. 1975 May;9(3):315–326. doi: 10.1002/jbm.820090307. [DOI] [PubMed] [Google Scholar]
- Hu C. L., Crombie G., Franzblau C. A new assay for collagenolytic activity. Anal Biochem. 1978 Aug 1;88(2):638–643. doi: 10.1016/0003-2697(78)90467-0. [DOI] [PubMed] [Google Scholar]
- KRAJICEK M., ZASTAVA V., CHVAPIL M. COLLAGEN--FABRIC VASCULAR PROSTHESES; BIOLOGICAL AND MORPHOLOGICAL EXPERIENCE. J Surg Res. 1964 Jul;4:290–296. doi: 10.1016/s0022-4804(64)80089-5. [DOI] [PubMed] [Google Scholar]
- Klebe R. J. Isolation of a collagen-dependent cell attachment factor. Nature. 1974 Jul 19;250(463):248–251. doi: 10.1038/250248a0. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Rosenberger P. G., Naylor S. L., Burns R. L., Novak R., Kleinman H. Cell attachment to collagen. Isolation of a cell attachment mutant. Exp Cell Res. 1977 Jan;104(1):119–125. doi: 10.1016/0014-4827(77)90074-x. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., McGoodwin E. B., Rennard S. I., Martin G. R. Preparation of collagen substrates for cell attachment: effect of collagen concentration and phosphate buffer. Anal Biochem. 1979 Apr 15;94(2):308–312. doi: 10.1016/0003-2697(79)90365-8. [DOI] [PubMed] [Google Scholar]
- Linsenmayer T. F., Gibney E., Toole B. P., Gross J. Cellular adhesion to collagen. Exp Cell Res. 1978 Oct 15;116(2):470–474. doi: 10.1016/0014-4827(78)90473-1. [DOI] [PubMed] [Google Scholar]
- Meier L., Hay E. D. Stimulation of corneal differentiation by interaction between cell surface and extracellular matrix. I. Morphometric analysis of transfilter "induction". J Cell Biol. 1975 Aug;66(2):275–291. doi: 10.1083/jcb.66.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier S., Hay E. D. Control of corneal differentiation by extracellular materials. Collagen as a promoter and stabilizer of epithelial stroma production. Dev Biol. 1974 Jun;38(2):249–270. doi: 10.1016/0012-1606(74)90005-0. [DOI] [PubMed] [Google Scholar]
- PEACOCK E. E., Jr, SEIGLER H. F., BIGGERS P. W. USE OF TANNED COLLAGEN SPONGES IN THE TREATMENT OF LIVER INJURIES. Ann Surg. 1965 Feb;161:238–247. doi: 10.1097/00000658-196502000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Rubin A. L., Powers M. J., Hopkins L. E., Stenzel K. H., Dunn M. W., Miyata T. Acellular corneal transparency. Nature. 1971 Mar 12;230(5289):120–121. doi: 10.1038/230120a0. [DOI] [PubMed] [Google Scholar]
- Rubin A. L., Riggio R. R., Nachman R. L., Schwartz G. H., Miyata T., Stenzel K. H. Collagen materials in dialysis and implantation. Trans Am Soc Artif Intern Organs. 1968;14:169–175. [PubMed] [Google Scholar]
- Sawyer P. N., Stanczewski B., Sivakoff S. I., Lucas T., Kirschenbaum D. Search for the ideal collagen vascular prosthesis. Trans Am Soc Artif Intern Organs. 1977;23:288–296. doi: 10.1097/00002480-197700230-00072. [DOI] [PubMed] [Google Scholar]
- Schachtschabel D. O., Blencke B. A. Effect of pulverized implantation materials (plastic and glass ceramic) on growth and metabolism of mammalian cell cultures. Eur Surg Res. 1976;8(1):71–80. doi: 10.1159/000127849. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Abe R., Teramatsu T., Okamura S., Hino T. Studies on copolymers of collagen and a synthetic polymer. First report--experimental study on biocompatibility of laminar copolymers of collagen and a synthetic polymer. Biomater Med Devices Artif Organs. 1977;5(1):49–66. doi: 10.3109/10731197709118665. [DOI] [PubMed] [Google Scholar]
- Stenzel K. H., Rubin A. L., Yamayoshi W., Miyata T., Suzuki T., Sonde T., Nishizawa M. Optimization of collagen dialysis membranes. Trans Am Soc Artif Intern Organs. 1971;17:293–298. [PubMed] [Google Scholar]
- Stenzel K. H., Sullivan J. F., Miyata T., Rubin A. L. Collagen dialysis membranes: initial clinical evaluation. Trans Am Soc Artif Intern Organs. 1969;15:114–117. [PubMed] [Google Scholar]




