Abstract
Historically, dosing regimens for the treatment of tuberculosis (TB) have been proposed in an empirical manner. Dose selection has often been the result of efficacy trials in which drugs were administered regardless of the magnitude of the effect of demographic factors on drug disposition. This has created challenges for the prescription of fixed-dose combinations with novel therapeutic agents. The objectives of this investigation were to evaluate the impact of body weight on the overall systemic exposure to pyrazinamide (PZA) and to assess whether the use of one fixed dose, without adjustment according to weight, would ensure target exposure and safety requirements across the overall patient population. Using a population pharmacokinetic model, simulation scenarios were explored based on population demographics from clinical trials in TB patients and on historical hepatotoxicity data. The systemic drug exposure (area under the concentration-time curve [AUC]), peak concentrations (the maximum concentration of drug in serum [Cmax]), the time above the MIC (t > MIC), and the risk of hepatotoxicity were evaluated for the current weight-banded regimen and compared to fixed doses under the assumption that pharmacokinetic differences are the primary drivers of toxicity. Evaluation of the standard weight banding reveals that more than 50% of subjects in the weight range of 45 to 55 kg remain below the proposed target exposure to PZA. In contrast, the use of a fixed 1,500-mg dose resulted in a lower proportion of subjects under the target value, with a 0.2% average overall increase in the risk of hepatotoxicity. Our results strongly support the use of a fixed-dose regimen for PZA in coformulation or combination with novel therapeutic agents.
INTRODUCTION
For the first time in 30 years, the antituberculosis drug development pipeline may be on the verge of delivering significant advances in therapy (32, 50). However, assessing the pharmacodynamics and efficacy of new agents in tuberculosis (TB) patients requires combination therapy with existing agents (1, 8, 9). The use of such combinations imposes the availability of suitable delivery and dosage forms, which do not only ensure adherence and ease of administration but also warrant enhanced efficacy.
One of the best ways of optimizing such regimens is to physically combine the requisite drugs into one preparation, a so-called fixed-dose combination (FDC) product. Indeed, the approach of combination therapy has proven to be successful in the treatment of cancer, infectious diseases, hypertension, and neurological disorders. For the treatment of tuberculosis, the FDC formulations consist of a combination of two or more first-line anti-TB drugs in a fixed proportion in a single-dosage form (2, 3). Pyrazinamide (PZA) is one of the drugs that may contribute to promising combinations with novel agents. It is an essential component of tuberculosis combination chemotherapy and is used during the initial 2 months of treatment for its sterilizing activity (6, 43).
The potential advantages associated with the use of FDCs include (i) a reduced risk of the emergence of drug-resistant strains, (ii) less risk of medication errors, (iii) better patient compliance, (iv) reduced cost of treatment, and (v) simplified drug supply management, shipping, and distribution. Nevertheless, there are major challenges in the formulation of FDCs, the most important of which lies in the choice of the dosage strength of each of the components to be contained in each combination tablet. This challenge is particularly relevant for PZA, which is currently prescribed according to a weight-banded regimen.
The objective of our investigation was therefore to demonstrate the feasibility of using a simplified dosing regimen for PZA as an option, in addition to the currently recommended dosing regimen based on weight bands, without significant changes in the risk associated with hepatotoxicity. It can be anticipated that the availability of an alternative fixed-dose regimen for PZA will provide a framework for the evaluation of novel therapeutic agents for the treatment of tuberculosis.
Historically, the evaluation of safety in clinical trials has relied on direct experimental evidence and the continuous or proactive monitoring of symptoms and signs. Until a decade ago, only a few examples existed of clinical pharmacology research in which pharmacostatistical models were used to support risk management, optimize protocol design, or even replace the need for empirical evidence where appropriate (52). In the current investigation, we applied a population pharmacokinetic model previously published by Wilkins and coworkers (50) as the basis for simulation scenarios in which the impact of body weight is evaluated for a range of doses and a new dosing regimen is proposed. Subsequently, these results are used to evaluate the implications of different dosing regimens for the risk of hepatotoxicity. This is performed by pooling toxicity data from the published literature and assessing the relationship between predicted drug exposure and liver toxicity events under the assumption that pharmacokinetic differences are the primary drivers of toxicity.
In contrast to the empirical evidence from clinical trials, the use of simulation scenarios allows the introduction of optimization concepts in medicine and medical research, which have been common practice in other areas of science for much longer (7, 39). Strangely, in TB research, limited attention has been given to how the use of optimized dosing regimens for drug combinations may contribute to improving efficacy or mitigating the risk of resistance (6, 12). In a number of different therapeutic areas, an increased understanding of pharmacokinetic-pharmacodynamic (PKPD) relationships has enabled a better selection of doses, schedules, and combinations of agents. Moreover, in conjunction with a model-based approach, the assessment of PKPD relationships has allowed better insight into the role of confounding factors and covariates on pharmacokinetics and treatment outcome (34).
MATERIALS AND METHODS
Patient demographic characteristics.
An overview of the study population used for modeling the risks associated with PZA-induced hepatotoxicity is provided in Table 1. The demographic information for body weight across different endemic regions was obtained from the REMoxTB trial (Table 2). For each region, the male-to-female ratio was assumed to be 60:40. A model was developed to describe body weight as normal and log-normal distributions, with gender as a colinearity factor, under the assumption that the average (geometric mean) weight of females is 82.4% of the average male, as has been observed in healthy subjects (23, 24). An estimation was performed for each region of endemicity using only the tail of the weight distribution (i.e., <40 kg and >75 kg). The remaining data were reserved for evaluating the goodness-of-fit and as diagnostics for the comparison between normal and log-normal models.
Table 1.
Overview of the studies used for modeling of the risk associated with PZA-induced hepatotoxicity
| Study no. | PZA dose | Treatment duration | No. of males | No. of females | Total no. of patients | Other drugs in treatment arms, including PZA | Ethnicity | No. of hepatotoxicity incidents at baseline | No. of hepatotoxicity incidents during treatment | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20–25 mg/kg | 6 mo | 278 | 193 | 471 | INH + RIFa | White, black, Hispanic, and Asian | NOb | 20/471 | 17 |
| 2 | 15 mg/kg | 2 mo | 63 | 47 | 110 | RIF | White, black, and Asian | 5/109 | 14/110 | 31 |
| 3 | 20–25 mg/kg | 6 mo | 1,953 | 1,054 | 3,007 | RIF and/or INH | Asian | NO | 113/3,007 | 10 |
| 4 | 25 mg/kg | 12 wk | NAc | NA | 101 | INH | White, black, Hispanic, and Asian | 3/122 | 3/101 | 48 |
| 5 | 40 mg/kg | 12 wk | NA | NA | 94 | INH | White, black, Hispanic, and Asian | NO | 2/94 | 48 |
| 6* | 50 mg/kg | ≥3 mo | NA | NA | 119 | INH | NA | NO | 9/119 | 29, 37 |
| 7 | 25 mg/kg | 24 wk | NA | NA | 160 | INH | NA | NO | 4/160 | 18 |
| 8 | 40 mg/kg | 24 wk | NA | NA | 167 | INH | NA | NO | 11/167 | 18 |
| 9 | 3g/day | ≥4 mo | NA | NA | 261 | INH | NA | NO | 21/261 | 28 |
| 10 | 20–25 mg/kg | 6 mo | 44 | 56 | 100 | INH + RIF + EMBd | NA | 4/26 | 27 |
INH, isoniazid; RIF, rifampin.
NO, not included or not defined in the original publication.
NA, not available.
EMB, ethambutol.
Table 2.
Actual distribution of weight bands in TB patients across different regions of endemicitya
| Range | Weight band (kg) | Distribution (%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Africa | Mexico | Peru | China | India | Korea | Taiwan | Thailand | Malaysia | ||
| WR1 | <40.0 | 4 | 10 | 3 | 5 | 19 | 4 | 7 | 9 | 5 |
| WR2 | 40.0–45.0 | 13 | 15 | 7 | 6 | 29 | 8 | 11 | 14 | 9 |
| WR3 | 45.1–55.0 | 41 | 25 | 37 | 37 | 37 | 18 | 29 | 44 | 20 |
| WR4 | 55.1–75.0 | 40 | 40 | 49 | 42 | 14 | 60 | 45 | 31 | 57 |
| WR5 | >75.0 | 3 | 10 | 4 | 10 | 1 | 10 | 8 | 2 | 10 |
Data are derived from the REMoxTB trial (Clinical Trials identifier NCT00864383 [http://ClinicalTrials.gov]). This is a randomized placebo-controlled double-blind trial comparing two treatment-shortening regimens with the standard regimen.
Pharmacokinetic model.
The population PK model (Fig. 1) proposed by Wilkins and coworkers (50) was used for all simulations in our analyses. Concentration-time measurements were obtained from 227 South African patients with pulmonary tuberculosis receiving oral doses of PZA. Gender and body weight were identified as important covariates for drug distribution and elimination. In addition, the modeling results also clearly indicated a significant contribution of interoccasion variability (IOV) in the absorption and elimination of PZA as the main driver for differences in systemic exposure. In the current analysis, IOV was set to zero to demonstrate the net impact of body weight on the overall variability in the pharmacokinetics of PZA.
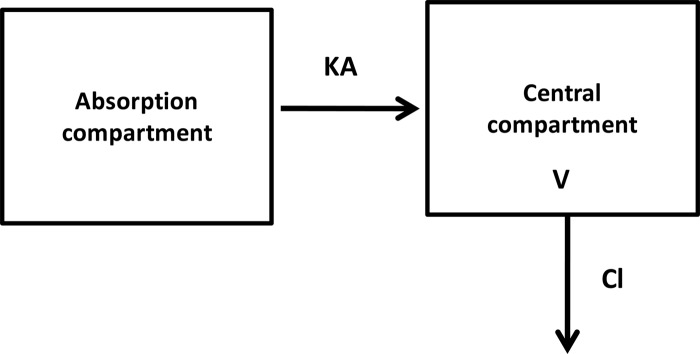
Fig 1.
One-compartment PK model of PZA with first-order absorption and first-order elimination data from Wilkins et al. (48). In this model, body weight is a covariate on clearance (CL), while sex and body weight were found to influence the volume of distribution (V). Most of the variability in drug absorption was characterized by interoccasion variability in the absorption rate constant (KA).
For the purposes of our evaluation, the currently recommended dosing regimens for PZA were used as references. The predicted area under the concentration-time curve over a 7-day PZA period (AUC), the predicted maximum concentration (Cmax), and the predicted percentage of time above the MIC (%t > MIC) over the corresponding period were used as pharmacokinetic measures of interest in the subsequent set of simulation scenarios. While the implications of the peak concentrations for the risk of hepatotoxicity may be evident, published findings suggest that the time above a threshold concentration can be considered a prognostic factor for events that show delayed onset, as in the case of PZA-induced hepatotoxicity (12, 19). For the sake of simplicity, the MIC was assumed to be 12.5 mg/ml (19) and constant throughout the course of therapy. Simulations were performed under the assumption of steady-state conditions, and the results were summarized according to different conditioning factors, including gender, regions of endemicity, and different weight bands. In addition, a target exposure range was defined according to the distributions of the parameters of interest. The assessment of the feasibility of alternative dosing regimens for PZA was based on the premise that the predicted exposure range should remain above the 25th percentile and not exceed the upper range of the current approved regimen.
Logistic model for the risk of hepatotoxicity.
Logistic regression and time-to-event analyses are widely accepted methods in clinical pharmacology. These approaches are often applied to establish whether drug levels can be considered an intermediate or a proxy for treatment response or a safety endpoint (13). In the current investigation, the use of a logistic regression was deemed sufficiently robust to address the underlying clinical question, i.e., to assess whether differences in drug exposure correlate with an increased risk of hepatotoxicity. Data from published literature on the incidence of hepatotoxicity from 1954 to 2010 were aggregated to support the evaluation of the risk associated with increasing exposure to PZA (5, 10, 11, 17, 18, 27, 31, 33, 46, 47). The data extraction for subsequent model fitting included, however, only studies that provided clear details on the treatment duration and combination therapy used in the trial, the hepatotoxicity incidence rates, and population demographics (4, 9, 14, 15, 26, 27, 29, 31, 43). The baseline incidence rates of hepatotoxicity were derived from studies in which data from different treatment arms, with and without PZA, were reported (27, 31, 48). Given the evolution of methods for quantifying changes in liver enzymes and of the clinical criteria applied for identifying hepatotoxicity, a conservative approach was taken for the evaluation of the risk, in which all definitions associated with liver damage and hepatotoxicity were considered (15, 44, 45). Furthermore, for the purposes of this analysis, it was assumed that (i) dropout was not informative during the first 8 weeks of treatment and (ii) no other factors or covariates (e.g., comedication, gender, ethnicity, disease status) directly altered the underlying risk of hepatotoxicity (14, 21, 25, 26). The data informing the estimation of baseline risk were meant to reflect the averaged risk of non-PZA-related hepatotoxicity. In fact, the use of a constant incidence rate for background events (baseline) relies on the assumption that comorbidities or other clinical factors may vary randomly and, as such, would not influence the results of our analysis. Model-based predictions for PZA-induced toxicity were therefore estimated independently of the baseline risk and its variations (including comorbidity rates and region-specific comedications).
The predicted PZA exposure at steady state, expressed as AUC0–168, was assumed to be the most relevant parameter for characterizing the relationship between differences in dosing regimens and the incidence of hepatotoxicity (21). The male-to-female ratios and regions were obtained for each study and used to infer individual body weight and to simulate body weight distributions. Exposure distributions were then simulated for groups of 1,000 patients within the weight range in each study and the predicted exposure values in the upper tail of the distribution linked in ascending order to the reported number of hepatotoxicity events. This procedure was performed by using the ppoints() function in R (version 2.11.1) (38a) with the enrolled number of subjects. The first n − 1 points were taken to be the quantiles of the exposure distribution for which no hepatotoxicity was manifest. The remaining quantiles were assumed to reflect hepatotoxicity. In a second phase, a logistic regression was performed using the mle() function on the total log-likelihood function on the aggregated data from all available studies, in which a wide range of PZA doses were investigated: P = exp (α + β × AUCγ)/[1 + exp (α + β × AUCγ)], where P is the probability associated with the event of interest (i.e., hepatotoxicity), α and β are coefficients, and γ is the exponent of the logistic model.
Simulation of PZA dosing regimens.
To evaluate the impact of body weight on PZA pharmacokinetics and on overall systemic exposure, all patients who responded to short-course therapy with antitubercular drugs were assumed to gain weight (49). A discrete time (daily) Weiner process was used to describe the average 5% increase in weight over a 2-month period while allowing for a 10% probability of weight loss on any given day. The roles of other demographic or clinical covariates were considered to be negligible, and the duration of treatment was set to 56 days (i.e., 8 weeks) for all patients. The data were summarized, taking into account the predefined clinical weight bands (i.e., <40 kg, 40 to 50 kg, 50 to 55 kg, 55 to 75 kg, and >75 kg) (8, 9, 51). Simulations were performed with equal numbers of individuals in each weight band. The standard weight-banded doses (mg/kg) were compared with fixed daily doses of 1,500 mg PZA. The calculated dosing amount was rounded up to the nearest 500 mg to mimic current prescription practice for tablets. If the resulting dose for an individual was more than 400 mg away from the expected regimen, the dose was rounded down.
Comparison of fixed-dose to weight-banded dosing regimens.
Based on the predicted exposure estimates obtained from the pharmacokinetic model, simulations were performed to assess the implications for the risk of hepatotoxicity associated with potential differences in PZA exposure. Scenarios were developed, taking into account population replicates of 1,000 patients each. The results were then summarized in terms of relative risk (RR). The use of the relative risk ratio as a metric of risk is based on the ease of interpretation, i.e., it provides context for the risks already faced by the population as a whole.
Except for the pharmacokinetic data, which were extracted from the literature, all simulation scenarios were performed using the statistical software R (version 2.11.1) (38a). The steady-state concentrations after different dosing regimens were simulated using nonlinear mixed-effects modeling as implemented in NONMEM (version 7.1.2). All the data manipulations and analyses were performed within a validated in-house statistical modeling environment (Predictive Modeling Environment, PME 2.4; Mango Solutions, Chippenham, United Kingdom).
RESULTS
Effect of body weight on PZA pharmacokinetics.
A log-normal distribution was identified as the best model to describe the body weight distribution across the population, enabling accurate replication of the proportion of patients within and outside the target exposure range.
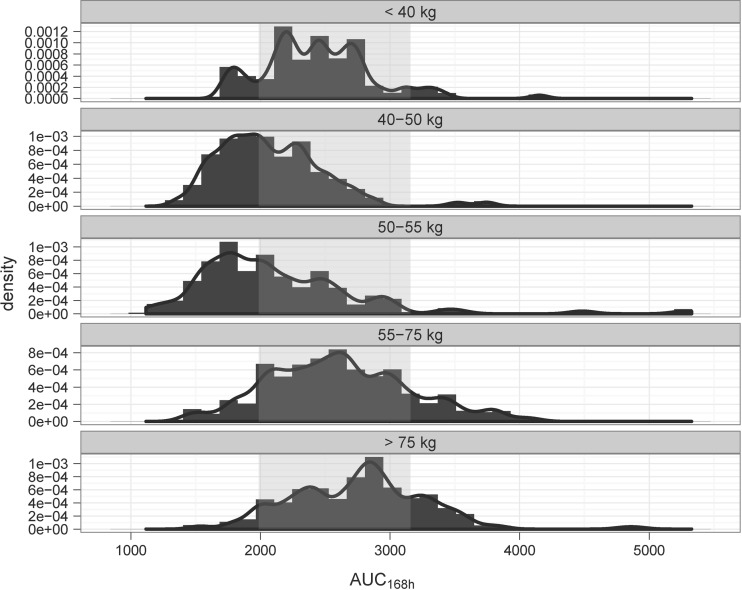
As shown in Fig. 2, different exposures to PZA are observed across the various weight bands despite correction of the dose for body weight. A variety of dose strengths were tested to explore the feasibility of a fixed-dose regimen. Based on the pharmacokinetic criteria, a 1,500-mg dose of PZA was identified as the best alternative to the currently recommended regimen. The predicted pharmacokinetic profiles were simulated for individual patients across the different body weight bands. A summary of the parameter estimates used in the simulation scenarios is presented in Table 3. The impact of body weight on systemic exposure following a fixed-dose regimen is depicted in Fig. 3. The details of the predicted exposure for the overall population and for individual weight groups are shown in Table S4 in the supplemental material. There is a major overlap in the AUC0-168 distributions between the currently recommended weight-banded and the fixed dose of 1,500 mg PZA. In addition, individual pharmacokinetic profiles, including concentration-time curves, indicated that the variability in exposure was not significantly different between regimens. Overall, the changes in the exposure to PZA associated with the fixed-dose regimen were found to be significant only in a small fraction of patients, with 13% of the patients showing a net shift from underexposure to the proposed target range. An increase in exposure to levels beyond the 90th percentile was also observed for a comparable proportion of patients (i.e., 13%). Yet, this increase does not yield exposure levels beyond those observed with the currently recommended dosing regimen.
Fig 2.
A skewed distribution of the exposure (AUC0–168 in mg · h/liter) to PZA is observed across the TB patient population despite dosing according to the currently recommended weight-banded regimen, i.e., from an 18.2 to a 26.3 mg/kg nominal dose. The AUC0–168 distributions include rounding to a whole-tablet dose (500 mg). The data illustrate pharmacokinetics in African TB patients. The shaded area represents the target exposure range between the 25th and 90th percentiles.
Table 3.
Summary of pharmacokinetic and logistic model parameter estimates
| Parameter description | Parametera | Unit | Value | Precision (%) |
|---|---|---|---|---|
| PK model | ||||
| Apparent oral clearance | TV(CL/F) | Liters/h | 3.42 | |
| Apparent vol of distribution | TV(V/F) | Liters | 29.2 | |
| Fast absorption rate constant | TV(ka, fast) | Liters/h | 3.56 | |
| Slow absorption rate constant | TV(ka, slow) | Liters/h | 1.25 | |
| Duration of zero-order absorption | TV(Dur) | h | 0.29 | |
| Proportion of fast absorbers | Pfast | 0.556 | ||
| Slope effect of covariate | θCL/F,WT | 0.0545 | ||
| Slope effect of covariate | θV/F,WT | 0.433 | ||
| Difference in V/F between males and females | θV/F,SEX | Liters | 4.55 | |
| Change in DUR following a fixed-dose combination | θDur,FDC | h | −0.0904 | |
| Stochastic model parameters | BSV(CL/F) | 0.0351 | ||
| BSV(V/F) | 0.0251 | |||
| BSV(Dur) | 0.957 | |||
| IOV(KA) | 0.623 | |||
| RES. ADD | mg/liter | 1.89 | ||
| RES. PROP | 0.0907 | |||
| PD model | ||||
| Probability of hepatotoxicity within 2 mo of comedication only | BASE | 0.0452 | 5 | |
| AUC at which 50% of the maximum probability (1) is reached | LAUCT50 | 8.4786 | 14 | |
| Exponent | EXPN | 3.45 | 7 |
TV, population-estimated typical value; θ, fixed-effect parameter; BSV, between-subject variability; IOV, interoccasion variability; RES. ADD, additive residual error; RES. PROP, proportional residual error.
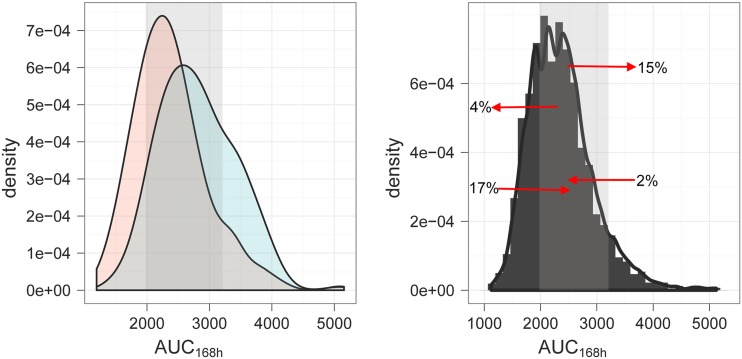
Fig 3.
(Left) Predicted AUC0–168 (mg · h/liter) distribution after a fixed dose of 1,500 mg PZA (blue) compared to that of the currently recommended weight-banded regimen (pink), including rounding to a whole-tablet dose (500 mg). The data illustrate pharmacokinetics in African TB patients. (Right) Overview of the changes in AUC0–168 following the administration of a fixed-dose regimen. The arrows indicate the direction of changes in individual exposure relative to the target range (shaded area) and the proportion (%) of patients affected by the administration of a fixed dose of 1,500 mg. The shaded area represents the target exposure range (i.e., between the 25th and 90th percentiles).
Evaluation of the impact of a fixed-dosing regimen of PZA on the risk of hepatotoxicity.
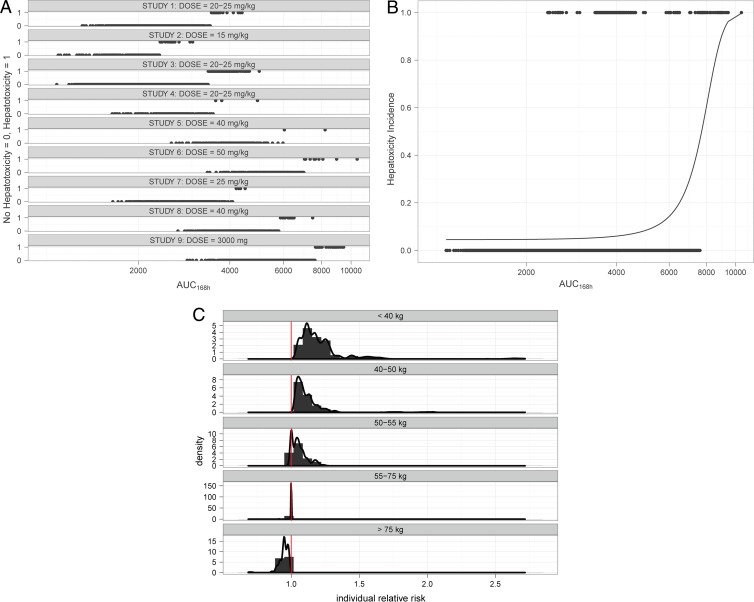
Data from nine studies were aggregated for the evaluation of the risk of hepatotoxicity (Table 1). Aggregations of the clinical trial data were limited to those publications in which treatment duration, drug combinations, demographic covariates, and incidence rates of hepatotoxicity were available (n = 4,490). An overview of the study population, the incidence of hepatotoxicity, and the predicted exposure associated with the adverse events is shown in Fig. 4. The baseline incidence of hepatotoxicity in TB patients was found to be 4.54%. This value was used as a reference for exploring the implications of an increase in systemic exposure to PZA and the intrinsic increase in the risk of hepatotoxicity due to the treatment.
Fig 4.
Aggregation of the clinical trial data in which treatment duration, drug combinations, demographic covariates, and incidence rates of hepatotoxicity were available (n = 4,490). (A) Overview of the dosing regimens and the corresponding incidence rates in each individual study (10, 17, 18, 28, 29, 31, 37, 48). (B) Logistic model. The dots represent the predicted individual exposures, expressed as AUC0–168s (mg · h/liter). Hepatotoxicity is shown as an event at probability P (1), and all patients with no hepatotoxicity are shown at P (0). (C) Predicted increase in the risk of hepatotoxicity for TB patients taking a fixed dose of 1,500 mg PZA compared to that of those taking the standard dosing regimen by weight band. Hepatotoxicity was linked to PZA exposure based on the assumption that these adverse events were entirely driven by increasing drug exposure.
From a pharmacodynamic perspective, the assessment of the predicted time above the MIC reveals that, in contrast to the current regimen, the majority of the patients remain above the MIC more than 50% of the time during the course of therapy. Only those patients weighing more than 75 kg (i.e., approximately 4% of the population in an African cohort) show a slight reduction the time above the MIC with the proposed regimen.
The goodness-of-fit and other diagnostic criteria showed that the use of a logistic model fitted the data adequately well. A summary of the final parameter estimates for the logistic model is presented in Table 3. As shown in Fig. 4, the exposure associated with 50% probability of hepatotoxicity was found to be approximately 7,000 ng/ml · h. However, it should be noted that the inflection of the logistic curve occurs at 5,000 ng/ml · h, a value which is observed at the extreme upper tail of the exposure distribution obtained with the current weight-banded regimen.
Based on the assumption that the increase in PZA exposure is the only driver for hepatotoxicity, it can be inferred that the use of a fixed dose of 1,500 mg PZA results in a minor increase in the risk of hepatotoxicity compared to that with the standard weight-banded dosing regimen. The differences in the absolute risk of hepatotoxicity are shown in Fig. 4 for the overall population and by weight band. Given the very small effect size, a cohort of more than 250,000 patients would be required to achieve 80% statistical power to detect an increase of 0.8% in the risk of hepatotoxicity in TB patients following a fixed dose of 1,500 mg PZA.
DISCUSSION
Given the burden of disease and often the resource-limited context in which TB prevails, effective interventions in which treatment can be delivered effectively in a combined dosage form and as a short-course therapy are needed (32, 43). Traditionally, however, the dosing regimens for first-line-therapy drugs have been based on weight bands. This practice has evolved regardless of the evidence for a clinically relevant effect of demographic factors on drug disposition (22, 30).
From a drug development perspective, the use of weight-banded regimens also carries implications for clinical programs where novel compounds may not be easily combined with background therapy. Such a hurdle may lead to adherence issues and potentially to the termination of efficacious compounds. On the other hand, proper consideration of the influence of body weight is critical for preventing under- or overexposure to the therapeutic agent. Even though the correlation between overexposure to PZA and the increased incidence of liver toxicity remains debatable, underexposure does represent a risk for treatment failure and resistance, both of which are undesirable (4, 9, 35) and deny the therapy area of much-needed innovation.
In this investigation, we have assessed the implications of a fixed-dose regimen on the pharmacokinetics of PZA, taking into account the effect of body weight as a covariate on drug disposition (30, 49). We emphasize that this exercise is not meant to lessen the potential relevance of demographic covariates when defining dosing recommendations. However, dose adjustment should be applied only if differences in exposure have direct clinical implications in terms of safety and efficacy. Hence, the feasibility of fixed-dose combinations cannot be properly addressed without a careful assessment of the covariate effects. This means that depending on the differences in pharmacokinetics, variable rather than fixed ratios may be required.
Although different publications exist in which the population pharmacokinetics of PZA have been described in healthy volunteers and patients (2, 3, 36), we have selected a pharmacokinetic model by Wilkins et al. (50) in which drug disposition was characterized in a South African population with the specific objective of investigating interindividual and interoccasion variability (IIV and IOV, respectively). Such differences in drug exposure may reflect differences in the drug effects, which in turn may have clinical importance. However, while IOV was used to explain the variability in clearance (CL/F) in the original publication, the authors seem to have ignored some important clinical aspects of tuberculosis. We have removed the IOV component from the model because we believe that the observed differences in CL/F were most likely caused by the changes in weight (WT) that occur during treatment. In fact, TB patients gain weight during the course of therapy but are dosed with the same regimen (i.e., based on body weight at baseline). We have instead incorporated time-dependent changes in body weight into our simulations, which lead to comparable variation in systemic exposure, as observed in the original publication.
The availability of PK and pharmacokinetic-pharmacodynamic (PKPD) models has enabled assessment of the clinical implications of covariates on drug exposure and, consequently, of the requirements for the dose and dosing regimen in a wide range of therapeutic indications (7, 39, 52). Unfortunately, the use of PK modeling has been rather limited in the field of tuberculosis, with very few attempts to describe the role of demographic covariates on the pharmacokinetics of first-line-therapy agents. Historically, no systematic evaluation of demographic covariates on the pharmacokinetics of PZA has been made. Yet, a strong covariate effect of body weight seems to have been inferred from the currently recommended dosing regimen based on weight bands (20). The empiricism upon which PZA doses are currently recommended can probably be explained by the confounding effects of comedications (e.g., isoniazid) and the longer treatment duration at the time it was introduced into clinical practice (1, 29, 37). PZA was responsible for shortening the duration of anti-TB rifampin-containing regimens to the current 6-month standard for drug-susceptible tuberculosis (TB) (8, 20, 43).
From a clinical pharmacology perspective, the use of dose titration, adjustment algorithms, or a variable dosing regimen is justifiable when the effects of influential covariates are clinically relevant and can be associated with interindividual differences in exposure (8). Surprisingly, our results show that the use of weight-banded regimens based on mg/kg doses does not effectively correct for the influence of body weight on the disposition of PZA. In fact, recent antimicrobial pharmacokinetic-pharmacodynamic studies suggested that doses higher than the dose of 15 to 30 mg/kg of body weight/day currently used would be more efficacious (19). In addition, the pharmacokinetic data show increased PZA serum clearance and volume of distribution with weight (36, 50), so higher doses than those currently used are required to achieve comparable exposures in heavier and leaner patients.
Despite the evidence for the discrepancies in systemic exposure to PZA across the different weight ranges, the justification underlying the use of a weight-banded regimen is the concern about tolerability and toxicity (38). In particular, rifampin and PZA each have hepatotoxic side effects which increase when the drugs are combined (14, 18, 22). The published data also show that the rate of recurrence of hepatotoxicity in the re-treatment of tuberculosis is lower when a regimen that does not include PZA is gradually reintroduced than when the full-dose regimen including PZA is given again (41, 44, 45). It is clear that one possible way in which the frequency of adverse events might be reduced and resistance prevented is by paying greater attention to the overall (systemic and target organ) exposure, as opposed to the delivery of an empirical dose related to the patient's body weight.
Interestingly, clinical studies in the 1970s included a fixed dose of 300 mg for isoniazid, whereas rifampin and PZA were prescribed in doses of 450 or 600 mg and 1.5 or 2.0 g, respectively; the higher doses were given to patients weighing 50 kg or more (18). In some studies, fixed doses of all 3 drugs have been given (16, 22), including 2 g PZA. This issue was also the subject of a recent meta-analysis in which Gumbo et al. assessed how often toxicity occurs with PZA in monotherapy versus in combination therapy for active TB. In their investigation, they also explored the role of dose and dose schedule on the frequency of adverse events (AEs) (19). For all the AEs in their investigation, the presence or absence of PZA did not result in a significantly higher frequency of side effects. Moreover, no relationship between PZA dose and hepatotoxicity could be demonstrated. In fact, a review of the available data regarding hepatotoxicity after administration of PZA suggests that this adverse event is most likely due to a combination of factors, including medical history and hypersensitivity to one or more of the drugs recommended during treatment (35, 42, 44).
This is the first time that a model-based approach has been used to identify a suitable dosing regimen for an antituberculosis drug and to support the subsequent use of a compound in fixed-dose combinations. Our results indicate that the requirement for a weight-banded regimen is not mandatory. Indeed, as was previously demonstrated by Wilkins et al. (50), interoccasion variability in absorption and elimination may play an even more important role in the variability of PZA pharmacokinetics than body weight itself. The current findings also concur with results of a meta-analysis performed by Pasipanodya and Gumbo (35) and support further questioning of prior attestations that the addition of PZA to a regimen, or increasing pyrazinamide doses, significantly increases anti-TB-drug-related adverse events. This represents an increasing body of evidence against previous case studies that suggested that PZA-containing regimens may entail large increases to the incidence and/or severity of drug-induced liver injury (11, 40). Nevertheless, our assessment of the implications of a fixed-dose regimen was based on a conservative assumption about causality (i.e., worst-case scenario) in which a relationship was hypothesized between the dose of PZA and hepatotoxicity.
The results from our simulations of the systemic exposure associated with a fixed dose of 1,500 mg PZA show that patients exposed to higher levels of PZA are unlikely to experience a clinically relevant increase in their overall risk of hepatotoxicity, while those showing a decrease in exposure are expected to continue to respond to treatment. Yet, the exposures predicted in the lightest individuals (and therefore the highest predicted risk) receiving the fixed-dose combination are similar to those predicted for 40- to 55-kg individuals with the current therapy. It should be noted that the estimated mean increase of 0.8% in the risk of hepatotoxicity for patients weighting <40 kg assumes that the only factors that alter risk are differences in PZA exposure. In reality, evidence exists to indicate that the risk of hepatotoxicity associated with the use of PZA is likely the result of a combination of factors, including comedications, underlying disease conditions, and previous medical history of liver disease (17, 25, 26, 42).
In summary, the use of a fixed-dose regimen with 1,500 mg PZA can be recommended as an alternative regimen for the treatment of TB. The changes in systemic exposure and the associated risk of hepatotoxicity due to a fixed-dose regimen are acceptable both from a pharmacokinetic and safety perspective. This finding enables further evaluation of fixed-dose combinations with PZA. While these combinations have the potential to make treatments safer, more effective, and more convenient to patients, they also entail a greater chance of demonstrating efficacy for new therapeutic agents, which are highly needed for TB.
Supplementary Material
Footnotes
Published ahead of print 9 July 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Acocella G, Angel JH. 1986. Short-course chemotherapy of pulmonary tuberculosis: a new approach to drug dosage in the initial intensive phase. Am. Rev. Respir. Dis. 134:1283–1286 [DOI] [PubMed] [Google Scholar]
- 2. Acocella G, et al. 1993. Bioavailability of isoniazid, rifampicin and pyrazinamide (in free combination or fixed-triple formulation) in intermittent antituberculous chemotherapy. Monaldi Arch. Chest Dis. 48:205–209 [PubMed] [Google Scholar]
- 3. Agrawal S, et al. 2004. Comparative bioavailability of rifampicin, isoniazid and pyrazinamide from a four drug fixed dose combination with separate formulations at the same dose levels. Int. J. Pharm. 276:41–49 [DOI] [PubMed] [Google Scholar]
- 4. Angel JH, Somner AR, Citron MK. 1979. Toxicity of antituberculosis drugs with special reference to hepatotoxicity. Bull. Int. Union Tuberc. 54:47–48 [Google Scholar]
- 5. Baghaei P, et al. 2010. Incidence, clinical and epidemiological risk factors, and outcome of drug-induced hepatitis due to antituberculous agents in new tuberculosis cases. Am. J. Ther. 17:17–22 [DOI] [PubMed] [Google Scholar]
- 6. Blumberg HM, et al. 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603–662 [DOI] [PubMed] [Google Scholar]
- 7. Cella M, Gorter de Vries F, Burger D, Danhof M, Della Pasqua O. 2010. A model-based approach to dose selection in early pediatric development. Clin. Pharmacol. Ther. 87:294–302 [DOI] [PubMed] [Google Scholar]
- 8. Chang KC, Leung CC, Grosset J, Yew WW. 2010. Treatment of tuberculosis and optimal dosing schedules. Thorax 66:997–1007 [DOI] [PubMed] [Google Scholar]
- 9. Chang KC, Leung CC, Yew WW, Chan SL, Tam CM. 2006. Dosing schedules of 6-month regimens and relapse for pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 174:1153–1158 [DOI] [PubMed] [Google Scholar]
- 10. Chang KC, Leung CC, Yew WW, Lau TY, Tam CM. 2008. Hepatotoxicity of pyrazinamide: cohort and case-control analyses. Am. J. Respir. Crit. Care Med. 177:1391–1396 [DOI] [PubMed] [Google Scholar]
- 11. Chang KC, Leung CC, Yew WW, Tam CM. 2007. Standard anti-tuberculosis treatment and hepatotoxicity: do dosing schedules matter? Eur. Respir. J. 29:347–351 [DOI] [PubMed] [Google Scholar]
- 12. Drusano GL. 2003. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin. Infect. Dis. 36 (Suppl 1):S42–S50 [DOI] [PubMed] [Google Scholar]
- 13. Duan JZ. 2009. Two commonly used methods for exposure–adverse events analysis: comparisons and evaluations. J. Clin. Pharmacol. 49:540–552 [DOI] [PubMed] [Google Scholar]
- 14. Durand F, et al. 1995. Deleterious influence of pyrazinamide on the outcome of patients with fulminant or subfulminant liver failure during antituberculous treatment including isoniazid. Hepatology 21:929–932 [PubMed] [Google Scholar]
- 15. Durand F, Jebrak G, Pessayre D, Fournier M, Bernuau J. 1996. Hepatotoxicity of antitubercular treatments. Rationale for monitoring liver status. Drug Saf. 15:394–405 [DOI] [PubMed] [Google Scholar]
- 16. Escreet BC, Cowie RL. 1981. Short-course chemotherapy for pulmonary tuberculosis. A 100-day interrupted regimen. S. Afr. Med. J. 60:951–955 [PubMed] [Google Scholar]
- 17. Fernandez-Villar A, et al. 2004. The influence of risk factors on the severity of anti-tuberculosis drug-induced hepatotoxicity. Int. J. Tuberc. Lung Dis. 8:1499–1505 [PubMed] [Google Scholar]
- 18. Girling DJ. 1978. The hepatic toxicity of antituberculosis regimens containing isoniazid, rifampicin and pyrazinamide. Tubercle 59:13–32 [DOI] [PubMed] [Google Scholar]
- 19. Gumbo T, Dona CS, Meek C, Leff R. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob. Agents Chemother. 53:3197–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hall RG, Leff RD, Gumbo T. 2009. Treatment of active pulmonary tuberculosis in adults: current standards and recent advances: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29:1468–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hussain Z, Kar P, Husain SA. 2003. Antituberculosis drug-induced hepatitis: risk factors, prevention and management. Indian J. Exp. Biol. 41:1226–1232 [PubMed] [Google Scholar]
- 22. Kleeberg HH, Swart DJ, Carey SM. 1983. The tolerability and efficacy of a 6-month antituberculosis regimen containing rifampicin, isoniazid and pyrazinamide. S. Afr. Med. J. 64:693–696 [PubMed] [Google Scholar]
- 23. Kuczmarski RJ, et al. 2000. CDC growth charts: United States. Adv. Data (314):1–27 [PubMed] [Google Scholar]
- 24. Kuczmarski RJ, et al. 2002. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. (246):111–190 [PubMed] [Google Scholar]
- 25. Lee AM, Mennone JZ, Jones RC, Paul WS. 2002. Risk factors for hepatotoxicity associated with rifampin and pyrazinamide for the treatment of latent tuberculosis infection: experience from three public health tuberculosis clinics. Int. J. Tuberc. Lung Dis. 6:995–1000 [PubMed] [Google Scholar]
- 26. Lee SW, et al. 2010. NAT2 and CYP2E1 polymorphisms and susceptibility to first-line anti-tuberculosis drug-induced hepatitis. Int. J. Tuberc. Lung Dis. 14:622–626 [PubMed] [Google Scholar]
- 27. Makhlouf HA, Helmy A, Fawzy E, El-Attar M, Rashed HA. 2008. A prospective study of antituberculous drug-induced hepatotoxicity in an area endemic for liver diseases. Hepatol. Int. 2:353–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matthews JH. 1960. Pyrazinamide and isoniazid used in the treatment of pulmonary tuberculosis. Am. Rev. Respir. Dis. 81:348–351 [Google Scholar]
- 29. McDermott W, et al. 1954. Pyrazinamide-isoniazid in tuberculosis. Am. Rev. Tuberc. 69:319–333 [DOI] [PubMed] [Google Scholar]
- 30. McIlleron H, et al. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob. Agents Chemother. 50:1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McNeill L, Allen M, Estrada C, Cook P. 2003. Pyrazinamide and rifampin vs isoniazid for the treatment of latent tuberculosis: improved completion rates but more hepatotoxicity. Chest 123:102–106 [DOI] [PubMed] [Google Scholar]
- 32. Mitchison DA, Davies GR. 2008. Assessment of the efficacy of new anti-tuberculosis drugs. Open Infect. Dis. J. 2:59–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mount FW, Wunderlich GS, Murray FJ, Ferebee SH. 1959. Hepatic toxicity of pyrazinamide used with isoniazid in tuberculous patients. Public Health Serv. Invest. 1(50):371–387 [Google Scholar]
- 34. Pasipanodya JG, Gumbo T. 2011. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob. Agents Chemother. 55:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pasipanodya JG, Gumbo T. 2010. Clinical and toxicodynamic evidence that high-dose pyrazinamide is not more hepatotoxic than the low doses currently used. Antimicrob. Agents Chemother. 54:2847–2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peloquin CA, et al. 1997. Population pharmacokinetic modeling of isoniazid, rifampin, and pyrazinamide. Antimicrob. Agents Chemother. 41:2670–2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Phillips S, Horton GE. 1956. Pyrazinamide-isoniazid: comparison with isoniazid-para-aminosalicylic acid in active pulmonary tuberculosis with the choice of regimens determined by chance. Am. Rev. Tuberc. 73:704–715 [DOI] [PubMed] [Google Scholar]
- 38. Ramakrishnan CV, et al. 1968. Toxicity of pyrazinamide, administered once weekly in high dosage, in tuberculous patients. Bull. World Health Organ. 39:775–779 [PMC free article] [PubMed] [Google Scholar]
- 38a. R Development Core Team 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 39. Santen G, Horrigan J, Danhof M, Della Pasqua O. 2009. From trial and error to trial simulation. Part 2: an appraisal of current beliefs in the design and analysis of clinical trials for antidepressant drugs. Clin. Pharmacol. Ther. 86:255–262 [DOI] [PubMed] [Google Scholar]
- 40. Saukkonen JJ, et al. 2006. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am. J. Respir. Crit. Care Med. 174:935–952 [DOI] [PubMed] [Google Scholar]
- 41. Sharma SK, et al. 2010. Safety of 3 different reintroduction regimens of antituberculosis drugs after development of antituberculosis treatment-induced hepatotoxicity. Clin. Infect. Dis. 50:833–839 [DOI] [PubMed] [Google Scholar]
- 42. Singh J, et al. 1995. Antituberculosis treatment-induced hepatotoxicity: role of predictive factors. Postgrad. Med. J. 71:359–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Steele MA, Des Prez RM. 1988. The role of pyrazinamide in tuberculosis chemotherapy. Chest 94:845–850 [DOI] [PubMed] [Google Scholar]
- 44. Tahaoglu K, et al. 2001. The management of anti-tuberculosis drug-induced hepatotoxicity. Int. J. Tuberc. Lung Dis. 5:65–69 [PubMed] [Google Scholar]
- 45. Teleman MD, Chee CB, Earnest A, Wang YT. 2002. Hepatotoxicity of tuberculosis chemotherapy under general programme conditions in Singapore. Int. J. Tuberc. Lung Dis. 6:699–705 [PubMed] [Google Scholar]
- 46. Tost JR, et al. 2005. Severe hepatotoxicity due to anti-tuberculosis drugs in Spain. Int. J. Tuberc. Lung Dis. 9:534–540 [PubMed] [Google Scholar]
- 47. Tostmann A, et al. 2008. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J. Gastroenterol. Hepatol. 23:192–202 [DOI] [PubMed] [Google Scholar]
- 48. United States Public Health Service 1959. Hepatic toxicity of pyrazinamide used with isoniazid in tuberculous patients. United States Public Health Service Tuberculosis Therapy Trial. Am. Rev. Respir. Dis. 80:371–387 [DOI] [PubMed] [Google Scholar]
- 49. Vasantha M, Gopi PG, Subramani R. 2009. Weight gain in patients with tuberculosis treated under directly observed treatment short-course (DOTS). Indian J. Tuberc. 56:5–9 [PubMed] [Google Scholar]
- 50. Wilkins JJ, et al. 2006. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur. J. Clin. Pharmacol. 62:727–735 [DOI] [PubMed] [Google Scholar]
- 51. World Health Organization 2010. WHO report: global tuberculosis control. World Health Organization, Geneva, Switzerland [Google Scholar]
- 52. Zhang L, et al. 2006. Model-based drug development: the road to quantitative pharmacology. J. Pharmacokinet. Pharmacodyn. 33:369–393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.