Abstract
Purpose
In preclinical models, the histone deacetylase inhibitor vorinostat sensitizes breast cancer cells to tubulin polymerizing agents and to anti-vascular endothelial growth factor (VEGF) directed therapies. We sought to determine the safety and efficacy of vorinostat plus paclitaxel and bevacizumab as first-line therapy in metastatic breast cancer (MBC), and the biological effects of vorinostat in vivo.
Patients and Methods
Fifty-four patients with measurable disease and no prior chemotherapy for MBC received vorinostat (200 or 300 mg PO BID) on days 1–3, 8–10, and 15–17, plus paclitaxel (90mg/m2) on days 2, 9, 16 and bevacizumab (10mg/kg) on days 2 and 16 every 28 days. The primary objective of the phase I study was to determine the recommended phase II dose (RPTD) of vorinostat, and for the phase II to detect an improvement of response rate from 40% to 60% (alpha =0.10, beta=0.10).
Results
No dose limiting toxicities were observed, and the RPTD of vorinostat was 300 mg BID. For the primary efficacy analysis in 44 patients at the RPTD, we observed 24 objective responses (55%, 95% confidence intervals [C.I.] 39%, 70%). The adverse event profile was consistent with paclitaxel-bevacizumab, with the exception of increased diarrhea with the addition of vorinostat. Analysis of serial tumor biopsies in 7 patients showed increased acetylation of Hsp 90 and -tubulin following vorinostat.
Conclusions
Vorinostat induces histone and alpha tubulin acetylation and functional inhibition of Hsp90 in breast cancer in vivo and can be safely combined with paclitaxel and bevacizumab.
Keywords: Metastatic breast cancer, paclitaxel, bevacizumab, vorinostat, HDAC inhibitors
Introduction
Histone deacetylase inhibitors (HDACi) belong to the novel class of anticancer drugs that modulate gene expression by altering chromatin structure[1]. They induce acetylation of histone proteins, rendering chromatin more accessible to transcription factors resulting in modulation of gene transcription. In addition, pan-HDAC inhibitors (HDACi) increase acetylation of non-histone proteins, including heat shock protein (Hsp90), which results in inhibition of its chaperone function [2–4]. This results in proteasome targeting and degradation of multiple Hsp90 client proteins, including HER2, estrogen receptor (ER), AKT and c-RAF. Other non-histone proteins that are acetylated by HDAC inhibitors include transcription factors, p53, α-tubulin, DNA repair enzymes and other structural proteins [5–7].
Vorinostat (Zolinza, Merck, Whitehouse station, New Jersey), a hydroxamic derivative pan-HDACi that inhibits class I and II HDACs, was the first HDACi to be approved by the Food and Drug Administration for treatment of cutaneous T-cell lymphoma [8]. Phase I studies of intravenous and oral vorinostat in advanced solid tumors demonstrated safety and accumulation of acetylated histones both in tumor biopsies and in peripheral blood mononuclear cells [9]. The most common side-effects included fatigue, diarrhea, hyperglycemia, and thrombocytopenia. Although vorinostat exhibits biological effects as a single agent in preclinical models, it has limited activity when used as a single agent in MBC. Objective response was not observed in a phase II study of vorinostat in patients with MBC resistant to conventional therapy, although four patients exhibited disease stabilization [10]. However, considerable evidence suggests that HDACi such as vorinostat sensitize cancer cells to cytotoxic and antiangiogenic agents in preclinical models. For example, by inhibiting HDAC6 and promoting tubulin acetylation, vorinostat synergistically enhances the antiapoptotic effects of paclitaxel in lung cancer cell lines [5, 6]. In addition, by inducing acetylation and disrupting the association of Hsp90 with client proteins such as AKT, c-RAF and HER2, vorinostat abrogates survival signals that promote recovery from cytotoxic induced injury [5, 11, 12]. Finally, HDAC inhibitors also alter vascular endothelial growth factor (VEGF) signaling, exhibit antiangiogenic activity, and enhance the activity of other VEGF-directed antiangiogenic agents [13].
Based upon these considerations, we initiated a phase I–II clinical trial evaluating the combination of an intermittent schedule of vorinostat plus paclitaxel and bevacizumab as first line therapy for MBC, which had previously been shown to be more effective than paclitaxel alone in this setting [14]. We also evaluated the effects of vorinostat on histone (eg, H3, H4) and non-histone (Hsp90, alpha tubulin) protein acetylation (a measure of HDAC inhibition) and both Hsp70 and Hsp90 client protein levels (functional measures of Hsp90 inhibition), and p27 induction (a measure of cell cycle arrest) in metastatic tumors.
Methods
Patient Eligibility
Patients with histologically or cytologically confirmed metastatic or surgically inoperable locally advanced HER2-negative breast cancer were eligible for the study. Patients were required to have no prior chemotherapy for MBC and at least one measurable lesion as per Response Evaluation Criteria in Solid Tumors (RECIST Version 1.0) [15].. Other eligibility criteria were similar to those used in the pivotal E2100 trial (14).
After fifty patients (2 with serial tumor biopsies) were enrolled on the study and preliminary analysis revealed that the primary efficacy endpoint was met, the study was amended to include only patients who had disease that was accessible to biopsy and were agreeable to two serial biopsies before therapy and approximately 2 hours after the third vorinostat dose given during the first cycle; five such patients were accrued.
The trial was reviewed, approved, and sponsored by the Cancer Therapy Evaluation Program of the National cancer Institute (Clinicaltrails.gov, identifier NCT00368875). The local institutional review board at each individual participating center approved the protocol. All patients were required to sign a written, voluntary, informed consent.
Treatment plan
Vorinostat dose (200 or 300 mg BID) was assigned at the time of registration. Vorinostat was administered orally twice daily within 30 minutes of food intake on days 1–3, 8–10, and 15–17 of each 28 day cycle (ie, the day before, day of, and day after each paclitaxel dose). All patients also received paclitaxel at 90mg/m2 as a one hour infusion on days 2, 9, and 16 of every 28 day cycle. Each paclitaxel dose was given approximately 4 hours (range 2–6 hours) after the third vorinostat dose of each 3-day course. Paclitaxel was administered before bevacizumab. Standard premedications including dexamethasone were administered prior to paclitaxel. The dose of paclitaxel was reduced (75 mg/m2 and 60 mg/m2) for grade 3 or 4 febrile neutropenia, and/or for grade 3 or 4 non-hematological toxicity. Bevacizumab was administered on day 2 and day 16 of the 28 day cycle at 10mg/kg dose. Premedication was not required for the first dose and was used at the discretion of the physician in the event of an infusional reaction. Bevacizumab was discontinued for grade 4 hypertension, proteinuria, hemorrhage, venous thrombosis and grade 2 arterial thrombosis. If bevacizumab was withheld for toxicity, paclitaxel and vorinostat could be continued. Primary prophylaxis with growth factors was not allowed. Vorinostat dose escalation was carried out in the standard 3+3 phase I trial design based upon toxicity observed during the first cycle of therapy. Dose-limiting toxicities (DLT) were defined as grade 3–4 febrile neutropenia, thrombocytopenia and non-hematological toxicity attributed to therapy (nausea, vomiting and diarrhea would be considered dose limiting only if not adequately controlled with therapy). Any toxicity occurring during cycle 1 that resulted in dose reduction of vorinostat or paclitaxel or failure to complete all protocol specified doses in the first cycle was also considered a DLT. Three dose reductions were allowed for vorinostat (200mg BID, 100 mg BID and 100 mg QD), after which patients was removed from study. Vorinostat dose was required to be reduced for grade 3 or 4 febrile neutropenia and any grade 3–4 vorinostat associated toxicity. If paclitaxel was held for toxicity for reasons discussed above, the 3 day course of vorinostat was held or discontinued.
Serial biopsies at baseline and 2–6 hours after the third vorinostat dose on day 2 were performed in consenting patients. Optional blood specimens were obtained before the first vorinostat dose and 4 hours after the third vorinostat dose and just prior to paclitaxel infusion of cycle 1 in patients undergoing the biopsy.
Treatment was continued until disease progression or severe intolerance to therapy or withdrawal of consent or by physician discretion to initiate alternate therapy. Concurrent bisphosphonate therapy was allowed in patients with bone involvement.
Safety and efficacy assessment
All patients were seen on day 1 of each cycle prior to administration of vorinostat for a history, physical examination, performance status assessment, toxicity assessment, routine laboratory tests, UPC ratio, and for drug adherence using pill counts. Toxic effects were graded according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC), version 3.0. Tumor response was assessed at baseline and every three cycles according to the RECIST criteria until disease progression using computed tomography of the chest, abdomen and bone scan (which could be repeated after 6 cycles instead of 3 if baseline did not show evidence of metastatic disease at the discretion of the treating physician).
Statistical considerations
The primary endpoint of the phase I part of the study was to identify the recommended phase II dose (RPTD) of vorinostat in combination with paclitaxel and bevacizumab. Dose-escalation of vorinostat was based on the standard 3+3 phase I trial design. The primary endpoint of the phase II portion was objective response rate (ORR defined as complete response (CR) plus partial response (PR)). The study was designed according to Simon’s two-stage Minimax design [16] to detect an improvement of ORR from 40% to 60% (alpha 0.10, beta 0.10). In the first stage, 28 patients were to be entered at the RPTD with the study proceeding to the second stage if there were at least 12 responses. In the second stage, if 21 or more had an objective response among 41 eligible patients, the regimen would be declared promising. Assuming 10% would be nonevaluable/ineligible, at least 45 patients were planned to be accrued on the RPTD. The 40% response rate was chosen because it was consistent with the response rate in patients with measurable disease that was initially reported from the E2100 trial in 2005 [17], although in the final analysis of the study the response rate was reported to be 49% in patients with measurable disease [14]. The primary endpoint of ORR was estimated with 95% confidence interval using binomial proportions. Secondary endpoints of progression-free survival (PFS), and overall survival (OS) was assessed by Kaplan-Meier survival analysis and the 95% CI was calculated by the Greenwood’s formulae. PFS was defined as the time from first treatment day until death or objective or symptomatic progression (whichever is earlier). Similar to the censoring rules followed in the E2100 trial, for the PFS analysis, patients who discontinued protocol therapy prior to a PFS event and given alternative therapy were censored. OS was defined as the time from first treatment day until death. All eligible patients were included in the efficacy analysis and all treated patients were included in the safety analysis.
A secondary trial objective was to perform exploratory analysis to determine the biologic effects of vorinostat in human breast cancer and peripheral blood mononuclear cells (PBMCs) in vivo. The specific objectives included to determine whether treatment with vorinostat in tumor and concurrently collected PBMCs modulates acetyl lysine, acetylated K69 hsp90, hsp90, hsp70, c-RAF, p-AKT, AKT, CDK4, p27, p21 and acetylated histone H3 and H4 using methods described below. This required core biopsies to be performed before and 4 hours after the third vorinostat dose, which were snap frozen.
Materials and Methods for correlative studies are described in the supplementary documents.
Results
Patient characteristics
Between July 14, 2006 and December 7, 2009, a total of 54 patients were registered on the study and of these patients 53 were eligible and received treatment. One patient was registered, found to be ineligible due to prior chemotherapy for MBC, and never received protocol therapy. One patient who received treatment was later found to be ineligible as the metastatic site biopsy done just prior to enrollment demonstrated Her2 neu overexpression. The characteristics of all 54 treated patients are shown in Table 1. The median age was 54 years (range 27–77 years), 70% of patients had ER positive breast cancer, 96% of patients had ECOG performance status of 0 or 1, 74% had at least 2 metastatic sites, 61% had visceral involvement and over half of the patients (65%) had a disease-free interval of > 12 months.
Table 1.
Patient Characteristics
| Baseline characteristics | N=54 (%) | |
|---|---|---|
| Age | ||
| Median Range |
54 27–77 |
|
| Ethnicity/Race | ||
| Hispanic White Black Asian Unknown |
8 (15%) 37 (69%) 11 (20%) 2 (4%) 4 (7%) |
|
| Hormone receptor status | ||
| ER+/PR+ ER+/PR− ER−/PR− |
26 (48%) 12 (22%) 16 (30%) |
|
| ECOG performance status | ||
| 0 1 2 Unk |
34 (63%) 18 (33%) 1 (2%) 1 (2%) |
|
| Disease- free interval | ||
| 0–12 months >12 months |
19 (35%) 35 (65%) |
|
| No. of metastatic sites | ||
| 1 2 ? 3 |
14 (26%) 22 (41%) 18 (33%) |
|
| Metastatic sites | ||
| Lung Liver Non-visceral only |
18 (33%) 24 (44%) 21 (39%) |
|
| Prior adjuvant taxane chemotherapy | 16 (29%) | |
Results of phase I part of the study
No dose limiting toxicity occurred during the first cycle of therapy in the 3 patients treated at the first vorinostat dose level of 200 mg BID, and in 3 the patients treated at the second dose level of 300 mg BID. After accrual of 6 patients at the second dose level, the recommended phase II dose of vorinostat was defined as 300 mg BID, and all subsequent patients were enrolled at that dose level.
Treatment administered and adverse events
A total of fifty-four patients (phase I and II) received a total of 474 cycles of therapy. The median number of cycles given was 9 and mean was 8.8, with a range from 1–27 cycles. Ten patients required a dose reduction for vorinostat (19%), of whom 7 had an objective response to the treatment. Reasons for vorinostat dose reduction include grade 2 or greater diarrhea (N=3), nausea and vomiting (N=2), thrombocytopenia (N=2), fatigue (N=2) and headache (N=1). Paclitaxel was dose reduced in seventeen patients (31%) because of grade 2 or greater neuropathy (N=9), neutropenia (N=3), or a variety other reasons (N=5). Growth factor (pegfilgastrim) was used in 9 patients (17%) after first cycle.
Reasons for discontinuation of protocol therapy included progressive disease in 30 patients (56%), adverse events in 12 patients (22%), patient refusal to continue therapy in 8 patients (14%), physician discretion and changing to alternative therapy after achieving maximum response in 2 patients (4%), and other reasons in 2 patients (4%). Adverse events that led to treatment discontinuation in 12 patients included gastrointestinal toxicity in 3 patients, a treatment associated death in 1 patient (described below), and a variety of other causes in 8 patients (including cerebral aneurysm, nasal septum perforation, thromboembolism, myalgias, neuropathy, headache, thrombocytopenia, and infection).
Adverse events on all treated patients are summarized in Table 2. One patient died from sepsis and respiratory failure during cycle 3 of therapy. She was admitted to the local county hospital with exacerbation of chronic obstructive pulmonary disease, respiratory failure and sepsis and died within 24 hours. She also had grade 4 neutropenia and grade 3 thrombocytopenia. The most common grade 4 toxicities include neutropenia (7%), musculoskeletal pain (6%), thrombosis (4%), fatigue (2%), hypocalcemia (2%), proteinuria (2%), anemia (2%), and nasal septum perforation (2%). The most frequent grade 2–3 adverse events potentially attributed to vorinostat included fatigue (52%), nausea (31%), diarrhea (24%), anemia (24%), anorexia (20%), taste disturbances (19%), and rash (15%). Grade 2–3 hypertension was seen in 15% and headache in 13% of patients.
Table 2.
Adverse events during therapy in all 54 treated patients.
| Adverse Event | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Any Grade | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| Hematologic | |||||||||||
| Neutropenia | 6 | 11 | 14 | 26 | 11 | 20 | 4 | 7 | 35 | 65 | |
| Febrile neutropenia | 0 | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 2 | 4 | |
| Anemia | 21 | 39 | 11 | 20 | 2 | 4 | 1 | 2 | 35 | 65 | |
| Thrombocytopenia | 15 | 28 | 3 | 6 | 1 | 2 | 0 | 0 | 19 | 35 | |
| Infection | 0 | 0 | 15 | 28 | 4 | 7 | 0 | 0 | 19 | 35 | |
| Gastrointestinal | |||||||||||
| Diarrhea | 30 | 56 | 10 | 19 | 3 | 6 | 0 | 0 | 43 | 80 | |
| Nausea | 23 | 43 | 16 | 30 | 1 | 2 | 0 | 0 | 40 | 74 | |
| Vomiting | 16 | 30 | 5 | 9 | 4 | 7 | 0 | 0 | 25 | 46 | |
| Mucositis | 20 | 37 | 3 | 6 | 1 | 2 | 0 | 0 | 24 | 44 | |
| Constitutional | |||||||||||
| Fatigue | 16 | 30 | 19 | 35 | 9 | 17 | 1 | 2 | 45 | 83 | |
| Anorexia | 17 | 31 | 10 | 19 | 1 | 2 | 0 | 0 | 28 | 52 | |
| Taste disturbance | 14 | 26 | 10 | 19 | 0 | 0 | 0 | 0 | 24 | 44 | |
| Pain | 21 | 39 | 11 | 20 | 4 | 7 | 3 | 6 | 39 | 72 | |
| Arthralgia | 18 | 33 | 6 | 11 | 0 | 0 | 0 | 0 | 24 | 44 | |
| Neurologic | |||||||||||
| Headache | 15 | 28 | 4 | 7 | 3 | 6 | 0 | 0 | 22 | 41 | |
| Neuropathy | 14 | 26 | 11 | 20 | 12 | 22 | 0 | 0 | 37 | 69 | |
| Muscle weakness (Generalized) | 9 | 17 | 3 | 6 | 2 | 4 | 0 | 0 | 14 | 26 | |
| Dizziness | 13 | 24 | 3 | 6 | 2 | 4 | 0 | 0 | 18 | 33 | |
| Renal/Metabolic | |||||||||||
| Creatinine | 4 | 7 | 3 | 6 | 0 | 0 | 0 | 0 | 7 | 13 | |
| AST | 15 | 28 | 3 | 6 | 0 | 0 | 0 | 0 | 18 | 33 | |
| ALT | 11 | 20 | 4 | 7 | 0 | 0 | 0 | 0 | 15 | 28 | |
| Hypocalcemia | 13 | 24 | 2 | 4 | 0 | 0 | 1 | 2 | 16 | 30 | |
| Hypokaelemia | 5 | 9 | 0 | 0 | 1 | 2 | 0 | 0 | 6 | 11 | |
| Hyponatremia | 13 | 24 | 0 | 0 | 1 | 2 | 0 | 0 | 14 | 26 | |
| Proteinuria | 1 | 2 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 4 | |
| Vascular | |||||||||||
| Hypertension | 6 | 11 | 4 | 7 | 4 | 7 | 0 | 0 | 14 | 26 | |
| Thrombosis | 0 | 0 | 1 | 2 | 4 | 7 | 2 | 4 | 7 | 13 | |
| Epistaxis | 29 | 54 | 2 | 4 | 1 | 2 | 0 | 0 | 32 | 59 | |
| Skin | |||||||||||
| Rash | 12 | 22 | 8 | 15 | 0 | 0 | 0 | 0 | 20 | 37 | |
| Pulmonary | |||||||||||
| Dyspnea | 13 | 24 | 3 | 6 | 6 | 11 | 0 | 0 | 22 | 41 | |
Correlatives
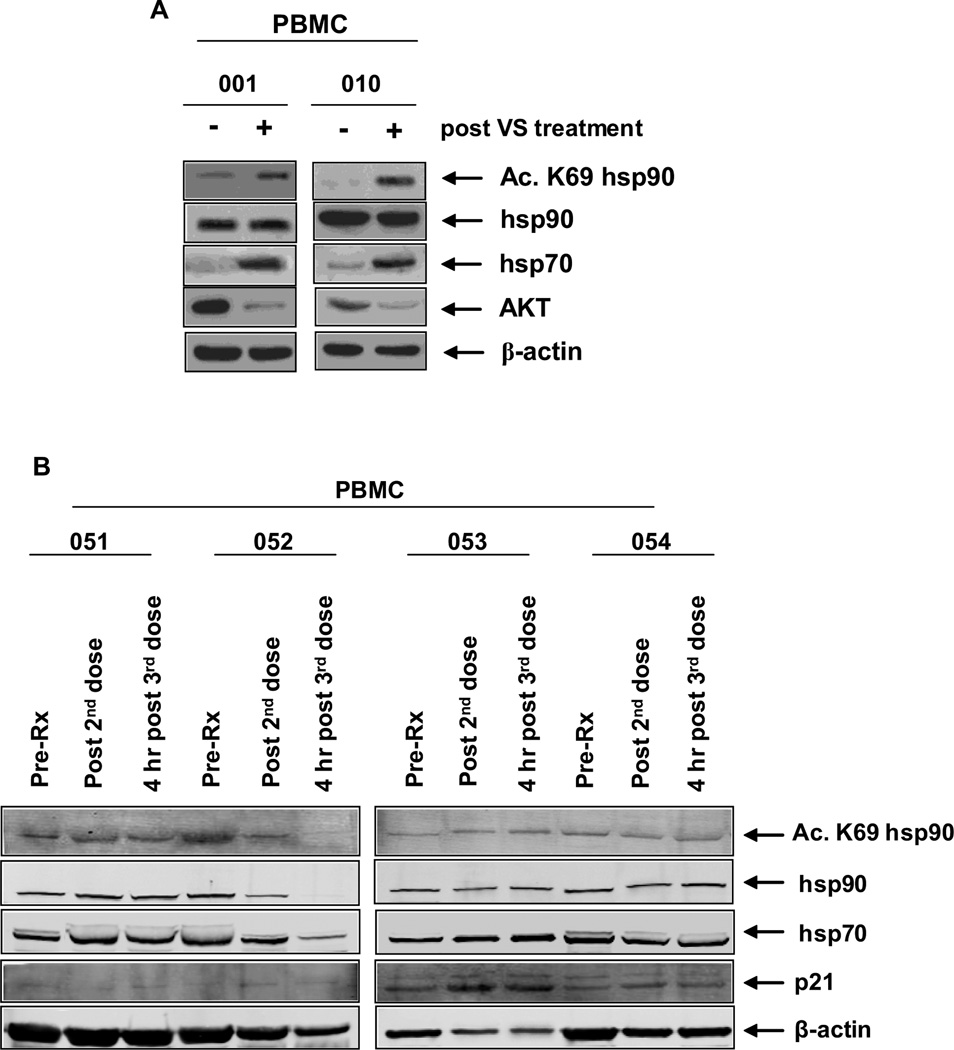
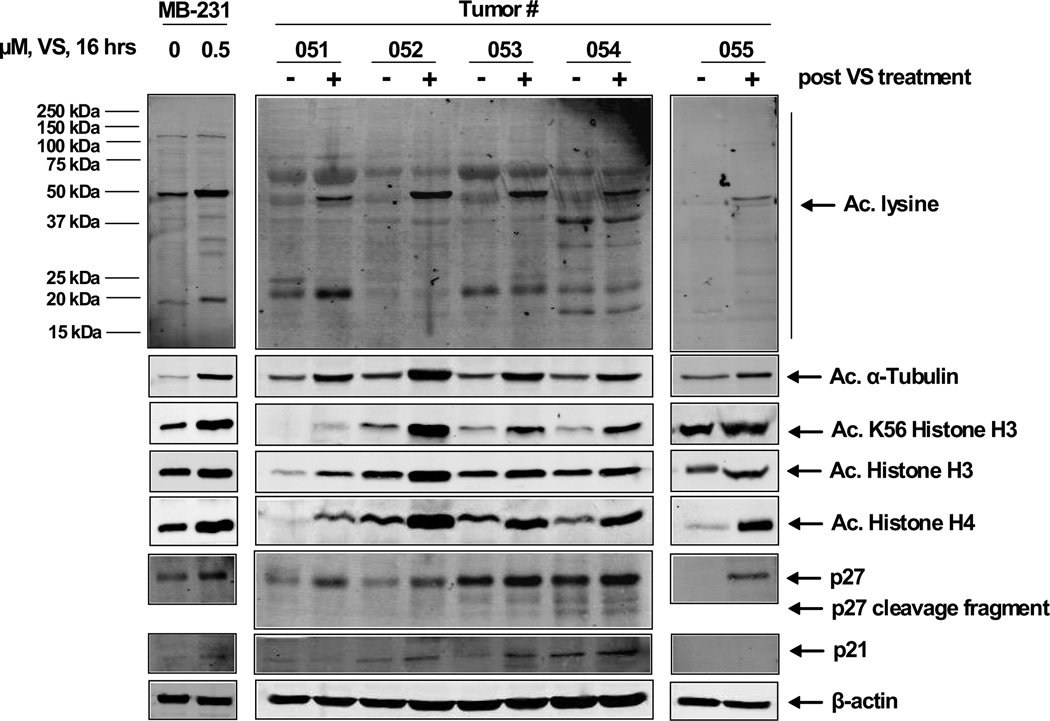
A total of seven patients (3 ER and/or PR positive and 4 ER/PR- negative) had pre and post-treatment tumor biopsy collected at baseline and on day 2, cycle 1 after third dose of vorinostat (of whom 6 also had PBMC collected), as shown in Figures 1–3. Comparison of the pre and post treatment (after exposure to vorinostat) tumor samples (N=6) revealed acetylation of the K69 residue of Hsp 90 chaperone protein by immunoblot analysis, demonstrating the in vivo effect of vorinostat (both in the 200 mg BID and 300 mg BID dose); similar changes were also seen in the PBMC of 3 patients. Hsp 70 was induced in 5 of 7 tumor samples (Table 3). Immunoblot analyses were performed for acetylated lysine, acetylated α-tubulin, acetylated K56 histone H3, acetylated histone H3, acetylated histone H4 in 5 tumor pairs and showed induction in all the samples. The cyclin-dependent kinase inhibitor p27 was induced in all the paired tumor tissue (N=5).
Figure 1. Treatment with vorinostat (VS) induces in vivo acetylation of heat shock protein (hsp) 90, induction of hsp70 and depletion of pAKT and AKT expression levels in ER positive and ER negative breast cancer cell.
A–B. Tumor biopsy specimens were collected from ER positive (#001, #010, and #53) and ER-negative (#51, 52, 54, and 55) patients prior to treatment with VS. Four hours following the third dose of VS, on Day 2, a second tumor biopsy was collected and cell lysates were prepared. Immunoblot analyses were performed for the acetylated-K69 of hsp90, total hsp90, hsp70, c-RAF, pAKT, AKT and CDK4 utilizing the tumor cell lysates. The expression levels of β-actin in the lysates served as the loading control.
Figure 3. Inconsistent in vivo effects of VS treatment in peripheral blood mononuclear cells (PBMCs) derived from ER-positive and ER-negative patients.
A. Peripheral blood was collected from patients #001 and #010 prior to the administration of VS on Day1 (Pre-Rx) and Day 2 (post-second dose) then 4 hours after dosing on Day 2 (post-third dose). PBMCs were separated by Ficoll Hypaque and cell lysates were prepared. Immunoblot analyses were performed for the acetylated-K69 of hsp90, hsp90, hsp70, and AKT on the total cell lysates. The expression levels of β-actin in the lysates served as the loading control. B. Peripheral blood was collected from patients #051, #052, #053 and #054 at the indicated time points and processed as in (A). Immunoblot analyses were performed for the acetylated-K69 of hsp90, hsp90, hsp70 and p21 on the total cell lysates. The expression levels of β-actin in the lysates served as the loading control.
Table 3.
Summary of molecular changes in the tumor on serial biopsy
| Patient # |
V- Dose (mg BID) |
ER/PR | # of Cycles |
BR | PFS (months) |
Ac. K69 Hsp90 |
Hsp90 | Hsp70 | c-Raf | AKT | pAKT | Ac. α- tubulin |
P27 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 001 | 200 | +/− | 12 | PR | 20.7 | ↑ | NC | ↑ | ND | ? | ND | ND | ND |
| 010 | 300 | +/+ | 8 | PR | 7.6 | ↑ | ND | ↑ | ND | ND | ND | ND | ND |
| 051 | 300 | −/− | 9 | SD | 8.9 | ↑ | NC | NC | ? | NC | ? | ↑ | ↑ |
| 052 | 300 | −/− | 3 | PR | 15.7 | ↑ | NC | ↑ | NC | NC | NC | ↑ | ↑ |
| 053 | 300 | +/− | 1 | NE | 14.0 | ↑ | NC | ↑ | NC | NC | NC | ↑ | ↑ |
| 054 | 300 | −/− | 6 | PR | 13.8 | ↑ | NC | NC | NC | NC | NC | ↑ | ↑ |
| 055 | 300 | −/− | 6 | SD | 5.6 | ND | NC | ↑ | NC | NC | NC | ↑ | ↑ |
V: Vorinostat; ER/PR: Estrogen and Progesterone receptor; BR: Best Response; PR: Partial Response; SD Stable Disease; NE: Non-evaluable; PFS: Progression-Free Survival; PBMC: Peripheral Blood Mononuclear Cells; Ac: Acetylation; NC: No change; ND: Not done
Efficacy
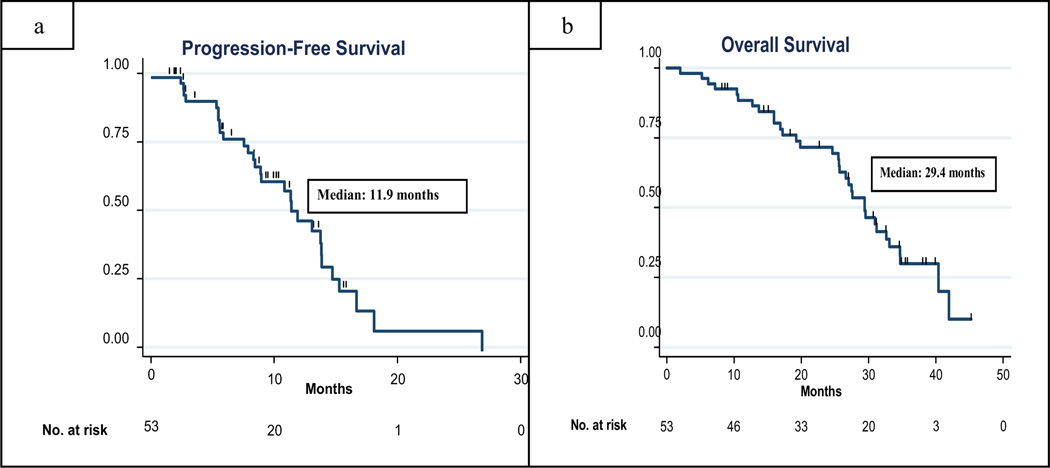
For the primary efficacy analysis in 44 eligible and evaluable patients treated at the recommended phase II dose, there were 24 objective responses (55%, 95% confidence intervals [C.I.] 39, 70%), indicating the achievement of a sufficient number of pre-specified responses required to consider the regimen promising. In an intention-to--treat-analysis including all 53 treated and eligible patients treated in the phase I and II portion of the trial, 26 (49%, 95% C.I. 37%, 60%) exhibited an objective response (2 CR and 24 confirmed PR) and 16 (30%) patients had stable disease for ≥ 24_weeks. Five (9%) patients had progressive disease as their best response. Six patients were considered not evaluable for response as they came off study prior to completion of 2 cycles (2 patients withdrew consent, 4 patients came off study for toxicity including grade 4 fatigue (n=1), grade 3 diarrhea and fatigue (n=1), grade 3 vomiting and fatigue (n=1), and grade 2 thrombocytopenia (n=1). Of the 26 patients who had an objective response, 7 had received adjuvant paclitaxel. Median progression-free survival in all 54 treated patients was 11.9 months (95% CI = 8.9, 13.9 months).and median overall survival was 29.4 months (95% CI. 25.6, 34.7 months). (Figure 4a–b).
Figure 4.
Progression-free survival (4A) and overall survival (4B) in all eligible patients were analyzed using Kaplan-Meier method.
Discussion
Numerous studies have evaluated the biological effects of vorinostat and other HDAC inhibitors in cell lines and animal models, but few have evaluated the in vivo effects of vorinostat in a solid tumor in humans[18, 19]. In a phase II study of vorinostat in recurrent glioblastoma which included 5 patients with paired samples before and after vorinostat (six doses of 200 mg), there was increased acetylation of histones H2B and H4 in 4 patients and H3 in 3 patients, and upregulation of E-cadherin in four patients. In another phase I study of vorinostat plus pelvic palliative radiation for gastrointestinal cancer, tumor samples obtained from 2 patients before and after vorinostat (after three doses of 100 and 400 mg respectively) exhibited increased acetylation of H3 and H4 histones and α-tubulin. In our study, we evaluated paired tumor and blood samples in seven patients before and after third vorinostat dose prior to administration of other antineoplastic therapy. There was increased acetylation of alpha tubulin and the K69 residue of Hsp90, induction of the stress protein Hsp70 (reflecting functional HsP90 inhibition), induction of p27 and p21, and downregulation of cyclin dependent kinase (CDK) 4, confirming the changes induced by vorinostat observed in preclinical studies.[12] [20] In contrast to consistent changes observed in tumor, there were variable changes in PBMCs, indicating that they cannot be used as a surrogates for vorinostat-induced intratumoral effects. These results provide a foundation for evaluation of vorinostat or other HDAC inhibitors in patients when supported by preclinical data.
The results of E2100 demonstrated that the addition of bevacizumab to paclitaxel as firstline therapy in metastatic breast cancer significantly improved objective response rate in the patients with measurable disease (49.2% vs 25.2%, P<0.001). Based on preclinical data demonstrating that vorinostat or other HDAC inhibitors sensitizes cancer cells to paclitaxel and anti-VEFG-directed therapy, we sought to determine the safety and efficacy of vorinostat combined with paclitaxel-bevacizumab. We found it was feasible to add vorinostat at a dose of 300 mg BID when using an intermittent three of seven day schedule, which minimized cumulative toxicities including diarrhea, fatigue, and thrombocytopenia The adverse event profile was consistent with what has been described for paclitaxel-bevacizumab alone, with the exception of more diarrhea, which usually was mild or moderate in severity, reversible and manageable.. In addition, 6 patients (11%) had grade 3 or 4 thromboembolic events, higher than the 2% rate observed for the paclitaxel-bevacizumab arm of E2100. The primary efficacy endpoint of distinguishing between a 60% versus 40% response rate was met based upon the response reported at the time the trial was designed[17]; however, the 55% response rate and median PFS of 11.9 months is similar to that observed for the final results of the E2100 trial for paclitaxel-bevacizumab [14]. In line with the E2100 study we found that the responses were similar between patients with ER positive and ER negative breast cancer (50% vs 44%). Although the role of bevacizumab for the treatment of metastatic breast cancer remains somewhat controversial [21], this study nevertheless provides evidence that combining this regimen with vorinostat is feasible, and may warrant further comparative studies. Other studies have found that even higher doses of vorinostat than used in our trial may be tolerable when using an intermittent schedule, and trials are ongoing evaluating this strategy (NCT01281176) [22].
Several phase III clinical trials are currently underway evaluating vorinostat in various cancers, including in mesothelioma (NCT00128102), in combination with bortezomib in multiple myeloma (NCT00773747), and in combination with radiation therapy (with temozolomide or bevacizumab) in pediatric high grade glioma (NCT01236560). A randomized study of paclitaxel and carboplatin with either placebo or vorinostat as first-line therapy in advanced non-small-cell lung cancer showed a 34% response rate in the vorinostat arm versus 12.5% response rate in the placebo arm. There was a trend toward improvement in median progression-free survival (6.0 months v 4.1 months; P = .48) and overall survival (13.0 months v 9.7 months; P = .17) in the vorinostat arm. [23]. Vorinostat was administered before chemotherapy (400mg daily) for a period of 14 days of a 21-day cycle, and was associated with a higher incidence of grade 4 thrombocytopenia (18% v 3%, P<0.05). A phase III trial failed to confirm a benefit for vorinostat (400mg daily) added to carboplatin/paclitaxel for non-small cell lung cancer using a continuous vorinostat dosing schedule, which was associated with more diarrhea, fatigue, and dehydration (NCT00473889)[24]. This study utilized a different schedule and slightly different eligibility criteria. Vorinostat was given on days 1 through 14 on each cycle, and carboplatin and paclitaxel were administered on day 5 of cycle 1 only and on day 1 of each subsequent cycle. In addition, only patients who were ineligible to receive bevacizumab, or those that did not have access to bevacizumab, were included. To what extent these differences contributed to the divergent results of these two studies are unclear. Additional studies evaluating the intermittent schedule used in our trial may be warranted in order to minimize the potential cumulative toxicity associated with continuous or more protracted drug schedules.
In summary, our study provides evidence that vorinostat may be safely combined with paclitaxel and bevacizumab using an intermittent schedule and does not substantially enhance toxicity of paclitaxel-bevacizumab combination. In addition, in the small paired sample size tested, vorinostat induces histone and non-histone protein hyperacetylation in vivo, resulting in functional Hsp90 inhibition, induction of p27 and p21, and inhibition of CKD4, providing a molecular basis for continued evaluation of this agent in this combination in a prospective comparative manner, and providing a foundation for evaluation of vorinostat with other cytotoxics or targeted therapies [25]when supported by preclinical data.
Supplementary Material
Figure 2. Treatment with VS induces in vivo hyper-acetylation of histone and non-histone proteins in ER-positive and ER-negative breast cancer cells.
Tumor biopsy specimens were collected from ER positive (#001, #010, and #53) and ER-negative (#51, 52, 54, and 55) patients prior to treatment with VS. Four hours following the third dose of VS, on Day 2, a second tumor biopsy was collected and cell lysates were prepared. Immunoblot analyses were performed for acetylated lysine, acetylated α-tubulin, acetylated K56 histone H3, acetylated histone H3, acetylated histone H4, p27 and p21 on the tumor cell lysates. The expression levels of β-actin in the lysates served as the loading control.
Acknowledgements
Supported by Supported by United States Department of Health and Human Service contract N01-CM-62204 (P.I. Joseph A. Sparano, MD) and N01-CM-62207 (PI: Miguel Villalona, MD) and N01 CM62205 (PI: Charles Erlichman MD)
Footnotes
Presented at San Antonio Breast Cancer Conference in December 2008 and at the 2009 Annual Meeting of American Association of Cancer Research at Denver, Colorado.
Disclosures:
The authors have no relevant disclosures except Dr. Vered Stearns who has received research funding from Merck.
REFERENCES
- 1.Marks PA, Richon VM, Breslow R, Rifkind RA. Histone deacetylase inhibitors as new cancer drugs. Curr Opin Oncol. 2001;13(6):477–483. doi: 10.1097/00001622-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Fuino LBP, Wittman S, et al. Histone deacetylse inhibitor LAQ824 down regulated Her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone. B Mol Cancer Ther. 2003;2:971–984. [PubMed] [Google Scholar]
- 3.Isaacs JS, Xu W, Neckers L. Heat shock protein 90 as a molecular target for cancer therapeutics. Cancer Cell. 2003;3(3):213–217. doi: 10.1016/s1535-6108(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Rao R, Shen J, Tang Y, Fiskus W, Nechtman J, Atadja P, Bhalla K. Role of acetylation and extracellular location of heat shock protein 90alpha in tumor cell invasion. Cancer Research. 2008;68(12):4833–4842. doi: 10.1158/0008-5472.CAN-08-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417(6887):455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 6.Marcus AI, Zhou J, O'Brate A, Hamel E, Wong J, Nivens M, El-Naggar A, Yao TP, Khuri FR, Giannakakou P. The synergistic combination of the farnesyl transferase inhibitor lonafarnib and paclitaxel enhances tubulin acetylation and requires a functional tubulin deacetylase. Cancer Research. 2005;65(9):3883–3893. doi: 10.1158/0008-5472.CAN-04-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owonikoko TK, Ramalingam SS, Kanterewicz B, Balius TE, Belani CP, Hershberger PA. Vorinostat increases carboplatin and paclitaxel activity in non-small-cell lung cancer cells. International Journal of Cancer. 126(3):743–755. doi: 10.1002/ijc.24759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duvic M, Talpur R, Ni X, Zhang C, Hazarika P, Kelly C, Chiao JH, Reilly JF, Ricker JL, Richon VM, et al. Phase 2 trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA) for refractory cutaneous T-cell lymphoma (CTCL).[erratum appears in Blood. 2007 Jun 15;109(12):5086] Blood. 2007;109(1):31–39. doi: 10.1182/blood-2006-06-025999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly WKOC,OA, Krug LM. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid in patients with advanced cancer. J Clin Oncol. 2005;23:3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luu THMR, Leong L, et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium Study. Clinical Cancer Research. 2008;14:7138–7142. doi: 10.1158/1078-0432.CCR-08-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bali PPM, Swaby R, Fiskus W, Yamaguchi H, Balasis M, Rocha K, Wang H, Richon V, Bhalla K. Activity of Suberoylanilide Hydroxamic acid against human breast cancer cells with amplificatoin of Her 2. Clinical Cancer Research. 2005;11(17):6382–6389. doi: 10.1158/1078-0432.CCR-05-0344. [DOI] [PubMed] [Google Scholar]
- 12.Fiskus W, Ren Y, Mohapatra A, Bali P, Mandawat A, Rao R, Herger B, Yang Y, Atadja P, Wu J, et al. Hydroxamic acid analogue histone deacetylase inhibitors attenuate estrogen receptor-alpha levels and transcriptional activity: a result of hyperacetylation and inhibition of chaperone function of heat shock protein 90. Clin Cancer Res. 2007;13(16):4882–4890. doi: 10.1158/1078-0432.CCR-06-3093. [DOI] [PubMed] [Google Scholar]
- 13.Deroanne CF, Bonjean K, Servotte S, Devy L, Colige A, Clausse N, Blacher S, Verdin E, Foidart JM, Nusgens BV, et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21(3):427–436. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 14.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. New England Journal of Medicine. 2007;357(26):2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92(3):205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Simon R. Optimal two-stage designs for phase II clinical trials. Controlled Clinical Trials. 1989;10(1):1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 17.Miller KDWM, Gralow J, et al. E2100: a randomized phase III trial of paclitaxel versus paclitaxel plus bevacizumab as first-line therapy for locally recurrent or metastatic breast cancer. Proc Am Soc Clin Oncol 2005. 2005 Late breaking session. [Google Scholar]
- 18.Ree AH, Dueland S, Folkvord S, Hole KH, Seierstad T, Johansen M, Abrahamsen TW, Flatmark K. Vorinostat, a histone deacetylase inhibitor, combined with pelvic palliative radiotherapy for gastrointestinal carcinoma: the Pelvic Radiation and Vorinostat (PRAVO) phase 1 study. Lancet Oncology. 11(5):459–464. doi: 10.1016/S1470-2045(10)70058-9. [DOI] [PubMed] [Google Scholar]
- 19.Galanis EJK, Maurer MJ, et al. Phase II trial of vorinostat in recurrent glioblastoma multiforme: A North Central cancer treatment Group Study. JCO. 2009;27(12):2052–2058. doi: 10.1200/JCO.2008.19.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angelucci A, Mari M, Millimaggi D, Giusti I, Carta G, Bologna M, Dolo V. Suberoylanilide hydroxamic acid partly reverses resistance to paclitaxel in human ovarian cancer cell lines. Gynecol Oncol. 119(3):557–563. doi: 10.1016/j.ygyno.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 21.Burstein HJ. Bevacizumab for advanced breast cancer: all tied up with a RIBBON? J Clin Oncol. 29(10):1232–1235. doi: 10.1200/JCO.2010.33.2684. [DOI] [PubMed] [Google Scholar]
- 22.Dickson MARD, Carvajal RD, et al. A phase I pharmacokinetic study of pulse-dosevorinostat with flavopiridol in solid tumors. Invest New Drugs (2011) 2011;29::1004–1012. doi: 10.1007/s10637-010-9447-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramalingam SS, Maitland ML, Frankel P, Argiris AE, Koczywas M, Gitlitz B, Thomas S, Espinoza-Delgado I, Vokes EE, Gandara DR, et al. Carboplatin and Paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer. Journal of Clinical Oncology. 28(1):56–62. doi: 10.1200/JCO.2009.24.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. NCT00473889: A Phase II/III Randomized, Double-Blind Study of Paclitaxel Plus Carboplatin in Combination With Vorinostat or Placebo in Patients With Stage IIIB (With Pleural Effusion) or Stage IV Non-Small-Cell Lung Cancer (NSCLC) clinicaltrialsgov [Google Scholar]
- 25.Ha K, Fiskus W, Rao R, Balusu R, Venkannagari S, Nalabothula NR, Bhalla KN. Hsp90 inhibitor-mediated disruption of chaperone association of ATR with hsp90 sensitizes cancer cells to DNA damage. Molecular Cancer Therapeutics. 10(7):1194–1206. doi: 10.1158/1535-7163.MCT-11-0094. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.